Pathobiology and Therapeutic Relevance of GSK-3 in Chronic Hematological Malignancies
Abstract
:1. Introduction
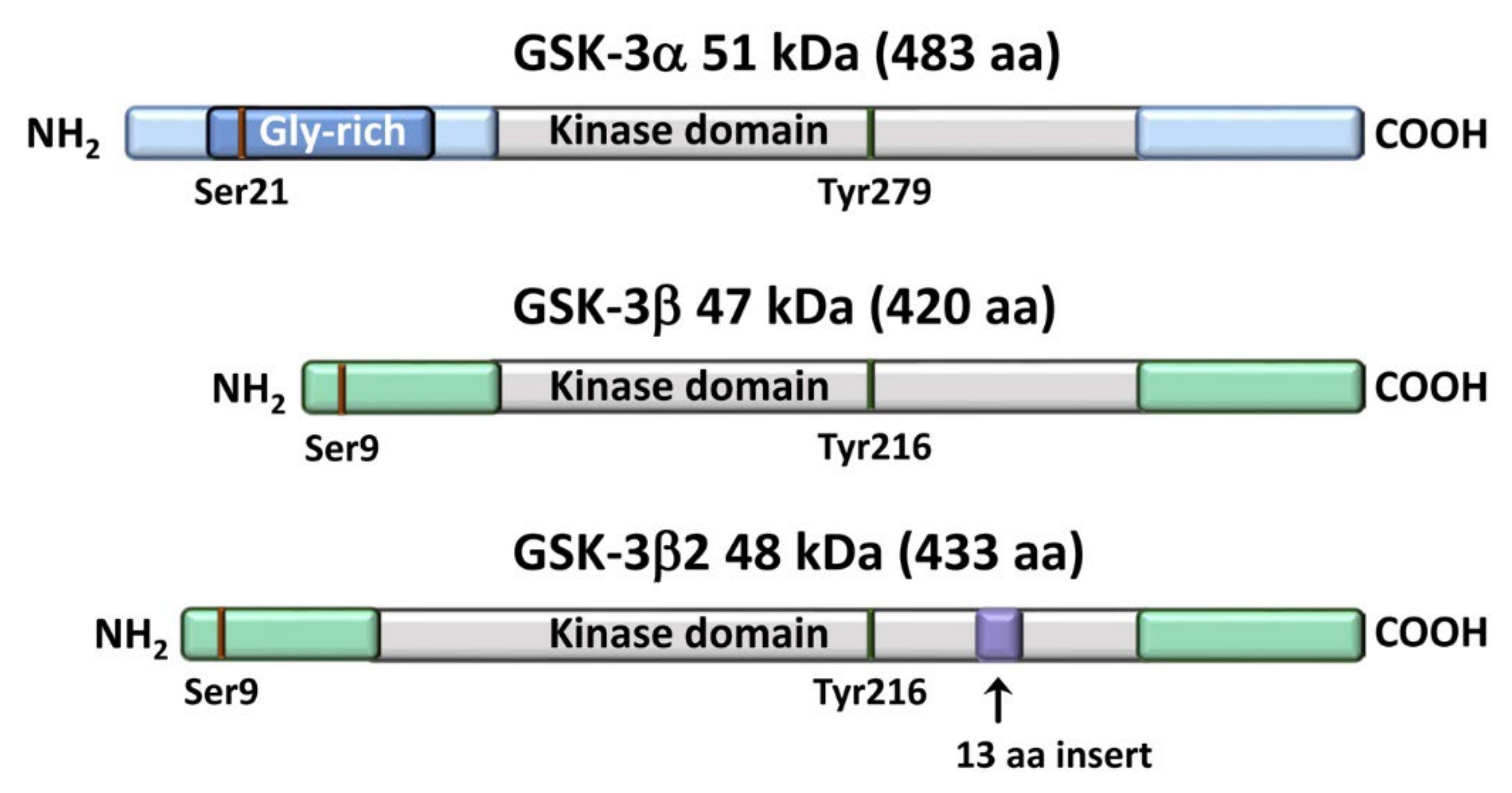
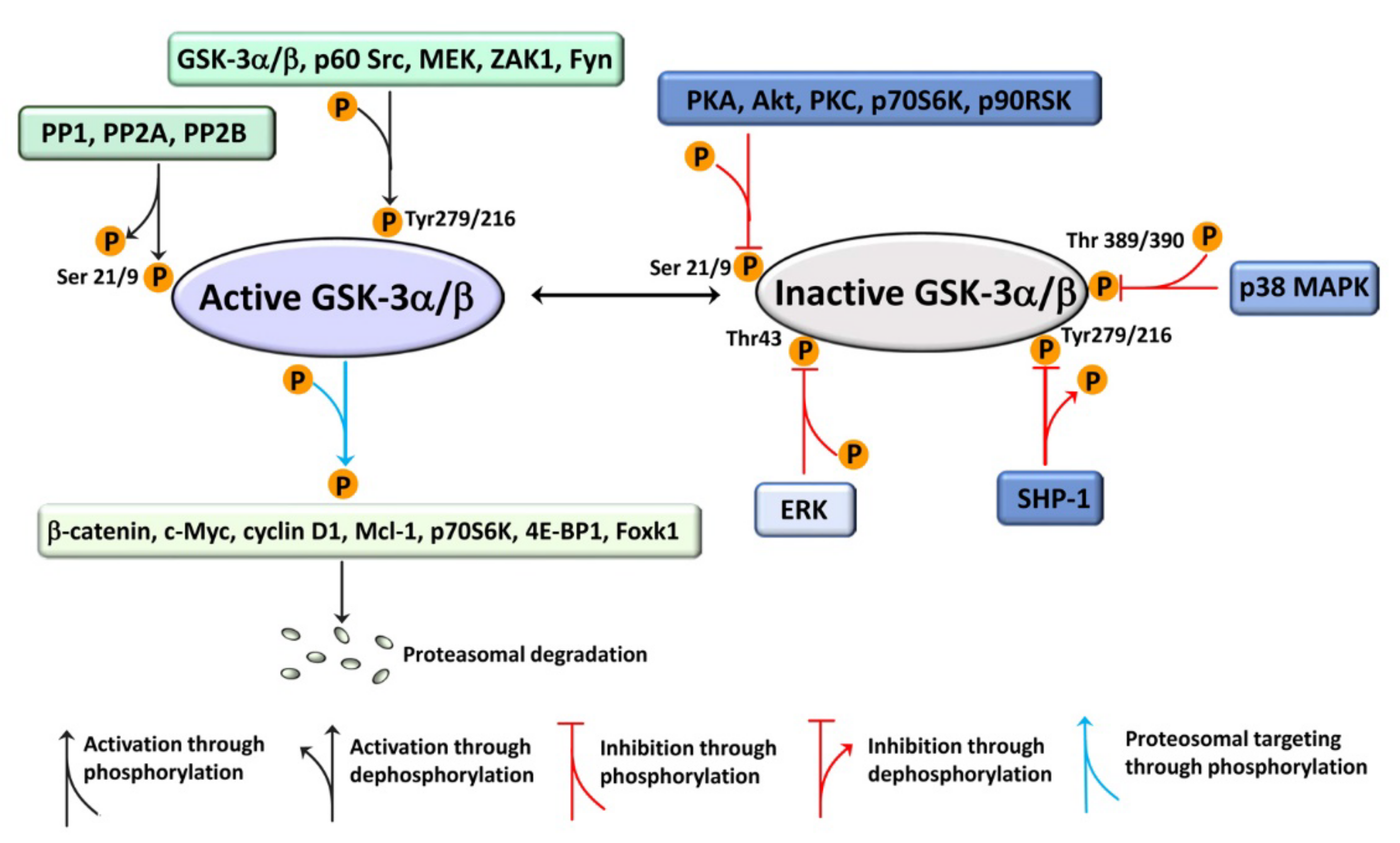
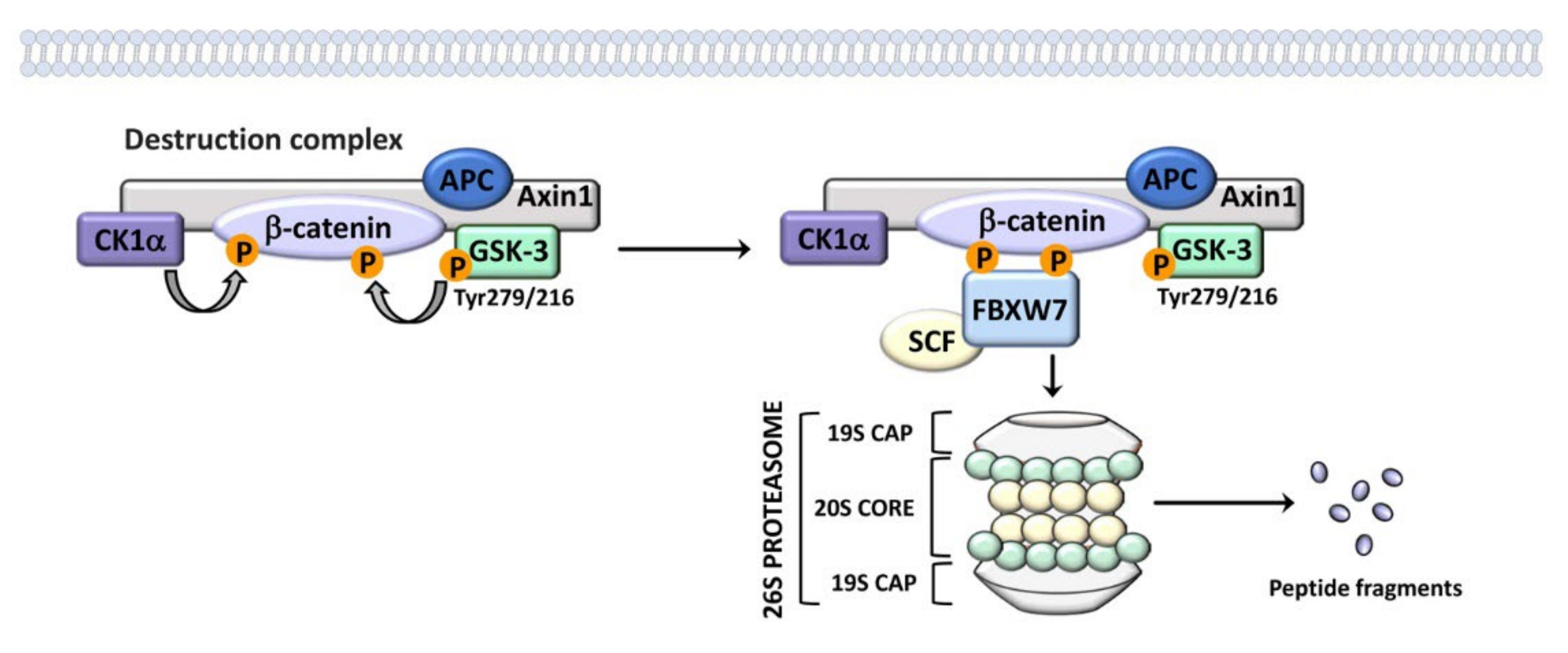
2. An Overview of GSK-3 Signaling
2.1. GSK-3 Signaling in Healthy B-Cells
3. GSK-3 Signaling in CML
4. GSK-3 Signaling in CLL
5. GSK-3 Signaling in MM
6. GSK-3 Signaling in B-Cell NHLs
7. Role of GSK-3 in the Immunosuppressive Microenvironment of Chronic Hematological Malignancies
8. New Strategies for Targeting GSK-3 in Cancer Cells
9. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, H.R.; Rein, L.A.M. The Evolving Landscape of Frontline Therapy in Chronic Phase Chronic Myeloid Leukemia (CML). Curr. Hematol. Malign. Rep. 2021, 16, 448–454. [Google Scholar] [CrossRef]
- Roeker, L.E.; Thompson, M.; Mato, A.R. Current Treatment of Chronic Lymphocytic Leukemia: The Diminishing Role of Chemoimmunotherapy. Drugs 2022, 82, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Elnair, R.A.; Holstein, S.A. Evolution of Treatment Paradigms in Newly Diagnosed Multiple Myeloma. Drugs 2021, 81, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Shingleton, J.; Wang, J.; Baloh, C.; Dave, T.; Davis, N.; Happ, L.; Jadi, O.; Kositsky, R.; Li, X.; Love, C.; et al. Non-Hodgkin Lymphomas: Malignancies Arising from Mature B Cells. Cold Spring Harb. Perspect. Med. 2021, 11, a034843. [Google Scholar] [CrossRef]
- Harrington, P.; Radia, D.; de Lavallade, H. What are the considerations for tyrosine kinase inhibitor discontinuation in chronic-phase chronic myeloid leukemia? Expert Rev. Hematol. 2020, 13, 213–222. [Google Scholar] [CrossRef]
- Delgado, J.; Nadeu, F.; Colomer, D.; Campo, E. Chronic lymphocytic leukemia: From molecular pathogenesis to novel therapeutic strategies. Haematologica 2020, 105, 2205–2217. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhao, L.; Zhou, Y.; Shi, J.; Chen, W.; Li, J. A review on the treatment of multiple myeloma with small molecular agents in the past five years. Eur. J. Med. Chem. 2021, 229, 114053. [Google Scholar] [CrossRef]
- Watanabe, T. Approaches of the Innate Immune System to Ameliorate Adaptive Immunotherapy for B-Cell Non-Hodgkin Lymphoma in Their Microenvironment. Cancers 2021, 14, 141. [Google Scholar] [CrossRef]
- Cho, S.F.; Xing, L.; Anderson, K.C.; Tai, Y.T. Promising Antigens for the New Frontier of Targeted Immunotherapy in Multiple Myeloma. Cancers 2021, 13, 6136. [Google Scholar] [CrossRef]
- Hallek, M.; Al-Sawaf, O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Goncalves, A.C.; Rutella, S.; Almeida, A.M.; De Las Rivas, J.; Trougakos, I.P.; Sarmento Ribeiro, A.B. Resistance to Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia-From Molecular Mechanisms to Clinical Relevance. Cancers 2021, 13, 4820. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2022 update. Pharmacol. Res. 2022, 175, 106037. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagaron, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Yeaman, S.J.; Armstrong, J.L.; Bonavaud, S.M.; Poinasamy, D.; Pickersgill, L.; Halse, R. Regulation of glycogen synthesis in human muscle cells. Biochem. Soc. Trans. 2001, 29, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Kim, N.G.; Gumbiner, B.M. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle 2009, 8, 4032–4039. [Google Scholar] [CrossRef] [Green Version]
- McCubrey, J.A.; Rakus, D.; Gizak, A.; Steelman, L.S.; Abrams, S.L.; Lertpiriyapong, K.; Fitzgerald, T.L.; Yang, L.V.; Montalto, G.; Cervello, M.; et al. Effects of mutations in Wnt/β-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-Diverse effects on cell growth, metabolism and cancer. Biochim. Biophys. Acta 2016, 1863, 2942–2976. [Google Scholar] [CrossRef]
- Dey, S.; Brothag, C.; Vijayaraghavan, S. Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals. Front. Cell Dev. Biol. 2019, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Seira, O.; Del Rio, J.A. Glycogen synthase kinase 3 β (GSK3β) at the tip of neuronal development and regeneration. Mol. Neurobiol. 2014, 49, 931–944. [Google Scholar] [CrossRef]
- Jaworski, T.; Banach-Kasper, E.; Gralec, K. GSK-3β at the Intersection of Neuronal Plasticity and Neurodegeneration. Neural Plast. 2019, 2019, 4209475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier, K.W.; Woodgett, J.R. Recent advances in understanding the cellular roles of GSK-3. F1000Res 2017, 6, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandekar, M.P.; Valvassori, S.S.; Dal-Pont, G.C.; Quevedo, J. Glycogen Synthase Kinase-3β as a Putative Therapeutic Target for Bipolar Disorder. Curr. Drug Metab. 2018, 19, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Arioka, M.; Takahashi-Yanaga, F. Glycogen synthase kinase-3 inhibitor as a multi-targeting anti-rheumatoid drug. Biochem. Pharmacol. 2019, 165, 207–213. [Google Scholar] [CrossRef]
- Huang, A.; Patel, S.; McAlpine, C.S.; Werstuck, G.H. The Role of Endoplasmic Reticulum Stress-Glycogen Synthase Kinase-3 Signaling in Atherogenesis. Int. J. Mol. Sci. 2018, 19, 1607. [Google Scholar] [CrossRef] [Green Version]
- Nagini, S.; Sophia, J.; Mishra, R. Glycogen synthase kinases: Moonlighting proteins with theranostic potential in cancer. Semin. Cancer Biol. 2019, 56, 25–36. [Google Scholar] [CrossRef]
- Demuro, S.; Di Martino, R.M.C.; Ortega, J.A.; Cavalli, A. GSK-3β, FYN, and DYRK1A: Master Regulators in Neurodegenerative Pathways. Int. J. Mol. Sci. 2021, 22, 9098. [Google Scholar] [CrossRef]
- D’Mello, S.R. When Good Kinases Go Rogue: GSK3, p38 MAPK and CDKs as Therapeutic Targets for Alzheimer’s and Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 5911. [Google Scholar] [CrossRef]
- Mancinelli, R.; Carpino, G.; Petrungaro, S.; Mammola, C.L.; Tomaipitinca, L.; Filippini, A.; Facchiano, A.; Ziparo, E.; Giampietri, C. Multifaceted Roles of GSK-3 in Cancer and Autophagy-Related Diseases. Oxid. Med. Cell Longev. 2017, 2017, 4629495. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118659. [Google Scholar] [CrossRef]
- Cole, A.R. GSK3 as a Sensor Determining Cell Fate in the Brain. Front. Mol. Neurosci. 2012, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.Y.; Snider, W.D. Functions of GSK-3 Signaling in Development of the Nervous System. Front. Mol. Neurosci. 2011, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, B.; Wiedau-Pazos, M.; Geschwind, D.H. Gene structure and alternative splicing of glycogen synthase kinase 3 β (GSK-3β) in neural and non-neural tissues. Gene 2003, 302, 73–81. [Google Scholar] [CrossRef]
- Wood-Kaczmar, A.; Kraus, M.; Ishiguro, K.; Philpott, K.L.; Gordon-Weeks, P.R. An alternatively spliced form of glycogen synthase kinase-3β is targeted to growing neurites and growth cones. Mol. Cell Neurosci. 2009, 42, 184–194. [Google Scholar] [CrossRef]
- Itoh, S.; Saito, T.; Hirata, M.; Ushita, M.; Ikeda, T.; Woodgett, J.R.; Algul, H.; Schmid, R.M.; Chung, U.I.; Kawaguchi, H. GSK-3α and GSK-3β proteins are involved in early stages of chondrocyte differentiation with functional redundancy through RelA protein phosphorylation. J. Biol. Chem. 2012, 287, 29227–29236. [Google Scholar] [CrossRef] [Green Version]
- Hoeflich, K.P.; Luo, J.; Rubie, E.A.; Tsao, M.S.; Jin, O.; Woodgett, J.R. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature 2000, 406, 86–90. [Google Scholar] [CrossRef]
- Liu, K.J.; Arron, J.R.; Stankunas, K.; Crabtree, G.R.; Longaker, M.T. Chemical rescue of cleft palate and midline defects in conditional GSK-3β mice. Nature 2007, 446, 79–82. [Google Scholar] [CrossRef]
- Kerkela, R.; Kockeritz, L.; Macaulay, K.; Zhou, J.; Doble, B.W.; Beahm, C.; Greytak, S.; Woulfe, K.; Trivedi, C.M.; Woodgett, J.R.; et al. Deletion of GSK-3β in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J. Clin. Investig. 2008, 118, 3609–3618. [Google Scholar] [CrossRef] [Green Version]
- Bechard, M.; Dalton, S. Subcellular localization of glycogen synthase kinase 3β controls embryonic stem cell self-renewal. Mol. Cell Biol. 2009, 29, 2092–2104. [Google Scholar] [CrossRef] [Green Version]
- Evangelisti, C.; Chiarini, F.; Paganelli, F.; Marmiroli, S.; Martelli, A.M. Crosstalks of GSK3 signaling with the mTOR network and effects on targeted therapy of cancer. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1867, 118635. [Google Scholar] [CrossRef] [Green Version]
- Ougolkov, A.V.; Fernandez-Zapico, M.E.; Bilim, V.N.; Smyrk, T.C.; Chari, S.T.; Billadeau, D.D. Aberrant nuclear accumulation of glycogen synthase kinase-3β in human pancreatic cancer: Association with kinase activity and tumor dedifferentiation. Clin. Cancer Res. 2006, 12, 5074–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.S.; Herreros-Villanueva, M.; Koenig, A.; Deng, Z.; de Narvajas, A.A.; Gomez, T.S.; Meng, X.; Bujanda, L.; Ellenrieder, V.; Li, X.K.; et al. Differential activity of GSK-3 isoforms regulates NF-κB and TRAIL- or TNFα induced apoptosis in pancreatic cancer cells. Cell Death Dis. 2014, 5, e1142. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.A.; Kinstrie, R.; Sibbet, G.; Rawjee, T.; Morrice, N.; Cleghon, V. A chaperone-dependent GSK3β transitional intermediate mediates activation-loop autophosphorylation. Mol. Cell 2006, 24, 627–633. [Google Scholar] [CrossRef]
- Goc, A.; Al-Husein, B.; Katsanevas, K.; Steinbach, A.; Lou, U.; Sabbineni, H.; DeRemer, D.L.; Somanath, P.R. Targeting Src-mediated Tyr216 phosphorylation and activation of GSK-3 in prostate cancer cells inhibit prostate cancer progression in vitro and in vivo. Oncotarget 2014, 5, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Yanaga, F.; Shiraishi, F.; Hirata, M.; Miwa, Y.; Morimoto, S.; Sasaguri, T. Glycogen synthase kinase-3β is tyrosine-phosphorylated by MEK1 in human skin fibroblasts. Biochem. Biophys. Res. Commun. 2004, 316, 411–415. [Google Scholar] [CrossRef]
- Dajani, R.; Fraser, E.; Roe, S.M.; Young, N.; Good, V.; Dale, T.C.; Pearl, L.H. Crystal structure of glycogen synthase kinase 3 β: Structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 2001, 105, 721–732. [Google Scholar] [CrossRef]
- Christian, S.L.; Sims, P.V.; Gold, M.R. The B cell antigen receptor regulates the transcriptional activator β-catenin via protein kinase C-mediated inhibition of glycogen synthase kinase-3. J. Immunol. 2002, 169, 758–769. [Google Scholar] [CrossRef] [Green Version]
- Kaidanovich-Beilin, O.; Woodgett, J.R. GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Thornton, T.M.; Pedraza-Alva, G.; Deng, B.; Wood, C.D.; Aronshtam, A.; Clements, J.L.; Sabio, G.; Davis, R.J.; Matthews, D.E.; Doble, B.; et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3β inactivation. Science 2008, 320, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Chiara, F.; Rasola, A. GSK-3 and mitochondria in cancer cells. Front. Oncol. 2013, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Simoneau, M.; Coulombe, G.; Vandal, G.; Vezina, A.; Rivard, N. SHP-1 inhibits β-catenin function by inducing its degradation and interfering with its association with TATA-binding protein. Cell Signal. 2011, 23, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Wang, C.N.; Zhu, X.Y.; Ni, X. Protein tyrosine phosphatase SHP-1 modulates osteoblast differentiation through direct association with and dephosphorylation of GSK3β. Mol. Cell Endocrinol. 2017, 439, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Peineau, S.; Taghibiglou, C.; Bradley, C.; Wong, T.P.; Liu, L.; Lu, J.; Lo, E.; Wu, D.; Saule, E.; Bouschet, T.; et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron 2007, 53, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Lambrecht, C.; Libbrecht, L.; Sagaert, X.; Pauwels, P.; Hoorne, Y.; Crowther, J.; Louis, J.V.; Sents, W.; Sablina, A.; Janssens, V. Loss of protein phosphatase 2A regulatory subunit B56δ promotes spontaneous tumorigenesis in vivo. Oncogene 2018, 37, 544–552. [Google Scholar] [CrossRef]
- Hernandez, F.; Langa, E.; Cuadros, R.; Avila, J.; Villanueva, N. Regulation of GSK3 isoforms by phosphatases PP1 and PP2A. Mol. Cell Biochem. 2010, 344, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Borden, B.A.; Baca, Y.; Xiu, J.; Tavora, F.; Winer, I.; Weinberg, B.A.; Vanderwalde, A.M.; Darabi, S.; Korn, W.M.; Mazar, A.P.; et al. The Landscape of Glycogen Synthase Kinase-3 β Genomic Alterations in Cancer. Mol. Cancer Ther. 2021, 20, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Duda, P.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Gizak, A.; Rakus, D.; McCubrey, J.A. GSK-3 and miRs: Master regulators of therapeutic sensitivity of cancer cells. Biochim Biophys. Acta Mol. Cell Res. 2020, 1867, 118770. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, J.; Wang, Y.; Liu, X.; Xu, K.; Xu, J.; Feng, F.; Sun, H. PROTACs suppression of GSK-3β, a crucial kinase in neurodegenerative diseases. Eur. J. Med. Chem. 2021, 210, 112949. [Google Scholar] [CrossRef]
- Fiol, C.J.; Mahrenholz, A.M.; Wang, Y.; Roeske, R.W.; Roach, P.J. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J. Biol. Chem. 1987, 262, 14042–14048. [Google Scholar] [CrossRef]
- Rubinfeld, B.; Albert, I.; Porfiri, E.; Fiol, C.; Munemitsu, S.; Polakis, P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 1996, 272, 1023–1026. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leis, H.; Segrelles, C.; Ruiz, S.; Santos, M.; Paramio, J.M. Expression, localization, and activity of glycogen synthase kinase 3β during mouse skin tumorigenesis. Mol. Carcinog. 2002, 35, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Wang, P.; Tan, S.; Chen, D.; Nikolovska-Coleska, Z.; Zou, F.; Yu, J.; Zhang, L. Mcl-1 Degradation Is Required for Targeted Therapeutics to Eradicate Colon Cancer Cells. Cancer Res. 2017, 77, 2512–2521. [Google Scholar] [CrossRef] [Green Version]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y.; Zhang, X.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, J.; Wu, X.; Mao, Z.; Khuri, F.R.; Sun, S.Y. Rictor Undergoes Glycogen Synthase Kinase 3 (GSK3)-dependent, FBXW7-mediated Ubiquitination and Proteasomal Degradation. J. Biol. Chem. 2015, 290, 14120–14129. [Google Scholar] [CrossRef] [Green Version]
- Stretton, C.; Hoffmann, T.M.; Munson, M.J.; Prescott, A.; Taylor, P.M.; Ganley, I.G.; Hundal, H.S. GSK3-mediated raptor phosphorylation supports amino-acid-dependent mTORC1-directed signalling. Biochem. J. 2015, 470, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Wolgamott, L.; Yu, Y.; Blenis, J.; Yoon, S.O. Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc. Natl. Acad. Sci. USA 2011, 108, E1204–E1213. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Wolgamott, L.; Tcherkezian, J.; Vallabhapurapu, S.; Yu, Y.; Roux, P.P.; Yoon, S.O. Glycogen synthase kinase-3β positively regulates protein synthesis and cell proliferation through the regulation of translation initiation factor 4E-binding protein 1. Oncogene 2014, 33, 1690–1699. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Gomes, A.P.; Wang, X.; Yoon, S.O.; Lee, G.; Nagiec, M.J.; Cho, S.; Chavez, A.; Islam, T.; Yu, Y.; et al. mTORC1 Promotes Metabolic Reprogramming by the Suppression of GSK3-Dependent Foxk1 Phosphorylation. Mol. Cell 2018, 70, 949–960.e944. [Google Scholar] [CrossRef] [Green Version]
- Linseman, D.A.; Butts, B.D.; Precht, T.A.; Phelps, R.A.; Le, S.S.; Laessig, T.A.; Bouchard, R.J.; Florez-McClure, M.L.; Heidenreich, K.A. Glycogen synthase kinase-3β phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J. Neurosci. 2004, 24, 9993–10002. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Robertson, H.; Hayes, J.D.; Sutherland, C. A partnership with the proteasome; the destructive nature of GSK3. Biochem. Pharmacol. 2018, 147, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Woodgett, J. Glycogen synthase kinase-3 and cancer: Good cop, bad cop? Cancer Cell 2008, 14, 351–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dajani, R.; Fraser, E.; Roe, S.M.; Yeo, M.; Good, V.M.; Thompson, V.; Dale, T.C.; Pearl, L.H. Structural basis for recruitment of glycogen synthase kinase 3β to the axin-APC scaffold complex. EMBO J. 2003, 22, 494–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamos, J.L.; Weis, W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; Maestro, R.; et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: Opportunities for therapeutic intervention. Leukemia 2014, 28, 15–33. [Google Scholar] [CrossRef] [Green Version]
- McManus, E.J.; Sakamoto, K.; Armit, L.J.; Ronaldson, L.; Shpiro, N.; Marquez, R.; Alessi, D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005, 24, 1571–1583. [Google Scholar] [CrossRef] [Green Version]
- Doble, B.W.; Patel, S.; Wood, G.A.; Kockeritz, L.K.; Woodgett, J.R. Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 2007, 12, 957–971. [Google Scholar] [CrossRef] [Green Version]
- Sancho, E.; Batlle, E.; Clevers, H. Signaling pathways in intestinal development and cancer. Annu. Rev. Cell Dev. Biol. 2004, 20, 695–723. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, F.F.; Benajiba, L.; Campbell, A.J.; Weiwer, M.; Sacher, J.R.; Gale, J.P.; Ross, L.; Puissant, A.; Alexe, G.; Conway, A.; et al. Exploiting an Asp-Glu “switch” in glycogen synthase kinase 3 to design paralog-selective inhibitors for use in acute myeloid leukemia. Sci. Transl. Med. 2018, 10, aam8460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reya, T.; O’Riordan, M.; Okamura, R.; Devaney, E.; Willert, K.; Nusse, R.; Grosschedl, R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000, 13, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Munguia-Fuentes, R.; Maqueda-Alfaro, R.A.; Chacon-Salinas, R.; Flores-Romo, L.; Yam-Puc, J.C. Germinal Center Cells Turning to the Dark Side: Neoplasms of B Cells, Follicular Helper T Cells, and Follicular Dendritic Cells. Front. Oncol. 2020, 10, 587809. [Google Scholar] [CrossRef]
- Basso, K. Biology of Germinal Center B Cells Relating to Lymphomagenesis. Hemasphere 2021, 5, e582. [Google Scholar] [CrossRef]
- Cato, M.H.; Chintalapati, S.K.; Yau, I.W.; Omori, S.A.; Rickert, R.C. Cyclin D3 is selectively required for proliferative expansion of germinal center B cells. Mol. Cell Biol. 2011, 31, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, H.; Lim, J.; Jin, H.S.; Park, Y.; Jung, Y.J.; Ko, H.J.; Yoon, S.I.; Lee, G.S.; Kim, P.H.; et al. GSK3 Restrains Germinal Center B Cells to Form Plasma Cells. J. Immunol. 2021, 206, 481–493. [Google Scholar] [CrossRef]
- Jellusova, J.; Cato, M.H.; Apgar, J.R.; Ramezani-Rad, P.; Leung, C.R.; Chen, C.; Richardson, A.D.; Conner, E.M.; Benschop, R.J.; Woodgett, J.R.; et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat. Immunol. 2017, 18, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Goetzman, E.S.; Prochownik, E.V. The Role for Myc in Coordinating Glycolysis, Oxidative Phosphorylation, Glutaminolysis, and Fatty Acid Metabolism in Normal and Neoplastic Tissues. Front. Endocrinol. 2018, 9, 129. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2018, 93, 442–459. [Google Scholar] [CrossRef] [Green Version]
- Rowley, J.D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.; Deininger, M.W.N.; Eide, C.A.; Clackson, T.; Druker, B.J. Targeting the BCR-ABL Signaling Pathway in Therapy-Resistant Philadelphia Chromosome-Positive Leukemia. Clin. Cancer Res. 2011, 17, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashihara, E.; Takada, T.; Maekawa, T. Targeting the canonical Wnt/-catenin pathway in hematological malignancies. Cancer Sci. 2015, 106, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Chen, A.; Jamieson, C.H.; Fereshteh, M.; Abrahamsson, A.; Blum, J.; Kwon, H.Y.; Kim, J.; Chute, J.P.; Rizzieri, D.; et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 2009, 460, 652. [Google Scholar] [CrossRef] [Green Version]
- Soverini, S.; Mancini, M.; Bavaro, L.; Cavo, M.; Martinelli, G. Chronic myeloid leukemia: The paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy. Mol. Cancer 2018, 17, 49. [Google Scholar] [CrossRef]
- Bavaro, L.; Martelli, M.; Cavo, M.; Soverini, S. Mechanisms of Disease Progression and Resistance to Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukemia: An Update. Int. J. Mol. Sci. 2019, 20, 6141. [Google Scholar] [CrossRef] [Green Version]
- Silver, R.T.; Woolf, S.H.; Hehlmann, R.; Appelbaum, F.R.; Anderson, J.; Bennett, C.; Goldman, J.M.; Guilhot, F.; Kantarjian, H.M.; Lichtin, A.E.; et al. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: Developed for the American Society of Hematology. Blood 1999, 94, 1517–1536. [Google Scholar]
- Gratwohl, A.; Baldomero, H.; Passweg, J. The role of hematopoietic stem cell transplantation in chronic myeloid leukemia. Ann. Hematol. 2015, 94, S177–S186. [Google Scholar] [CrossRef]
- Cohen, M.H.; Williams, G.; Johnson, J.R.; Duan, J.; Gobburu, J.; Rahman, A.; Benson, K.; Leighton, J.; Kim, S.K.; Wood, R.; et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin. Cancer Res. 2002, 8, 935–942. [Google Scholar]
- Hehlmann, R. How I treat CML blast crisis. Blood 2012, 120, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.; Kantarjian, H.M.; Ghorab, A.; Sasaki, K.; Jabbour, E.J.; Nogueras Gonzalez, G.; Kanagal-Shamanna, R.; Issa, G.C.; Garcia-Manero, G.; Kc, D.; et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: Cohort study of 477 patients. Cancer 2017, 123, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Mostazo, M.G.C.; Kurrle, N.; Casado, M.; Fuhrmann, D.; Alshamleh, I.; Haupl, B.; Martin-Sanz, P.; Brune, B.; Serve, H.; Schwalbe, H.; et al. Metabolic Plasticity Is an Essential Requirement of Acquired Tyrosine Kinase Inhibitor Resistance in Chronic Myeloid Leukemia. Cancers 2020, 12, 3443. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.N.; Jamieson, C.H. Molecular pathways to CML stem cells. Int. J. Hematol. 2010, 91, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.; Panuzzo, C.; Fava, C.; Saglio, G. Kinase-inhibitor-insensitive cancer stem cells in chronic myeloid leukemia. Expert Opin. Biol. Ther. 2014, 14, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Fava, C.; Rege-Cambrin, G.; Dogliotti, I.; Cerrano, M.; Berchialla, P.; Dragani, M.; Rosti, G.; Castagnetti, F.; Gugliotta, G.; Martino, B.; et al. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica 2019, 104, 1589–1596. [Google Scholar] [CrossRef]
- Jamieson, C.H.; Ailles, L.E.; Dylla, S.J.; Muijtjens, M.; Jones, C.; Zehnder, J.L.; Gotlib, J.; Li, K.; Manz, M.G.; Keating, A.; et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 2004, 351, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, A.E.; Geron, I.; Gotlib, J.; Dao, K.H.; Barroga, C.F.; Newton, I.G.; Giles, F.J.; Durocher, J.; Creusot, R.S.; Karimi, M.; et al. Glycogen synthase kinase 3β missplicing contributes to leukemia stem cell generation. Proc. Natl. Acad. Sci. USA 2009, 106, 3925–3929. [Google Scholar] [CrossRef] [Green Version]
- Coluccia, A.M.; Vacca, A.; Dunach, M.; Mologni, L.; Redaelli, S.; Bustos, V.H.; Benati, D.; Pinna, L.A.; Gambacorti-Passerini, C. Bcr-Abl stabilizes β-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007, 26, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Crews, L.A.; Barrett, C.L.; Chun, H.J.; Court, A.C.; Isquith, J.M.; Zipeto, M.A.; Goff, D.J.; Minden, M.; Sadarangani, A.; et al. ADAR1 promotes malignant progenitor reprogramming in chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA 2013, 110, 1041–1046. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.F.; Lu, Y.; Fang, Z.G.; Guo, X.Y.; Chen, Y.X.; Xu, Y.C.; Lei, Y.M.; Liu, K.F.; Lin, D.J.; Liu, L.L.; et al. PIM-1 kinase inhibitor SMI-4a exerts antitumor effects in chronic myeloid leukemia cells by enhancing the activity of glycogen synthase kinase 3beta. Mol. Med. Rep. 2017, 16, 4603–4612. [Google Scholar] [CrossRef] [Green Version]
- Inoue, C.; Sobue, S.; Kawamoto, Y.; Nishizawa, Y.; Ichihara, M.; Abe, A.; Hayakawa, F.; Suzuki, M.; Nozawa, Y.; Murate, T. Involvement of MCL1, c-myc, and cyclin D2 protein degradation in ponatinib-induced cytotoxicity against T315I(+) Ph+leukemia cells. Biochem. Biophys. Res. Commun. 2020, 525, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Reddiconto, G.; Toto, C.; Palama, I.; De Leo, S.; de Luca, E.; De Matteis, S.; Dini, L.; Passerini, C.G.; Di Renzo, N.; Maffia, M.; et al. Targeting of GSK3β promotes imatinib-mediated apoptosis in quiescent CD34+ chronic myeloid leukemia progenitors, preserving normal stem cells. Blood 2012, 119, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Konig, H.; Copland, M.; Chu, S.; Jove, R.; Holyoake, T.L.; Bhatia, R. Effects of dasatinib on SRC kinase activity and downstream intracellular signaling in primitive chronic myelogenous leukemia hematopoietic cells. Cancer Res. 2008, 68, 9624–9633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, A.K.; Lin, H.; Sun, T.; Kantarjian, H.; Arlinghaus, R.B. Janus kinase 2: A critical target in chronic myelogenous leukemia. Cancer Res. 2006, 66, 6468–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawstron, A.C.; Bennett, F.L.; O’Connor, S.J.; Kwok, M.; Fenton, J.A.; Plummer, M.; de Tute, R.; Owen, R.G.; Richards, S.J.; Jack, A.S.; et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N. Engl. J. Med. 2008, 359, 575–583. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [Green Version]
- Pula, B.; Golos, A.; Gorniak, P.; Jamroziak, K. Overcoming Ibrutinib Resistance in Chronic Lymphocytic Leukemia. Cancers 2019, 11, 1834. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhao, J. Frontline therapies for untreated chronic lymphoid leukemia. Exp. Hematol. Oncol. 2019, 8, 15. [Google Scholar] [CrossRef]
- Molica, S.; Gianfelici, V.; Levato, L. Emerging bruton tyrosine kinase inhibitors for chronic lymphocytic leukaemia: One step ahead ibrutinib. Expert Opin. Emerg. Drugs 2020, 25, 25–35. [Google Scholar] [CrossRef]
- Woyach, J.A. Treatment-naive CLL: Lessons from phase 2 and phase 3 clinical trials. Blood 2019, 134, 1796–1801. [Google Scholar] [CrossRef]
- Brown, J.R. Phosphatidylinositol 3 Kinase δ Inhibitors: Present and Future. Cancer J. 2019, 25, 394–400. [Google Scholar] [CrossRef]
- Luan, C.; Chen, B. Clinical application of obinutuzumab for treating chronic lymphocytic leukemia. Drug Des. Devel. Ther. 2019, 13, 2899–2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brander, D.; Islam, P.; Barrientos, J.C. Tailored Treatment Strategies for Chronic Lymphocytic Leukemia in a Rapidly Changing Era. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Kittai, A.S.; Woyach, J.A. Resistance Mechanisms to Targeted Agents in Chronic Lymphocytic Leukemia. Cancer J. 2019, 25, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, G.; Montserrat, E. Critical molecular pathways in CLL therapy. Mol. Med. 2018, 24, 9. [Google Scholar] [CrossRef]
- Lu, D.; Zhao, Y.; Tawatao, R.; Cottam, H.B.; Sen, M.; Leoni, L.M.; Kipps, T.J.; Corr, M.; Carson, D.A. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2004, 101, 3118–3123. [Google Scholar] [CrossRef] [Green Version]
- Mangolini, M.; Gotte, F.; Moore, A.; Ammon, T.; Oelsner, M.; Lutzny-Geier, G.; Klein-Hitpass, L.; Williamson, J.C.; Lehner, P.J.; Durig, J.; et al. Notch2 controls non-autonomous Wnt-signalling in chronic lymphocytic leukaemia. Nat. Commun. 2018, 9, 3839. [Google Scholar] [CrossRef] [Green Version]
- Ougolkov, A.V.; Bone, N.D.; Fernandez-Zapico, M.E.; Kay, N.E.; Billadeau, D.D. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood 2007, 110, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, H.; Novis Durie, S.; Meckl, A.; Hinke, A.; Durie, B. Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist 2020, 25, e1406–e1413. [Google Scholar] [CrossRef] [Green Version]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, J.J.; Brailey, M. Emerging small molecule approaches to enhance the antimyeloma benefit of proteasome inhibitors. Cancer Metastasis Rev. 2017, 36, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Uddin, S.; Zimmerman, T.; Kang, J.A.; Ulaszek, J.; Wickrema, A. Growth control of multiple myeloma cells through inhibition of glycogen synthase kinase-3. Leuk. Lymphoma 2008, 49, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Piazza, F.; Manni, S.; Tubi, L.Q.; Montini, B.; Pavan, L.; Colpo, A.; Gnoato, M.; Cabrelle, A.; Adami, F.; Zambello, R.; et al. Glycogen Synthase Kinase-3 regulates multiple myeloma cell growth and bortezomib-induced cell death. BMC Cancer 2010, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Yanaga, F. Activator or inhibitor? GSK-3 as a new drug target. Biochem. Pharmacol. 2013, 86, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.Z.; Mandelkow, E.M.; et al. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Santo, L.; Vallet, S.; Hideshima, T.; Cirstea, D.; Ikeda, H.; Pozzi, S.; Patel, K.; Okawa, Y.; Gorgun, G.; Perrone, G.; et al. AT7519, A novel small molecule multi-cyclin-dependent kinase inhibitor, induces apoptosis in multiple myeloma via GSK-3beta activation and RNA polymerase II inhibition. Oncogene 2010, 29, 2325–2336. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Morgan, D.; Garg, M.; Tergaonkar, V.; Tan, S.Y.; Sethi, G. Pharmacological significance of the non-canonical NF-κB pathway in tumorigenesis. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188449. [Google Scholar] [CrossRef]
- Busino, L.; Millman, S.E.; Scotto, L.; Kyratsous, C.A.; Basrur, V.; O’Connor, O.; Hoffmann, A.; Elenitoba-Johnson, K.S.; Pagano, M. Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat. Cell Biol. 2012, 14, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.M.; Gilmore, T.D. Looking Down on NF-κB. Mol. Cell Biol. 2020, 40, e00104-20. [Google Scholar] [CrossRef]
- Franic, D.; Zubcic, K.; Boban, M. Nuclear Ubiquitin-Proteasome Pathways in Proteostasis Maintenance. Biomolecules 2021, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Spokoini, R.; Kfir-Erenfeld, S.; Yefenof, E.; Sionov, R.V. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol. Endocrinol. 2010, 24, 1136–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uno, Y.; Iwashita, H.; Tsukamoto, T.; Uchiyama, N.; Kawamoto, T.; Kori, M.; Nakanishi, A. Efficacy of a novel, orally active GSK-3 inhibitor 6-Methyl-N-[3-[[3-(1-methylethoxy)propyl]carbamoyl]-1H-pyrazol-4-yl]pyridine-3-ca rboxamide in tau transgenic mice. Brain Res. 2009, 1296, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Functional analysis of large MAF transcription factors and elucidation of their relationships with human diseases. Exp. Anim. 2021, 70, 264–271. [Google Scholar] [CrossRef]
- Chesi, M.; Bergsagel, P.L. Many multiple myelomas: Making more of the molecular mayhem. Hematology Am. Soc. Hematol. Edu. Program 2011, 2011, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Herath, N.I.; Rocques, N.; Garancher, A.; Eychene, A.; Pouponnot, C. GSK3-mediated MAF phosphorylation in multiple myeloma as a potential therapeutic target. Blood Cancer J. 2014, 4, e175. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, C.M.; Hernandez, L.; Davis, R.E.; Zingone, A.; Lamy, L.; Lam, L.T.; Hurt, E.M.; Shaffer, A.L.; Kuehl, W.M.; Staudt, L.M. A mechanistic rationale for MEK inhibitor therapy in myeloma based on blockade of MAF oncogene expression. Blood 2011, 117, 2396–2404. [Google Scholar] [CrossRef]
- Hurt, E.M.; Wiestner, A.; Rosenwald, A.; Shaffer, A.L.; Campo, E.; Grogan, T.; Bergsagel, P.L.; Kuehl, W.M.; Staudt, L.M. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell 2004, 5, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Iida, S.; Kato-Uranishi, M.; Tajima, E.; Zhan, F.; Hanamura, I.; Huang, Y.; Ogura, T.; Takahashi, S.; Ueda, R.; et al. ARK5 is transcriptionally regulated by the Large-MAF family and mediates IGF-1-induced cell invasion in multiple myeloma: ARK5 as a new molecular determinant of malignant multiple myeloma. Oncogene 2005, 24, 6936–6944. [Google Scholar] [CrossRef] [Green Version]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef] [Green Version]
- Zeissig, M.N.; Hewett, D.R.; Panagopoulos, V.; Mrozik, K.M.; To, L.B.; Croucher, P.I.; Zannettino, A.C.W.; Vandyke, K. Expression of the chemokine receptor CCR1 promotes the dissemination of multiple myeloma plasma cells in vivo. Haematologica 2021, 106, 3176–3187. [Google Scholar] [CrossRef] [PubMed]
- Rocques, N.; Abou Zeid, N.; Sii-Felice, K.; Lecoin, L.; Felder-Schmittbuhl, M.P.; Eychene, A.; Pouponnot, C. GSK-3-mediated phosphorylation enhances Maf-transforming activity. Mol. Cell 2007, 28, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.W.; Ye, S.; Chen, Y.; Buros, A.F.; Edmonson, R.; van Rhee, F.; Barlogie, B.; Epstein, J.; Morgan, G.J.; Davies, F.E. MAF protein mediates innate resistance to proteasome inhibition therapy in multiple myeloma. Blood 2016, 128, 2919–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, Y.W.; Ye, S.; Huang, Y.; Chen, Y.; Van Rhee, F.; Epstein, J.; Walker, B.A.; Morgan, G.J.; Davies, F.E. MAFb protein confers intrinsic resistance to proteasome inhibitors in multiple myeloma. BMC Cancer 2018, 18, 724. [Google Scholar] [CrossRef] [Green Version]
- Van Woerkom, A.E. A fully integrated new paradigm for lithium’s mode of action—Lithium utilizes latent cellular fail-safe mechanisms. Neuropsychiatr. Dis. Treat. 2017, 13, 275–302. [Google Scholar] [CrossRef] [Green Version]
- Snitow, M.E.; Bhansali, R.S.; Klein, P.S. Lithium and Therapeutic Targeting of GSK-3. Cells 2021, 10, 255. [Google Scholar] [CrossRef]
- Bloedjes, T.A.; de Wilde, G.; Maas, C.; Eldering, E.; Bende, R.J.; van Noesel, C.J.M.; Pals, S.T.; Spaargaren, M.; Guikema, J.E.J. AKT signaling restrains tumor suppressive functions of FOXO transcription factors and GSK3 kinase in multiple myeloma. Blood Adv. 2020, 4, 4151–4164. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Q. JMJD2C triggers the growth of multiple myeloma cells via activation of betacatenin. Oncol. Rep. 2021, 45, 1162–1170. [Google Scholar] [CrossRef]
- Goel, U.; Usmani, S.; Kumar, S. Current Approaches to Management of Newly Diagnosed Multiple Myeloma. Am. J. Hematol. 2022, 97, 1. [Google Scholar] [CrossRef]
- Bjorklund, C.C.; Ma, W.; Wang, Z.Q.; Davis, R.E.; Kuhn, D.J.; Kornblau, S.M.; Wang, M.; Shah, J.J.; Orlowski, R.Z. Evidence of a role for activation of Wnt/β-catenin signaling in the resistance of plasma cells to lenalidomide. J. Biol. Chem. 2011, 286, 11009–11020. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Laversanne, M.; Brewster, D.H.; Gombe Mbalawa, C.; Kohler, B.; Pineros, M.; Steliarova-Foucher, E.; Swaminathan, R.; Antoni, S.; et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int. J. Cancer 2015, 137, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Grande, B.M.; Gerhard, D.S.; Jiang, A.; Griner, N.B.; Abramson, J.S.; Alexander, T.B.; Allen, H.; Ayers, L.W.; Bethony, J.M.; Bhatia, K.; et al. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood 2019, 133, 1313–1324. [Google Scholar] [CrossRef]
- Robaina, M.C.; Mazzoccoli, L.; Klumb, C.E. Germinal Centre B Cell Functions and Lymphomagenesis: Circuits Involving MYC and MicroRNAs. Cells 2019, 8, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurner, L.; Hartmann, S.; Neumann, F.; Hoth, M.; Stilgenbauer, S.; Kuppers, R.; Preuss, K.D.; Bewarder, M. Role of Specific B-Cell Receptor Antigens in Lymphomagenesis. Front. Oncol. 2020, 10, 604685. [Google Scholar] [CrossRef] [PubMed]
- Young, R.M.; Shaffer, A.L., 3rd; Phelan, J.D.; Staudt, L.M. B-cell receptor signaling in diffuse large B-cell lymphoma. Semin. Hematol. 2015, 52, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Varano, G.; Raffel, S.; Sormani, M.; Zanardi, F.; Lonardi, S.; Zasada, C.; Perucho, L.; Petrocelli, V.; Haake, A.; Lee, A.K.; et al. The B-cell receptor controls fitness of MYC-driven lymphoma cells via GSK3β inhibition. Nature 2017, 546, 302–306. [Google Scholar] [CrossRef]
- Vecchio, E.; Nistico, N.; Golino, G.; Iaccino, E.; Maisano, D.; Mimmi, S.; Aloisio, A.; Renna, M.; Avagliano, A.; Arcucci, A.; et al. IBtkalpha Activates the beta-Catenin-Dependent Transcription of MYC through Ubiquitylation and Proteasomal Degradation of GSK3β in Cancerous B Cells. Int. J. Mol. Sci. 2022, 23, 2044. [Google Scholar] [CrossRef]
- Karmali, R.; Chukkapalli, V.; Gordon, L.I.; Borgia, J.A.; Ugolkov, A.; Mazar, A.P.; Giles, F.J. GSK-3β inhibitor, 9-ING-41, reduces cell viability and halts proliferation of B-cell lymphoma cell lines as a single agent and in combination with novel agents. Oncotarget 2017, 8, 114924–114934. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Stenson, M.; Abeykoon, J.; Nowakowski, K.; Zhang, L.; Lawson, J.; Wellik, L.; Li, Y.; Krull, J.; Wenzl, K.; et al. Targeting glycogen synthase kinase 3 for therapeutic benefit in lymphoma. Blood 2019, 134, 363–373. [Google Scholar] [CrossRef]
- Anraku, T.; Kuroki, H.; Kazama, A.; Bilim, V.; Tasaki, M.; Schmitt, D.; Mazar, A.; Giles, F.J.; Ugolkov, A.; Tomita, Y. Clinically relevant GSK3β inhibitor 9ING41 is active as a single agent and in combination with other antitumor therapies in human renal cancer. Int. J. Mol. Med. 2020, 45, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, R.; Coveler, A.L.; Cavalcante, L.; Saeed, A. GSK-3beta in Pancreatic Cancer: Spotlight on 9-ING-41, Its Therapeutic Potential and Immune Modulatory Properties. Biology 2021, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.T.; Sotillo, E.; Robert, A.; Hayer, K.E.; Bogusz, A.M.; Psathas, J.; Yu, D.; Taylor, D.; Dang, C.V.; Klein, P.S.; et al. Transient stabilization, rather than inhibition, of MYC amplifies extrinsic apoptosis and therapeutic responses in refractory B-cell lymphoma. Leukemia 2019, 33, 2429–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, I.; Eturi, A.; De Souza, A.; Pamarthy, S.; Tavora, F.; Giles, F.J.; Carneiro, B.A. Glycogen synthase kinase-3 β inhibitors as novel cancer treatments and modulators of antitumor immune responses. Cancer Biol. Ther. 2019, 20, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Augello, G.; Emma, M.R.; Cusimano, A.; Azzolina, A.; Montalto, G.; McCubrey, J.A.; Cervello, M. The Role of GSK-3 in Cancer Immunotherapy: GSK-3 Inhibitors as a New Frontier in Cancer Treatment. Cells 2020, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Rudd, C.E. Glycogen Synthase Kinase 3 Inactivation Compensates for the Lack of CD28 in the Priming of CD8+ Cytotoxic T-Cells: Implications for anti-PD-1 Immunotherapy. Front. Immunol. 2017, 8, 1653. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Rothstein, D.; Rudd, C.E. Small-Molecule Inhibition of PD-1 Transcription Is an Effective Alternative to Antibody Blockade in Cancer Therapy. Cancer Res. 2018, 78, 706–717. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.D.; Liu, X.; Jiang, J.; Liao, Y.P.; Chang, C.H.; Nel, A.E.; Meng, H. Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor. Biomaterials 2021, 269, 120635. [Google Scholar] [CrossRef]
- Appleman, L.J.; van Puijenbroek, A.A.; Shu, K.M.; Nadler, L.M.; Boussiotis, V.A. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 2002, 168, 2729–2736. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Katz, S.C.; Sengupta, S.; Sampath, P. Glycogen synthase kinase 3 inhibition lowers PD-1 expression, promotes long-term survival and memory generation in antigen-specific CAR-T cells. Cancer Lett. 2018, 433, 131–139. [Google Scholar] [CrossRef]
- Cichocki, F.; Valamehr, B.; Bjordahl, R.; Zhang, B.; Rezner, B.; Rogers, P.; Gaidarova, S.; Moreno, S.; Tuininga, K.; Dougherty, P.; et al. GSK3 Inhibition Drives Maturation of NK Cells and Enhances Their Antitumor Activity. Cancer Res. 2017, 77, 5664–5675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.; Kim, M.Y.; Choi, W.S.; Yi, E.; Lee, H.J.; Kim, H.S. GSK-3alpha Inhibition in Drug-Resistant CML Cells Promotes Susceptibility to NK Cell-Mediated Lysis in an NKG2D- and NKp30-Dependent Manner. Cancers 2021, 13, 1802. [Google Scholar] [CrossRef]
- Phung, S.K.; Miller, J.S.; Felices, M. Bi-specific and Tri-specific NK Cell Engagers: The New Avenue of Targeted NK Cell Immunotherapy. Mol. Diagn. Ther. 2021, 25, 577–592. [Google Scholar] [CrossRef]
- Held, S.A.; Heine, A.; Kesper, A.R.; Schonberg, K.; Beckers, A.; Wolf, D.; Brossart, P. Interferon gamma modulates sensitivity of CML cells to tyrosine kinase inhibitors. Oncoimmunology 2016, 5, e1065368. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, M.R.; Nazemidashtarjandi, S.; Ghazanfari, D.; Allen, A.E.; Reynolds, N.M.; Faik, A.; Burdick, M.M.; McCall, K.D.; Goetz, D.J. Evidence that knock down of GSK-3beta in Chronic Myelogenous Leukemia cells augments IFN-γ-induced apoptosis. Leuk. Res. 2020, 99, 106464. [Google Scholar] [CrossRef]
- Fionda, C.; Malgarini, G.; Soriani, A.; Zingoni, A.; Cecere, F.; Iannitto, M.L.; Ricciardi, M.R.; Federico, V.; Petrucci, M.T.; Santoni, A.; et al. Inhibition of glycogen synthase kinase-3 increases NKG2D ligand MICA expression and sensitivity to NK cell-mediated cytotoxicity in multiple myeloma cells: Role of STAT3. J. Immunol. 2013, 190, 6662–6672. [Google Scholar] [CrossRef] [PubMed]
- Waitkus, M.S.; Chandrasekharan, U.M.; Willard, B.; Tee, T.L.; Hsieh, J.K.; Przybycin, C.G.; Rini, B.I.; Dicorleto, P.E. Signal integration and gene induction by a functionally distinct STAT3 phosphoform. Mol. Cell Biol. 2014, 34, 1800–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Li, S.; Duan, X.; Gu, Z.; Ma, Z.; Yuan, X.; Feng, X.; Wang, H. Inhibition of glycogen synthase kinase 3 β (GSK3β) suppresses the progression of esophageal squamous cell carcinoma by modifying STAT3 activity. Mol. Carcinog. 2017, 56, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Panagi, I.; Jennings, E.; Zeng, J.; Gunster, R.A.; Stones, C.D.; Mak, H.; Jin, E.; Stapels, D.A.C.; Subari, N.Z.; Pham, T.H.M.; et al. Salmonella Effector SteE Converts the Mammalian Serine/Threonine Kinase GSK3 into a Tyrosine Kinase to Direct Macrophage Polarization. Cell Host Microbe 2020, 27, 41–53.e46. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Woodgett, J.R. Emerging roles of GSK-3α in pathophysiology: Emphasis on cardio-metabolic disorders. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118616. [Google Scholar] [CrossRef]
- Neumann, T.; Benajiba, L.; Goring, S.; Stegmaier, K.; Schmidt, B. Evaluation of Improved Glycogen Synthase Kinase-3α Inhibitors in Models of Acute Myeloid Leukemia. J. Med. Chem. 2015, 58, 8907–8919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Dou, X.; Jiang, L.; Jin, H.; Zhang, L.; Zhang, L.; Liu, Z. Discovery of novel glycogen synthase kinase-3α inhibitors: Structure-based virtual screening, preliminary SAR and biological evaluation for treatment of acute myeloid leukemia. Eur. J. Med. Chem. 2019, 171, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wu, T.Y.; Brinker, A.; Peters, E.C.; Hur, W.; Gray, N.S.; Schultz, P.G. Synthetic small molecules that control stem cell fate. Proc. Natl. Acad. Sci. USA 2003, 100, 7632–7637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, A.M.; Evangelisti, C.; Paganelli, F.; Chiarini, F.; McCubrey, J.A. GSK-3: A multifaceted player in acute leukemias. Leukemia 2021, 35, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Smaill, J.B.; Patterson, A.V.; Ding, K. Discovery of Cysteine-targeting Covalent Protein Kinase Inhibitors. J. Med. Chem. 2022, 65, 58–83. [Google Scholar] [CrossRef] [PubMed]
- De Vita, E. 10 years into the resurgence of covalent drugs. Future Med. Chem. 2021, 13, 193–210. [Google Scholar] [CrossRef]
- Hacker, S.M. Promising reversible protein inhibitors kept on target. Nature 2022, 603, 583–584. [Google Scholar] [CrossRef]
- Serafimova, I.M.; Pufall, M.A.; Krishnan, S.; Duda, K.; Cohen, M.S.; Maglathlin, R.L.; McFarland, J.M.; Miller, R.M.; Frodin, M.; Taunton, J. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat. Chem. Biol. 2012, 8, 471–476. [Google Scholar] [CrossRef]
- Yan, T.; Desai, H.S.; Boatner, L.M.; Yen, S.L.; Cao, J.; Palafox, M.F.; Jami-Alahmadi, Y.; Backus, K.M. SP3-FAIMS Chemoproteomics for High-Coverage Profiling of the Human Cysteinome*. Chembiochem 2021, 22, 1841–1851. [Google Scholar] [CrossRef]
- Abbasov, M.E.; Kavanagh, M.E.; Ichu, T.A.; Lazear, M.R.; Tao, Y.; Crowley, V.M.; Am Ende, C.W.; Hacker, S.M.; Ho, J.; Dix, M.M.; et al. A proteome-wide atlas of lysine-reactive chemistry. Nat. Chem. 2021, 13, 1081–1092. [Google Scholar] [CrossRef]
- Reja, R.M.; Wang, W.; Lyu, Y.; Haeffner, F.; Gao, J. Lysine-Targeting Reversible Covalent Inhibitors with Long Residence Time. J. Am. Chem. Soc. 2022, 144, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Gajjar, A.; Basha, S.H. Pharmacophore feature-based virtual screening for finding potent GSK-3 inhibitors using molecular docking and dynamics simulations. Bioinformation 2016, 12, 391–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, J.; Perez, C.; van Otterlo, W.A.L.; Martinez, A.; Blackie, M.A.L. 1-Aryl-3-(4-methoxybenzyl)ureas as potentially irreversible glycogen synthase kinase 3 inhibitors: Synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 1597–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Min, Z.; Gao, Y.; Bian, J.; Lin, X.; He, J.; Ye, D.; Li, Y.; Peng, C.; Cheng, Y.; et al. Discovery of Novel Benzothiazepinones as Irreversible Covalent Glycogen Synthase Kinase 3β Inhibitors for the Treatment of Acute Promyelocytic Leukemia. J. Med. Chem. 2021, 64, 7341–7358. [Google Scholar] [CrossRef]
- Palomo, V.; Perez, D.I.; Roca, C.; Anderson, C.; Rodriguez-Muela, N.; Perez, C.; Morales-Garcia, J.A.; Reyes, J.A.; Campillo, N.E.; Perez-Castillo, A.M.; et al. Subtly Modulating Glycogen Synthase Kinase 3 β: Allosteric Inhibitor Development and Their Potential for the Treatment of Chronic Diseases. J. Med. Chem. 2017, 60, 4983–5001. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; Borges, R.S.; Santos, K.L.B.; Federico, L.B.; Francischini, I.A.G.; Gomes, S.Q.; Barcelos, M.P.; Silva, R.C.; Santos, C.B.R.; Silva, C. Revisiting the Proposition of Binding Pockets and Bioactive Poses for GSK-3β Allosteric Modulators Addressed to Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 8252. [Google Scholar] [CrossRef]
- Li, H.; Dong, J.; Cai, M.; Xu, Z.; Cheng, X.D.; Qin, J.J. Protein degradation technology: A strategic paradigm shift in drug discovery. J. Hematol. Oncol. 2021, 14, 138. [Google Scholar] [CrossRef]
- Wu, P.; Manna, D. Optochemical Control of Protein Degradation. ChemBioChem 2020, 21, 2250–2252. [Google Scholar] [CrossRef]
- Qu, L.; Li, S.; Ji, L.; Luo, S.; Ding, M.; Yin, F.; Wang, C.; Luo, H.; Lu, D.; Liu, X.; et al. Discovery of PT-65 as a highly potent and selective Proteolysis-targeting chimera degrader of GSK3 for treating Alzheimer’s disease. Eur. J. Med. Chem. 2021, 226, 113889. [Google Scholar] [CrossRef]
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; et al. Smart Nanocarriers as an Emerging Platform for Cancer Therapy: A Review. Molecules 2021, 27, 146. [Google Scholar] [CrossRef]
- Alexis, F. Nano-polypharmacy to treat tumors: Coencapsulation of drug combinations using nanoparticle technology. Mol. Ther. 2014, 22, 1239–1240. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.S. Nanotechnology-Based Targeting of mTOR Signaling in Cancer. Int. J. Nanomed. 2020, 15, 5767–5781. [Google Scholar] [CrossRef]
- Wang, Y.; Newman, M.R.; Ackun-Farmmer, M.; Baranello, M.P.; Sheu, T.J.; Puzas, J.E.; Benoit, D.S.W. Fracture-Targeted Delivery of beta-Catenin Agonists via Peptide-Functionalized Nanoparticles Augments Fracture Healing. ACS Nano 2017, 11, 9445–9458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loukogeorgakis, S.P.; Fachin, C.G.; Dias, A.; Li, H.; Tang, L.; Kim, A.G.; Vrecenak, J.D.; Stratigis, J.D.; Ahn, N.J.; Nissim, I.; et al. Donor cell engineering with GSK3 inhibitor-loaded nanoparticles enhances engraftment after in utero transplantation. Blood 2019, 134, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, A.; Matteis, L.; Serrano-Sevilla, I.; Tortiglione, C.; De La Fuente, J.M. Glycogen Synthase Kinase 3β Inhibitor Delivered by Chitosan Nanocapsules Promotes Safe, Fast, and Efficient Activation of Wnt Signaling In Vivo. ACS Biomater. Sci. Eng. 2020, 6, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Zoulikha, M.; He, W. Targeted Drug Delivery for Chronic Lymphocytic Leukemia. Pharm. Res. 2022, 39, 441–461. [Google Scholar] [CrossRef]
- Bhat, R.V.; Andersson, U.; Andersson, S.; Knerr, L.; Bauer, U.; Sundgren-Andersson, A.K. The Conundrum of GSK3 Inhibitors: Is it the Dawn of a New Beginning? J. Alzheimers Dis. 2018, 64, S547–S554. [Google Scholar] [CrossRef] [PubMed]
- Rizzieri, D.A.; Cooley, S.; Odenike, O.; Moonan, L.; Chow, K.H.; Jackson, K.; Wang, X.; Brail, L.; Borthakur, G. An open-label phase 2 study of glycogen synthase kinase-3 inhibitor LY2090314 in patients with acute leukemia. Leuk. Lymphoma. 2016, 57, 1800–1806. [Google Scholar] [CrossRef]
- Sun, J.; Park, C.; Guenthner, N.; Gurley, S.; Zhang, L.; Lubben, B.; Adebayo, O.; Bash, H.; Chen, Y.; Maksimos, M.; et al. Tumor-associated macrophages in multiple myeloma: Advances in biology and therapy. J. Immunother. Cancer 2022, 10, e003975. [Google Scholar] [CrossRef]
- Lv, J.; Feng, Z.P.; Chen, F.K.; Liu, C.; Jia, L.; Liu, P.J.; Yang, C.Z.; Hou, F.; Deng, Z.Y. M2-like tumor-associated macrophages-secreted Wnt1 and Wnt3a promotes dedifferentiation and metastasis via activating β-catenin pathway in thyroid cancer. Mol. Carcinog. 2021, 60, 25–37. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Wang, J.; Si, T.; Xing, W. Tumor-associated macrophage-derived transforming growth factor-β promotes colorectal cancer progression through HIF1-TRIB3 signaling. Cancer Sci. 2021, 112, 4198–4207. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martelli, A.M.; Paganelli, F.; Evangelisti, C.; Chiarini, F.; McCubrey, J.A. Pathobiology and Therapeutic Relevance of GSK-3 in Chronic Hematological Malignancies. Cells 2022, 11, 1812. https://doi.org/10.3390/cells11111812
Martelli AM, Paganelli F, Evangelisti C, Chiarini F, McCubrey JA. Pathobiology and Therapeutic Relevance of GSK-3 in Chronic Hematological Malignancies. Cells. 2022; 11(11):1812. https://doi.org/10.3390/cells11111812
Chicago/Turabian StyleMartelli, Alberto M., Francesca Paganelli, Camilla Evangelisti, Francesca Chiarini, and James A. McCubrey. 2022. "Pathobiology and Therapeutic Relevance of GSK-3 in Chronic Hematological Malignancies" Cells 11, no. 11: 1812. https://doi.org/10.3390/cells11111812
APA StyleMartelli, A. M., Paganelli, F., Evangelisti, C., Chiarini, F., & McCubrey, J. A. (2022). Pathobiology and Therapeutic Relevance of GSK-3 in Chronic Hematological Malignancies. Cells, 11(11), 1812. https://doi.org/10.3390/cells11111812






