Autophagy Protects against Eosinophil Cytolysis and Release of DNA
Abstract
:1. Introduction
2. Methods
2.1. Subjects, Eosinophil Preparation and Cultures
2.2. Adhesion Assays
2.3. Western Blot
2.4. Reactive Oxygen Species (ROS) Measurement
2.5. Microscopy
2.6. Cytolysis Measurement of Eosinophils on HA-IgG
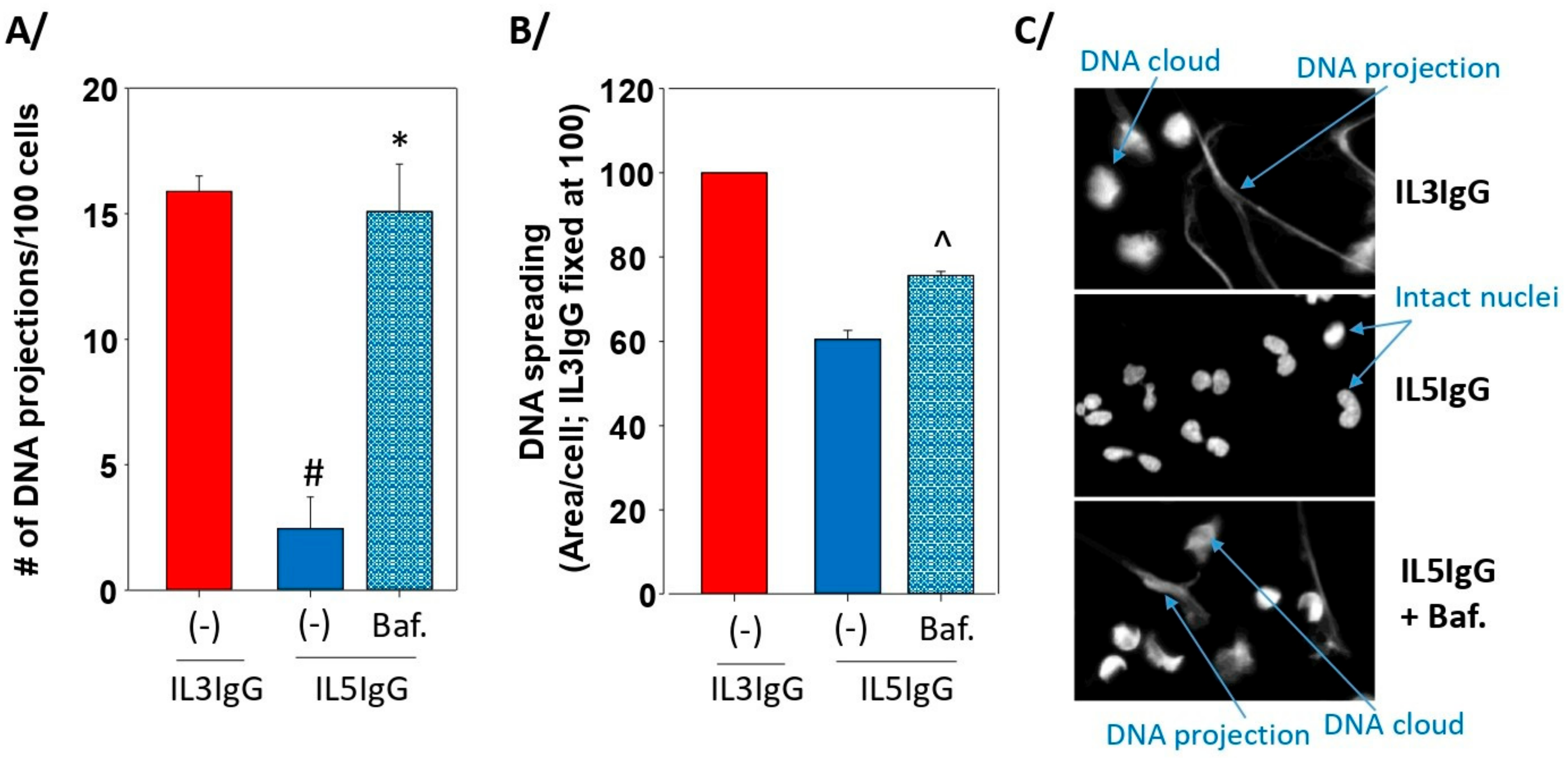
2.7. DNA Spreading and Projections
2.8. Statistical Analyses
3. Results
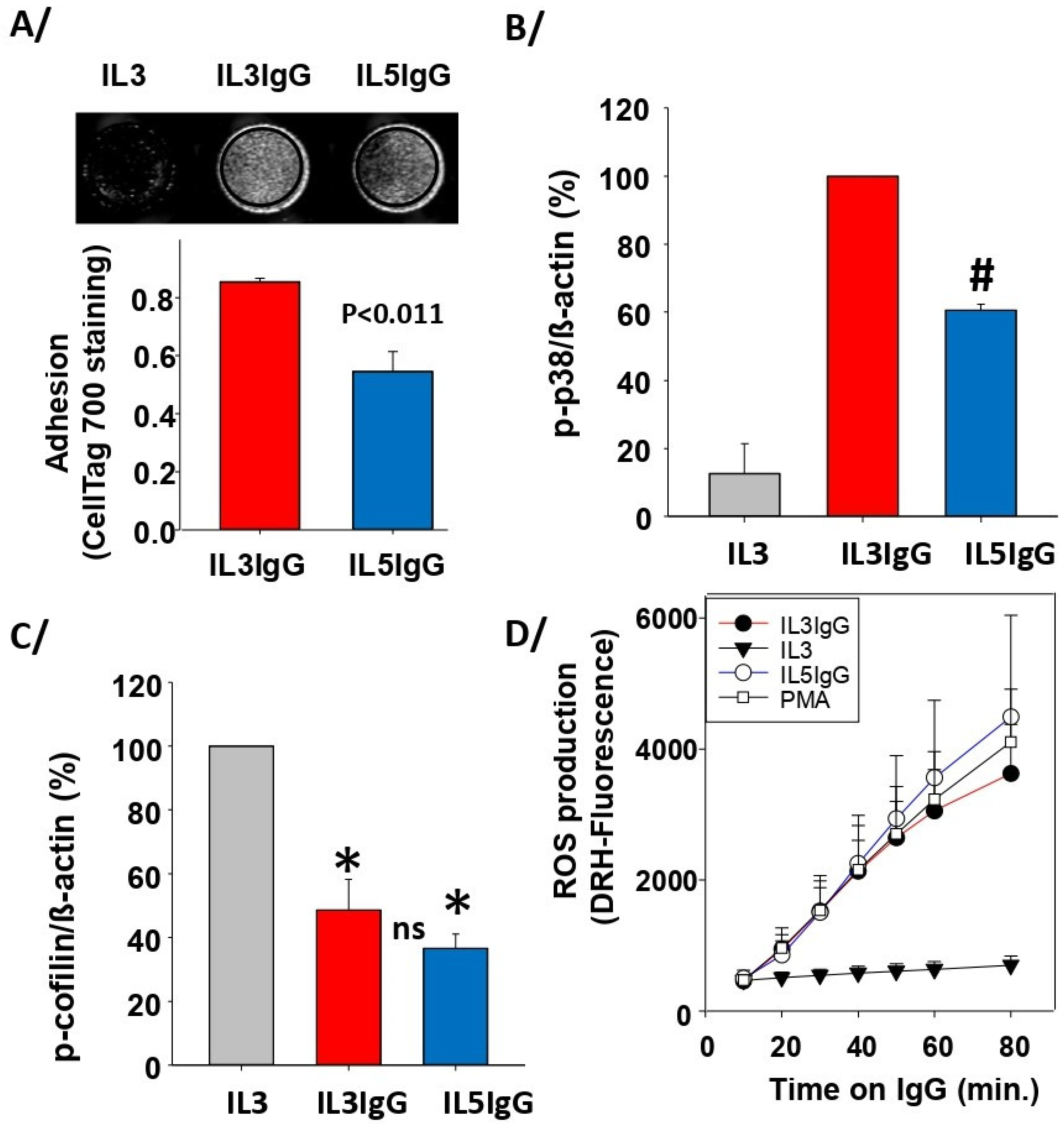
3.1. Both IL5 and IL3 Priming Led to Adhesion on IgG, Phosphorylation of p38, Dephosphorylation of Cofilin and to High Production of ROS after Interaction with IgG
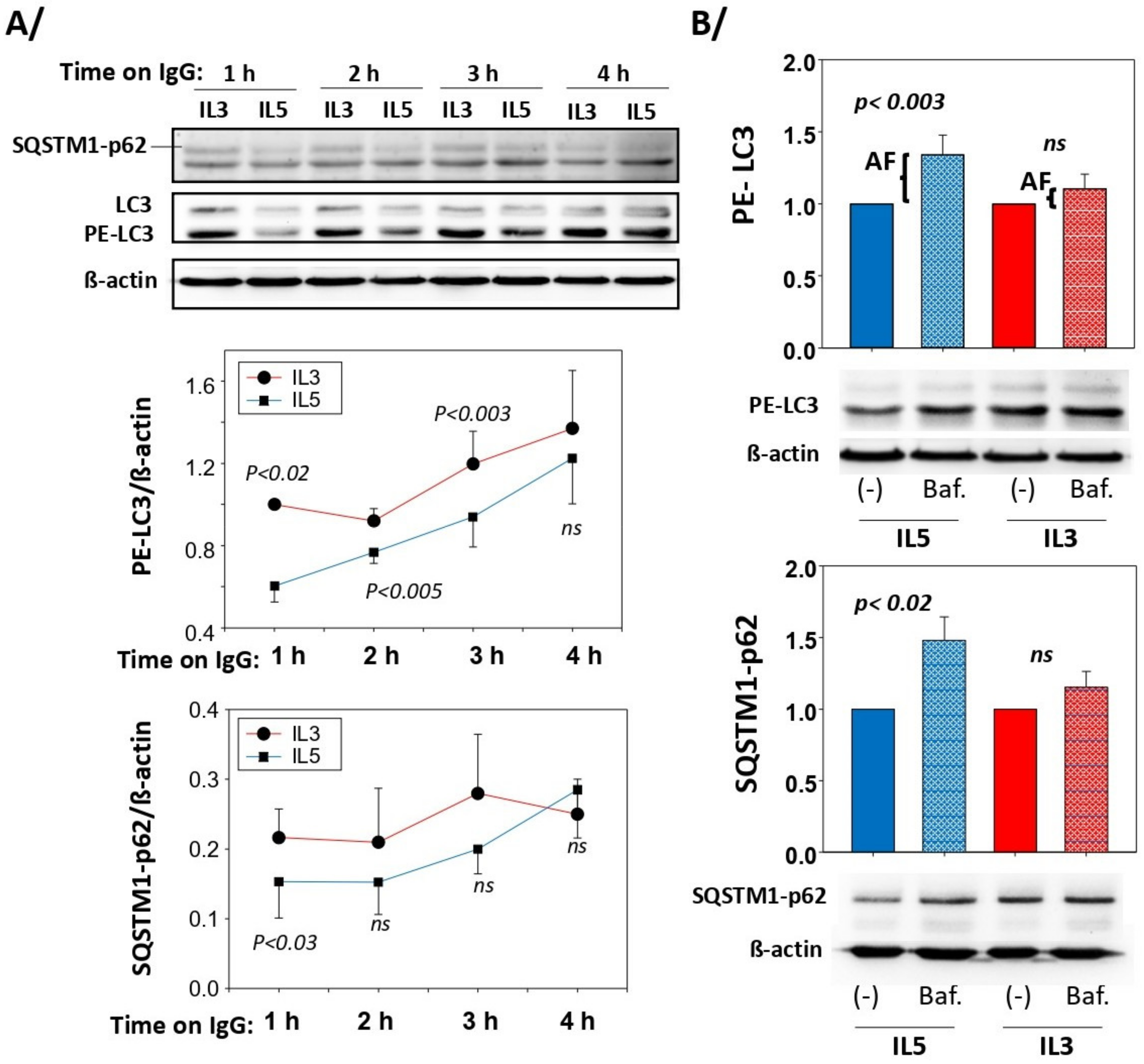
3.2. Autophagic Flux Is Higher after IL5 Priming than IL3 Priming
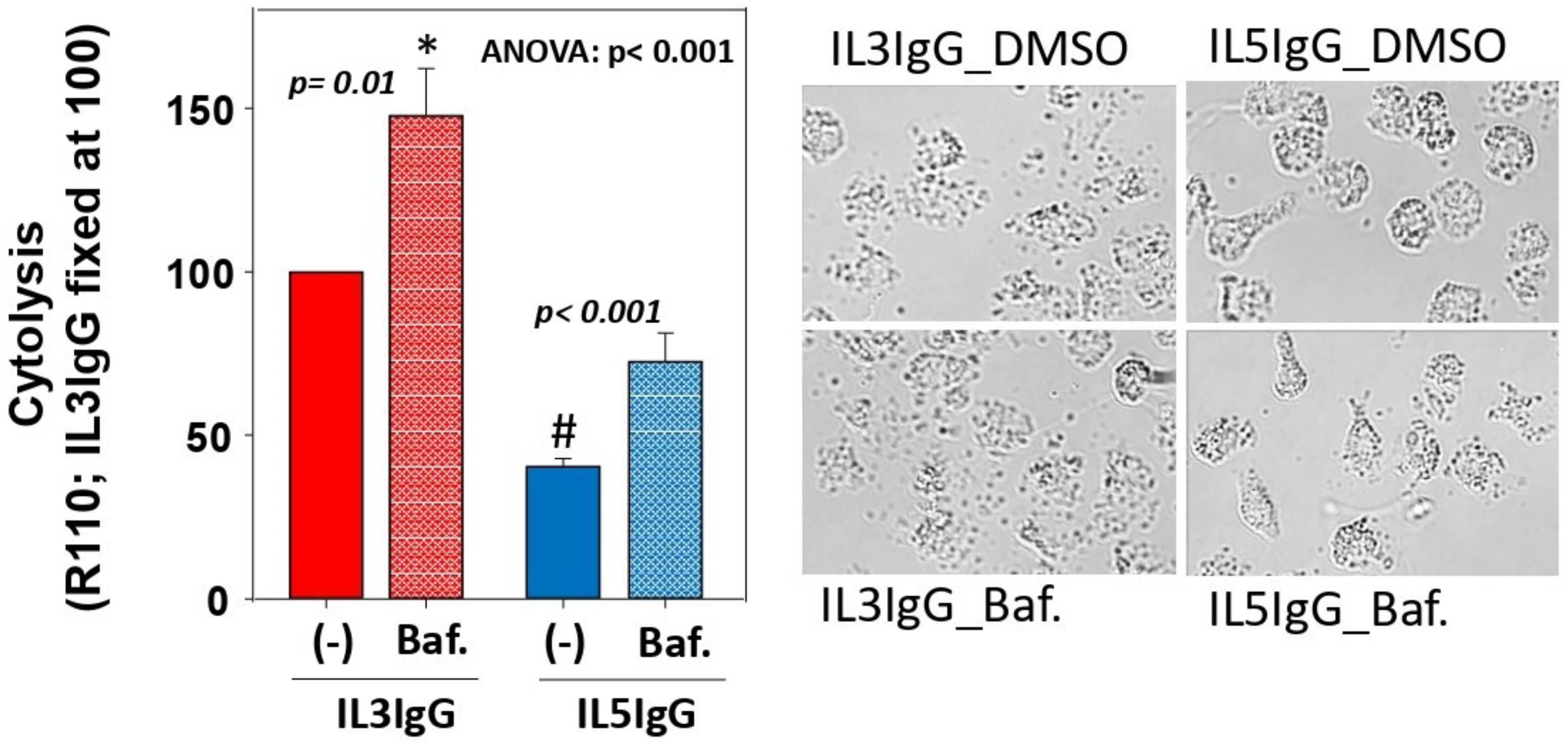
3.3. Blockade of Autophagolysosome Formation Increases IL5-Primed Eosinophil Lysis on IgG
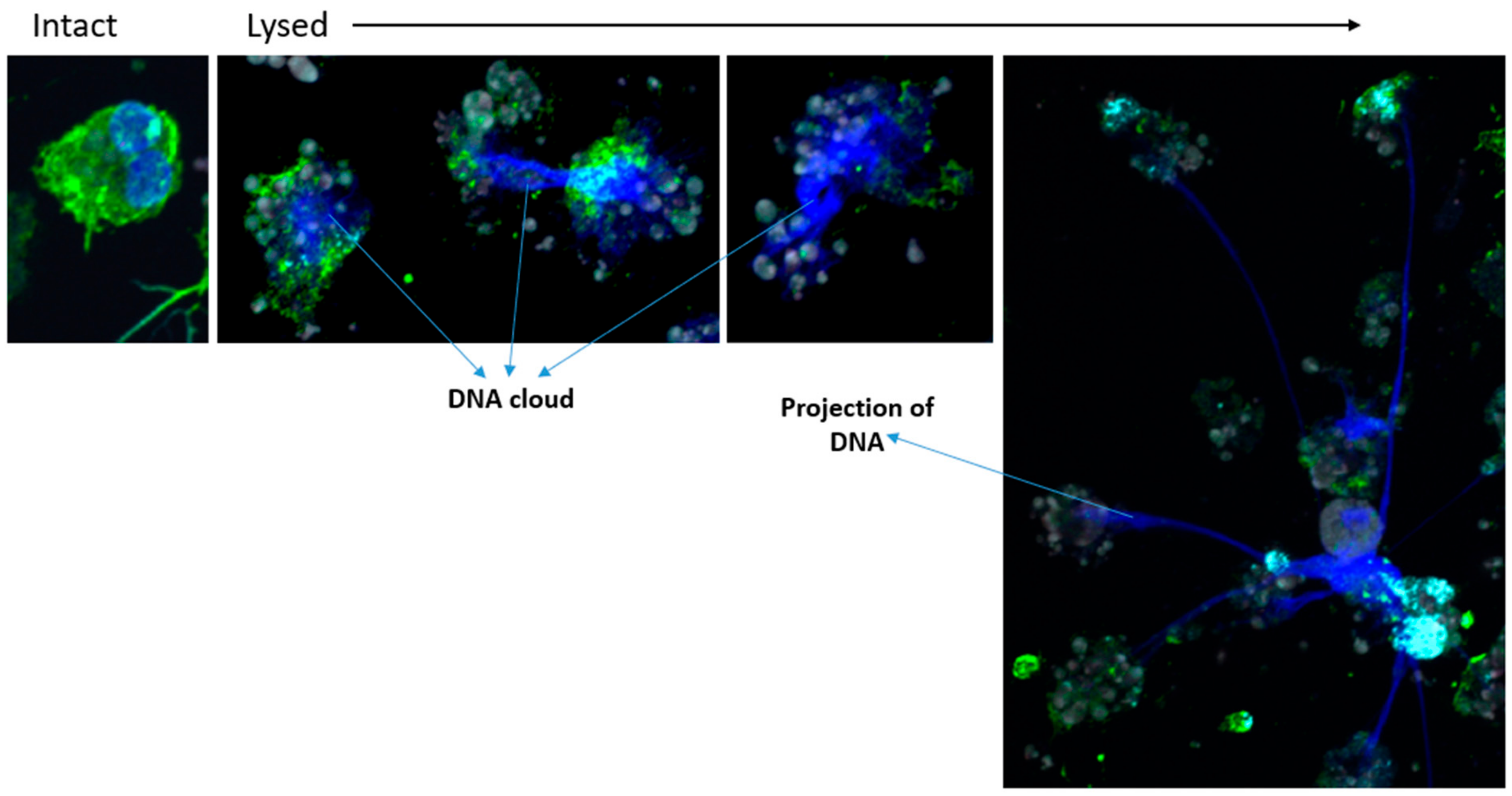
3.4. The Formation of Autophagolysosomes Controls the Release of DNA Traps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masterson, J.C.; Menard-Katcher, C.; Larsen, L.D.; Furuta, G.T.; Spencer, L.A. Heterogeneity of Intestinal Tissue Eosinophils: Potential Considerations for Next-Generation Eosinophil-Targeting Strategies. Cells 2021, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Salter, B.M.; Ju, X.; Sehmi, R. Eosinophil Lineage-Committed Progenitors as a Therapeutic Target for Asthma. Cells 2021, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clinic. Proc. 2021, 96, 2694–2707. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.F.; Ott, N.L.; Peterson, E.A.; George, T.J.; Hukee, M.J.; Gleich, G.J.; Leiferman, K.M. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J. Allergy Clin. Immunol. 1997, 99, 683–692. [Google Scholar] [CrossRef]
- Erjefält, J.S.; Andersson, M.; Greiff, L.; Korsgren, M.; Gizycki, M.; Jeffery, P.K.; Persson, G.A. Cytolysis and piecemeal degranulation as distinct modes of activation of airway mucosal eosinophils. J. Allergy Clin. Immunol. 1998, 102, 286–294. [Google Scholar] [CrossRef]
- Erjefält, J.S.; Greiff, L.; Andersson, M.; Matsson, E.; Petersen, H.; Linden, M.; Ansari, T.; Jeffery, P.K.; Persson, C.G. Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am. J. Respir. Crit. Care Med. 1999, 160, 304–312. [Google Scholar] [CrossRef]
- Uller, L.; Andersson, M.; Greiff, L.; Persson, C.G.; Erjefalt, J.S. Occurrence of apoptosis, secondary necrosis, and cytolysis in eosinophilic nasal polyps. Am. J. Respir. Crit. Care Med. 2004, 170, 742–747. [Google Scholar] [CrossRef]
- Kelly, E.A.; Esnault, S.; Liu, L.Y.; Evans, M.D.; Johansson, M.W.; Mathur, S.; Mosher, D.F.; Denlinger, L.C.; Jarjour, N.N. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 1385–1395. [Google Scholar] [CrossRef]
- Yousefi, S.; Simon, D.; Stojkov, D.; Karsonova, A.; Karaulov, A.; Simon, H.U. In vivo evidence for extracellular DNA trap formation. Cell Death Dis. 2020, 11, 300. [Google Scholar] [CrossRef]
- Miyabe, Y.; Kobayashi, Y.; Fukuchi, M.; Saga, A.; Moritoki, Y.; Saga, T.; Akuthota, P.; Ueki, S. Eosinophil-mediated inflammation in the absence of eosinophilia. Asia Pac. Allergy 2021, 11, e30. [Google Scholar] [CrossRef]
- Erjefält, J.S.; Korsgren, M.; Nilsson, M.C.; Sundler, F.; Persson, C.G. Association between inflammation and epithelial damage-restitution processes in allergic airways in vivo. Clin. Exp. Allergy 1997, 27, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Kamide, Y.; Ueki, S.; Miyabe, Y.; Konno, Y.; Oka, N.; Takeuchi, H.; Koyota, S.; Hirokawa, M.; Yamada, T.; et al. Eosinophil ETosis-Mediated Release of Galectin-10 in Eosinophilic Granulomatosis With Polyangiitis. Arthritis Rheumatol. 2021, 73, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Tokunaga, T.; Melo, R.C.N.; Saito, H.; Honda, K.; Fukuchi, M.; Konno, Y.; Takeda, M.; Yamamoto, Y.; Hirokawa, M.; et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood 2018, 132, 2183–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueki, S.; Melo, R.C.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 2013, 121, 2074–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, J.S.; Perez, S.A.; Spencer, L.A.; Melo, R.C.; Reynolds, L.; Ghiran, I.; Mahmudi-Azer, S.; Odemuyiwa, S.O.; Dvorak, A.M.; Moqbel, R.; et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc. Natl. Acad. Sci. USA 2008, 105, 18478–18483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radonjic-Hoesli, S.; Wang, X.; de Graauw, E.; Stoeckle, C.; Styp-Rekowska, B.; Hlushchuk, R.; Simon, D.; Spaeth, P.J.; Yousefi, S.; Simon, H.U. Adhesion-induced eosinophil cytolysis requires the receptor-interacting protein kinase 3 (RIPK3)-mixed lineage kinase-like (MLKL) signaling pathway, which is counterregulated by autophagy. J. Allergy Clin. Immunol. 2017, 140, 1632–1642. [Google Scholar] [CrossRef] [Green Version]
- Esnault, S.; Johansson, M.W.; Kelly, E.A.; Koenderman, L.; Mosher, D.F.; Jarjour, N.N. IL-3 up-regulates and activates human eosinophil CD32 and alphaMbeta2 integrin causing degranulation. Clin. Exp. Allergy 2017, 47, 488–498. [Google Scholar] [CrossRef]
- Esnault, S.; Leet, J.P.; Johansson, M.W.; Barretto, K.T.; Fichtinger, P.S.; Fogerty, F.J.; Bernau, K.; Mathur, S.K.; Mosher, D.F.; Sandbo, N.; et al. Eosinophil cytolysis on Immunoglobulin G is associated with microtubule formation and suppression of rho-associated protein kinase signalling. Clin. Exp. Allergy 2020, 50, 198–212. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A. Essential mechanisms of differential activation of eosinophils by IL-3 compared to GM-CSF and IL-5. Crit. Rev. Immunol. 2016, 36, 429–444. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.Y.; Sedgwick, J.B.; Bates, M.E.; Vrtis, R.F.; Gern, J.E.; Kita, H.; Jarjour, N.N.; Busse, W.W.; Kelly, E.A.B. Decreased expression of membrane IL-5R alpha on human eosinophils: I. Loss of membrane IL-5 alpha on eosinophils and increased soluble IL-5R alpha in the airway after antigen challenge. J. Immunol. 2002, 169, 6452–6458. [Google Scholar]
- Liu, L.Y.; Sedgwick, J.B.; Bates, M.E.; Vrtis, R.F.; Gern, J.E.; Kita, H.; Jarjour, N.N.; Busse, W.W.; Kelly, E.A.B. Decreased expression of membrane IL-5R alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J. Immunol. 2002, 169, 6459–6466. [Google Scholar]
- Esnault, S.; Kelly, E.A.; Shen, Z.J.; Johansson, M.W.; Malter, J.S.; Jarjour, N.N. IL-3 maintains activation of the p90S6K/RPS6 pathway and increases translation in human eosinophils. J. Immunol. 2015, 195, 2529–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esnault, S.; Bernau, K.; Torr, E.E.; Bochkov, Y.A.; Jarjour, N.N.; Sandbo, N. RNA-sequencing analysis of lung primary fibroblast response to eosinophil-degranulation products predicts downstream effects on inflammation, tissue remodeling and lipid metabolism. Respir. Res. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; del Razo, L.M.; Quintanilla-Vega, B.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Oxidative stress, redox signaling, and autophagy: Cell death versus survival. Antioxid. Redox Signal. 2014, 21, 66–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, N.Y.; Suh, Y.A.; Lee, C. Involvement of ROS in Curcumin-induced Autophagic Cell Death. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2011, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martin, P.; Komatsu, M. p62/SQSTM1-steering the cell through health and disease. J. Cell Sci. 2018, 131, jcs222836. [Google Scholar] [CrossRef] [Green Version]
- Orhon, I.; Reggiori, F. Assays to Monitor Autophagy Progression in Cell Cultures. Cells 2017, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Daubeuf, F.; Schall, N.; Petit-Demouliere, N.; Frossard, N.; Muller, S. An Autophagy Modulator Peptide Prevents Lung Function Decrease and Corrects Established Inflammation in Murine Models of Airway Allergy. Cells 2021, 10, 2468. [Google Scholar] [CrossRef]
- Germic, N.; Hosseini, A.; Stojkov, D.; Oberson, K.; Claus, M.; Benarafa, C.; Calzavarini, S.; Angelillo-Scherrer, A.; Arnold, I.C.; Muller, A.; et al. ATG5 promotes eosinopoiesis but inhibits eosinophil effector functions. Blood 2021, 137, 2958–2969. [Google Scholar] [CrossRef]
- Corcelle, E.; Djerbi, N.; Mari, M.; Nebout, M.; Fiorini, C.; Fenichel, P.; Hofman, P.; Poujeol, P.; Mograbi, B. Control of the autophagy maturation step by the MAPK ERK and p38, lessons from environmental carcinogens. Autophagy 2007, 3, 57–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Su, J.; Li, M.; Li, T.; Wang, X.; Zhao, M.; Hu, X. Myricetin Induces Autophagy and Cell Cycle Arrest of HCC by Inhibiting MARCH1-Regulated Stat3 and p38 MAPK Signaling Pathways. Front. Pharmacol. 2021, 12, 709526. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.C.; Yu, L.; Wang, H.J.; Tashiro, S.; Onodera, S.; Ikejima, T. TNFalpha-induced necroptosis and autophagy via supression of the p38-NF-kappaB survival pathway in L929 cells. J. Pharmacol. Sci. 2011, 117, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celia, A.I.; Colafrancesco, S.; Barbati, C.; Alessandri, C.; Conti, F. Autophagy in Rheumatic Diseases: Role in the Pathogenesis and Therapeutic Approaches. Cells 2022, 11, 1359. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Choi, Y.J.; Lee, H.K. The Role of Autophagy in the Function of CD4(+) T Cells and the Development of Chronic Inflammatory Diseases. Front. Pharmacol. 2022, 13, 860146. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Handa, K.; Murakami, T.; Aizawa, T.; Ozawa, H. Chaperone-Mediated Autophagy in Neurodegenerative Diseases and Acute Neurological Insults in the Central Nervous System. Cells 2022, 11, 1205. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, J.; Liu, Q.; Su, J.; Zhao, Y.; Zheng, G.; Yang, Z.; Zhuo, D.; Ma, C.; Fan, G. The Induction of Endothelial Autophagy and Its Role in the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 831847. [Google Scholar] [CrossRef]
- Gundamaraju, R.; Lu, W.; Paul, M.K.; Jha, N.K.; Gupta, P.K.; Ojha, S.; Chattopadhyay, I.; Rao, P.V.; Ghavami, S. Autophagy and EMT in cancer and metastasis: Who controls whom? Biochim. Biophys. Acta Mol. Basis. Dis. 2022, 1868, 166431. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef]
- Ban, G.Y.; Pham, D.L.; Trinh, T.H.; Lee, S.I.; Suh, D.H.; Yang, E.M.; Ye, Y.M.; Shin, Y.S.; Chwae, Y.J.; Park, H.S. Autophagy mechanisms in sputum and peripheral blood cells of patients with severe asthma: A new therapeutic target. Clin. Exp. Allergy 2016, 46, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.S.; Antunes, G.L.; Kaiber, D.B.; da Costa, M.S.; Marques, E.P.; Ferreira, F.S.; Gassen, R.B.; Breda, R.V.; Wyse, A.T.S.; Pitrez, P.; et al. Reactive oxygen species are involved in eosinophil extracellular traps release and in airway inflammation in asthma. J. Cell Physiol. 2019, 234, 23633–23646. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pliyev, B.K.; Menshikov, M. Differential effects of the autophagy inhibitors 3-methyladenine and chloroquine on spontaneous and TNF-alpha-induced neutrophil apoptosis. Apoptosis Int. J. Program. Cell Death 2012, 17, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Suh, D.H.; Trinh, H.K.; Chwae, Y.J.; Park, H.S.; Shin, Y.S. The role of autophagy in allergic inflammation: A new target for severe asthma. Exp. Mol. Med. 2016, 48, e243. [Google Scholar] [CrossRef]
- Kurashima, K.; Numata, M.; Yachie, A.; Sai, Y.; Ishizaka, N.; Fijimura, M.; Matsuda, T.; Ohkuma, S. The role of vascular H+-ATPase in the control of intragranular pH and exocytosis in eosinophils. Lab. Investig. 1996, 75, 689–698. [Google Scholar]
- Bankers-Fulbright, J.L.; Kephart, G.M.; Bartemes, K.R.; Kita, H.; O’Grady, S.M. Platelet-activating factor stimulates cytoplasmic alkalinization and granule acidification in human eosinophils. J. Cell Sci. 2004, 117, 5749–5757. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esnault, S.; Fichtinger, P.S.; Barretto, K.T.; Fogerty, F.J.; Bernau, K.; Mosher, D.F.; Mathur, S.K.; Sandbo, N.; Jarjour, N.N. Autophagy Protects against Eosinophil Cytolysis and Release of DNA. Cells 2022, 11, 1821. https://doi.org/10.3390/cells11111821
Esnault S, Fichtinger PS, Barretto KT, Fogerty FJ, Bernau K, Mosher DF, Mathur SK, Sandbo N, Jarjour NN. Autophagy Protects against Eosinophil Cytolysis and Release of DNA. Cells. 2022; 11(11):1821. https://doi.org/10.3390/cells11111821
Chicago/Turabian StyleEsnault, Stephane, Paul S. Fichtinger, Karina T. Barretto, Frances J. Fogerty, Ksenija Bernau, Deane F. Mosher, Sameer K. Mathur, Nathan Sandbo, and Nizar N. Jarjour. 2022. "Autophagy Protects against Eosinophil Cytolysis and Release of DNA" Cells 11, no. 11: 1821. https://doi.org/10.3390/cells11111821
APA StyleEsnault, S., Fichtinger, P. S., Barretto, K. T., Fogerty, F. J., Bernau, K., Mosher, D. F., Mathur, S. K., Sandbo, N., & Jarjour, N. N. (2022). Autophagy Protects against Eosinophil Cytolysis and Release of DNA. Cells, 11(11), 1821. https://doi.org/10.3390/cells11111821






