Immune Microenvironment of Muscular-Invasive Urothelial Carcinoma: The Link to Tumor Immune Cycle and Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection and Patient Characteristics
2.2. Tissue Processing and Immunohistochemistry
2.3. Methodology of Tumor–Host Immunity Assessment
2.4. Statistical Analysis
3. Results
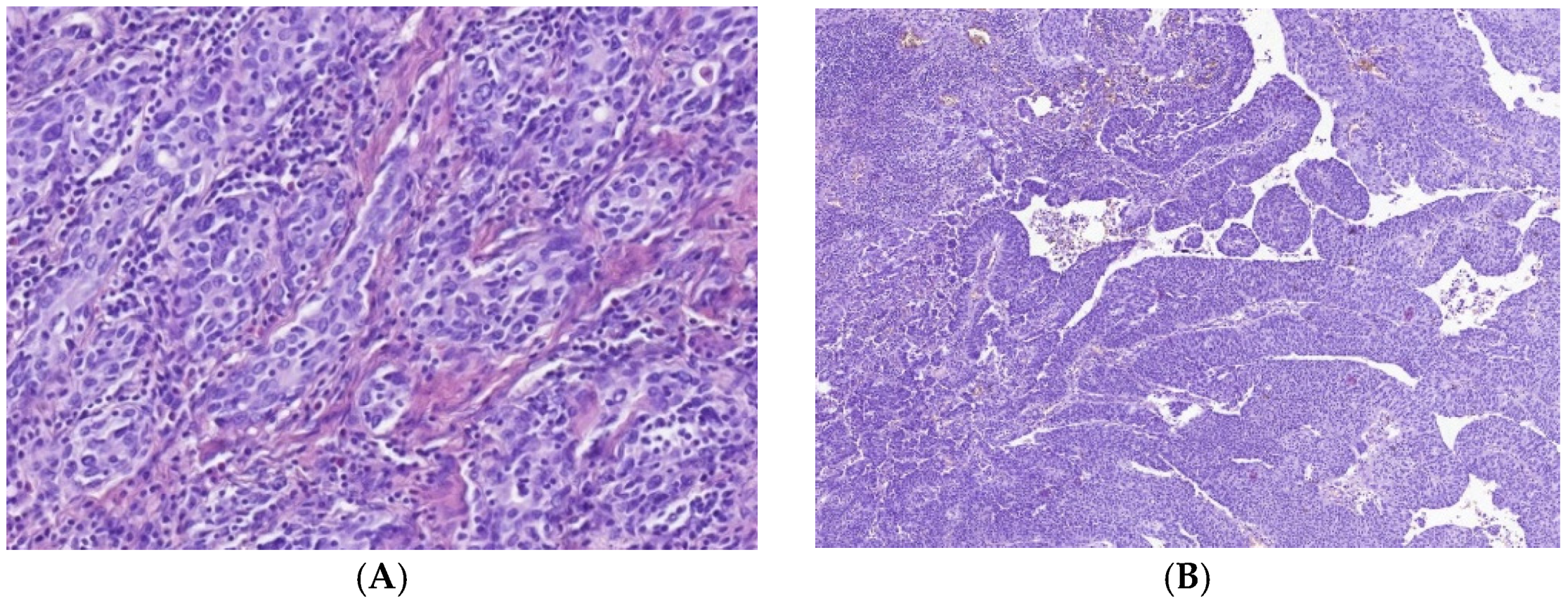
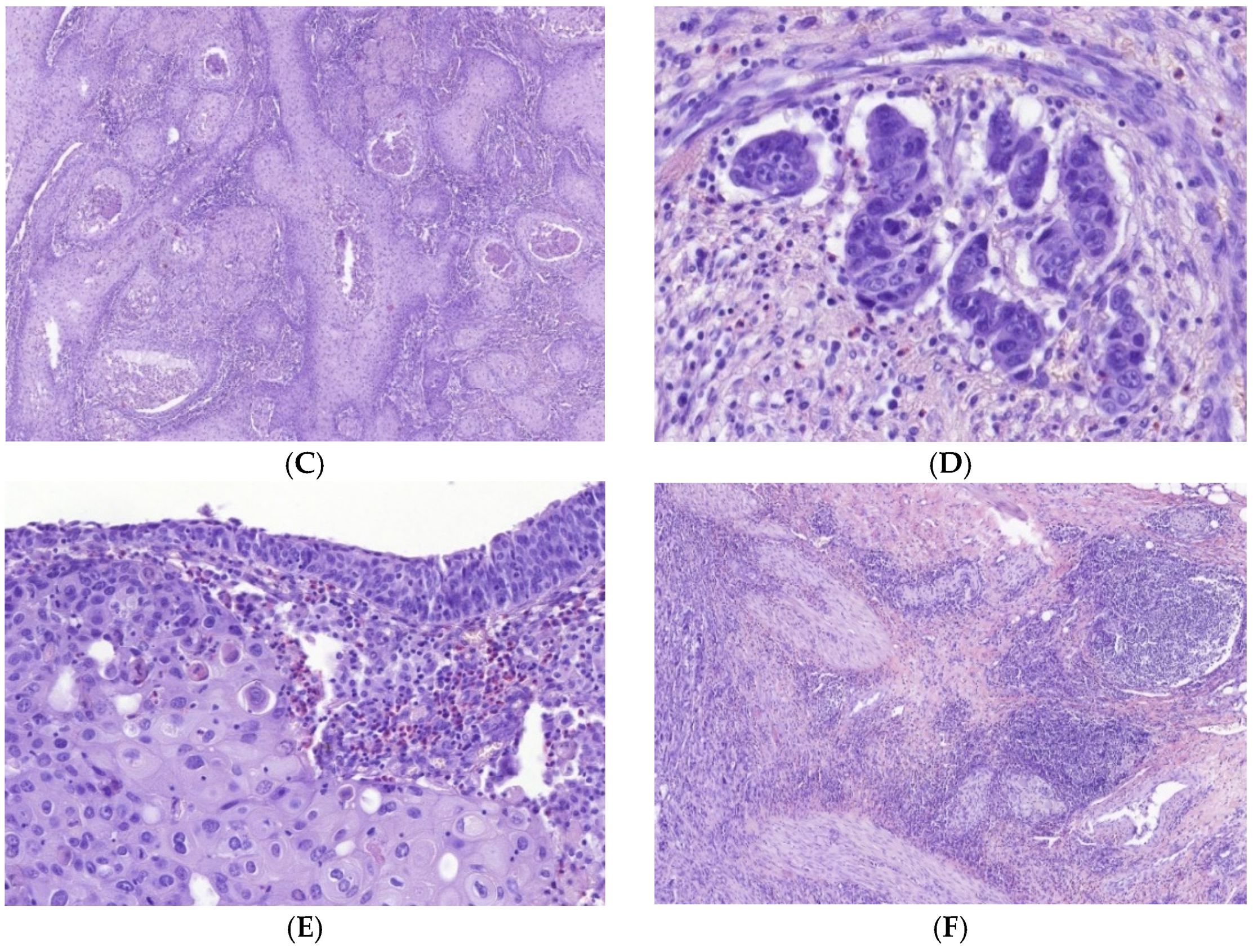
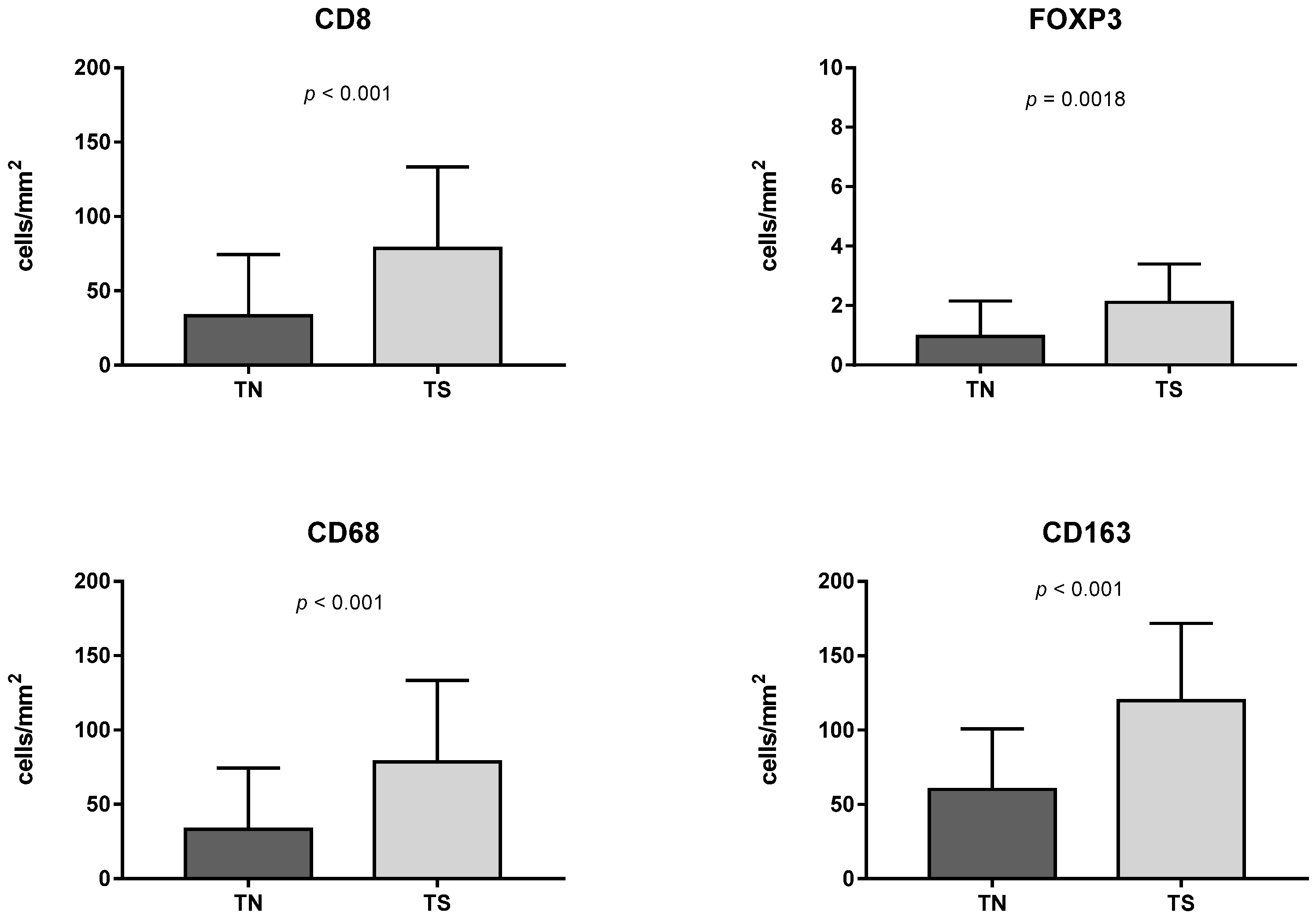
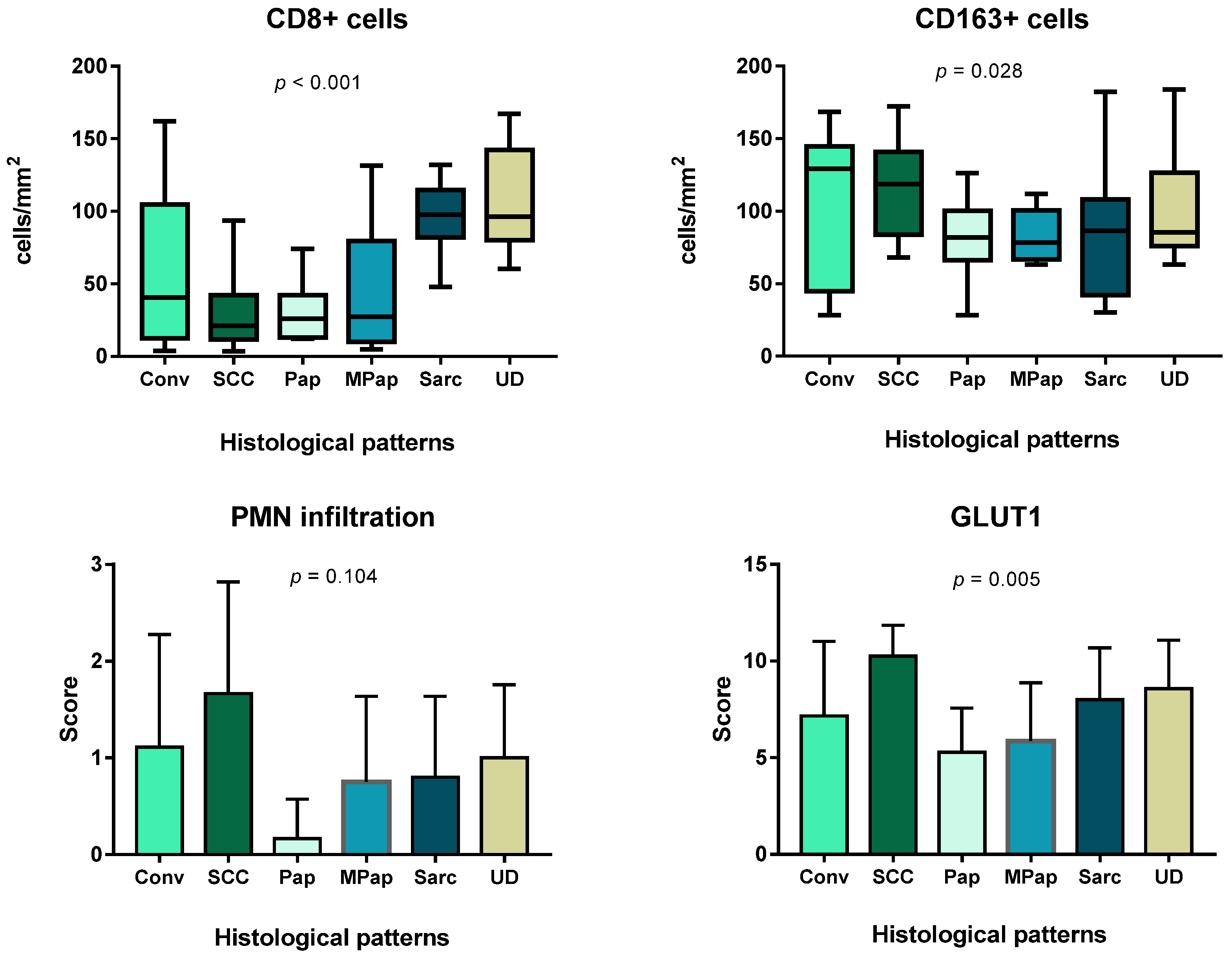
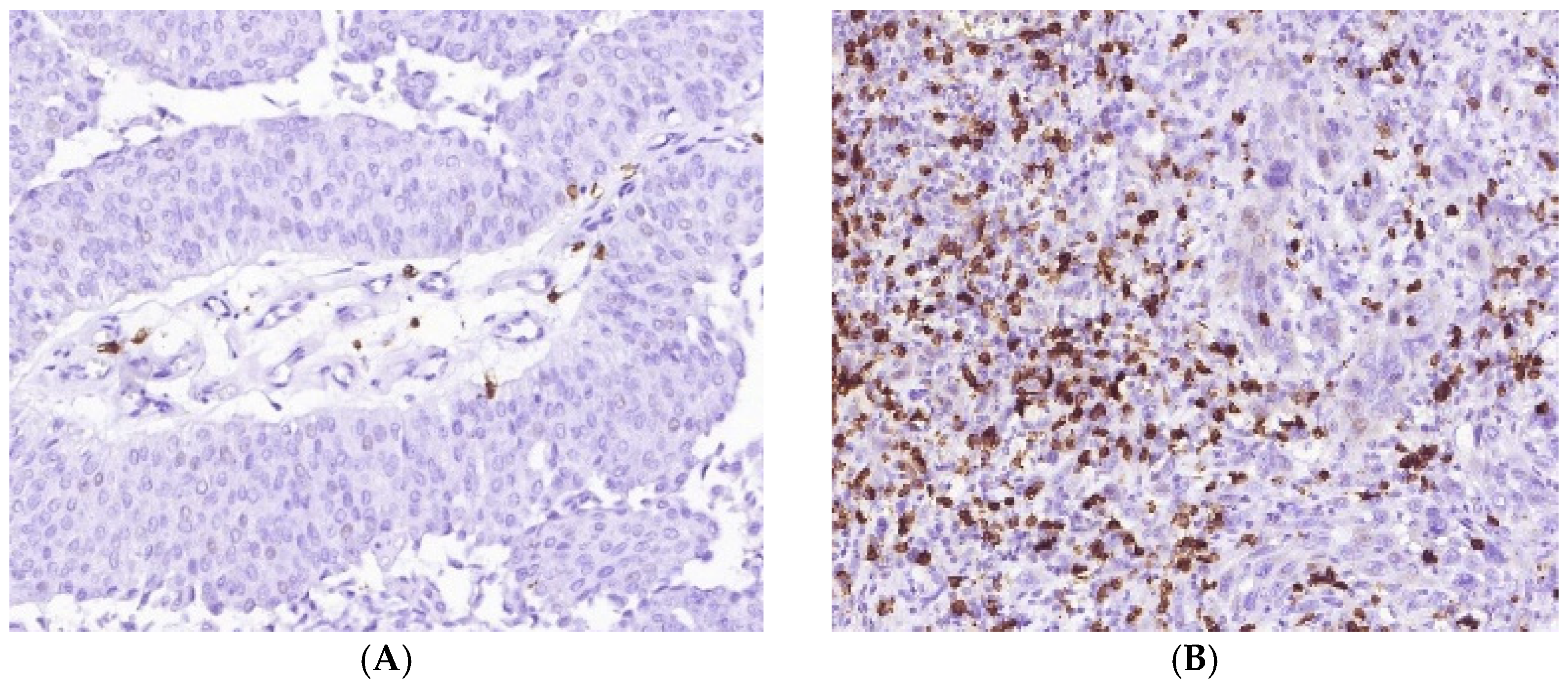
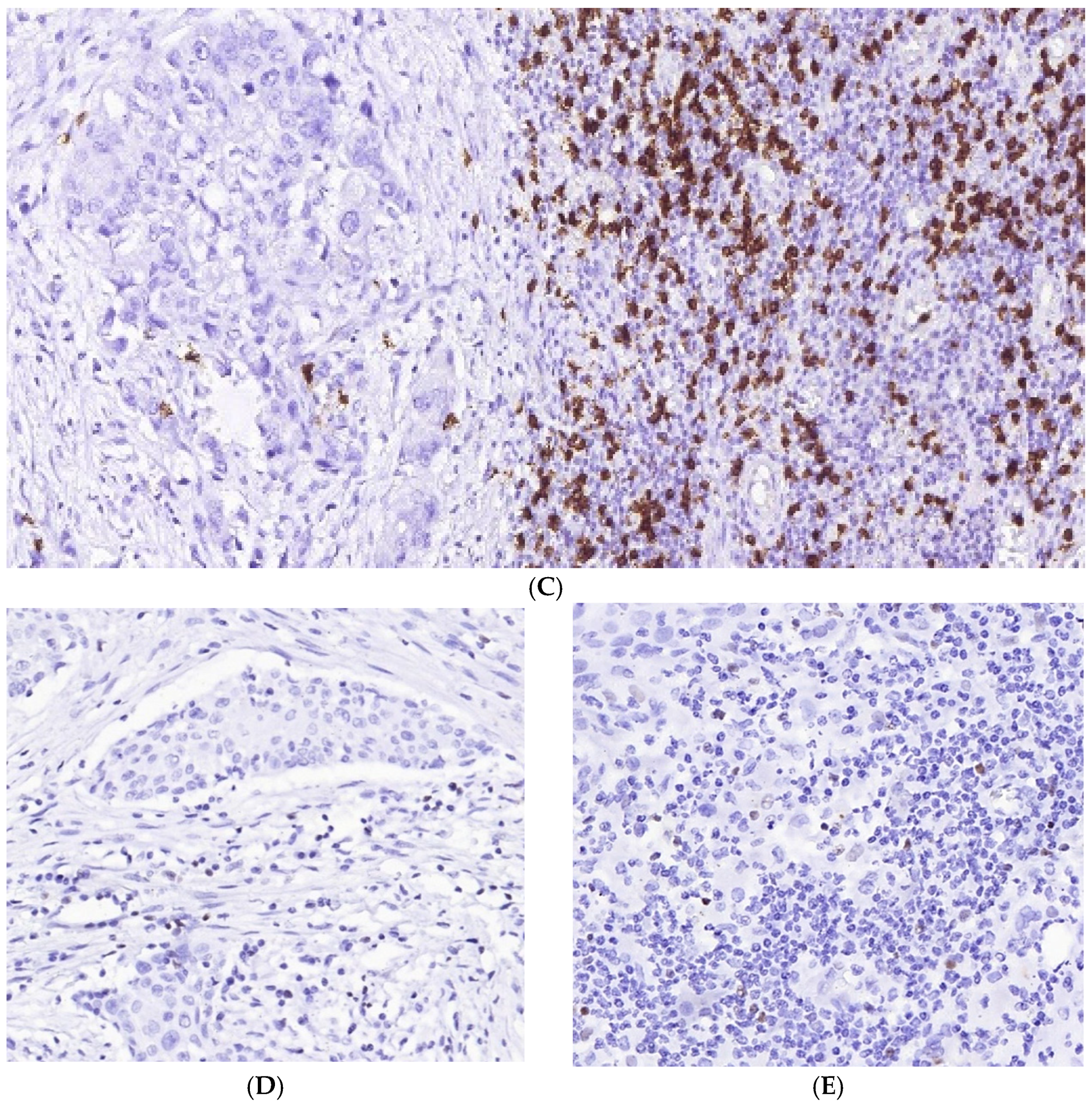
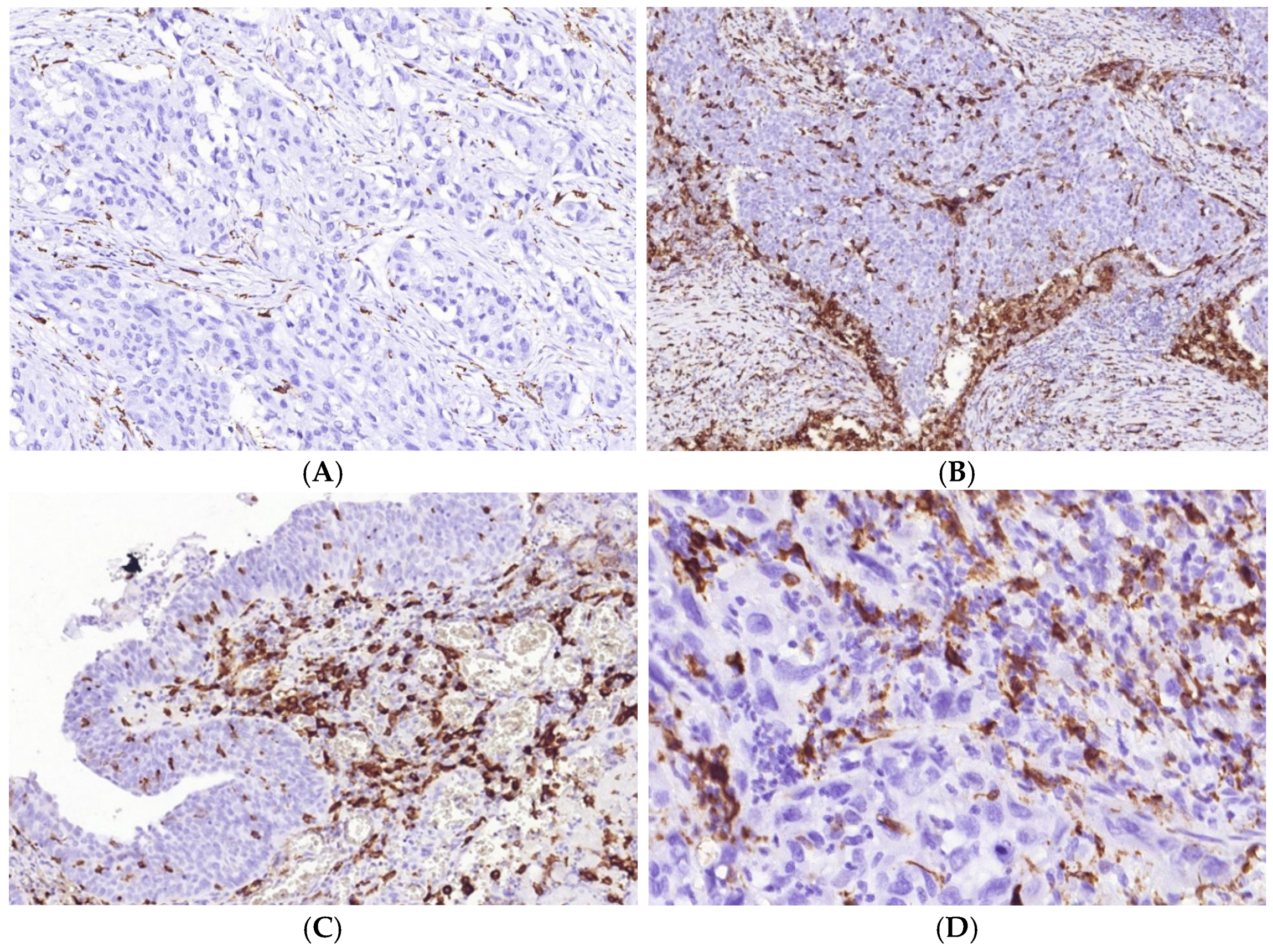
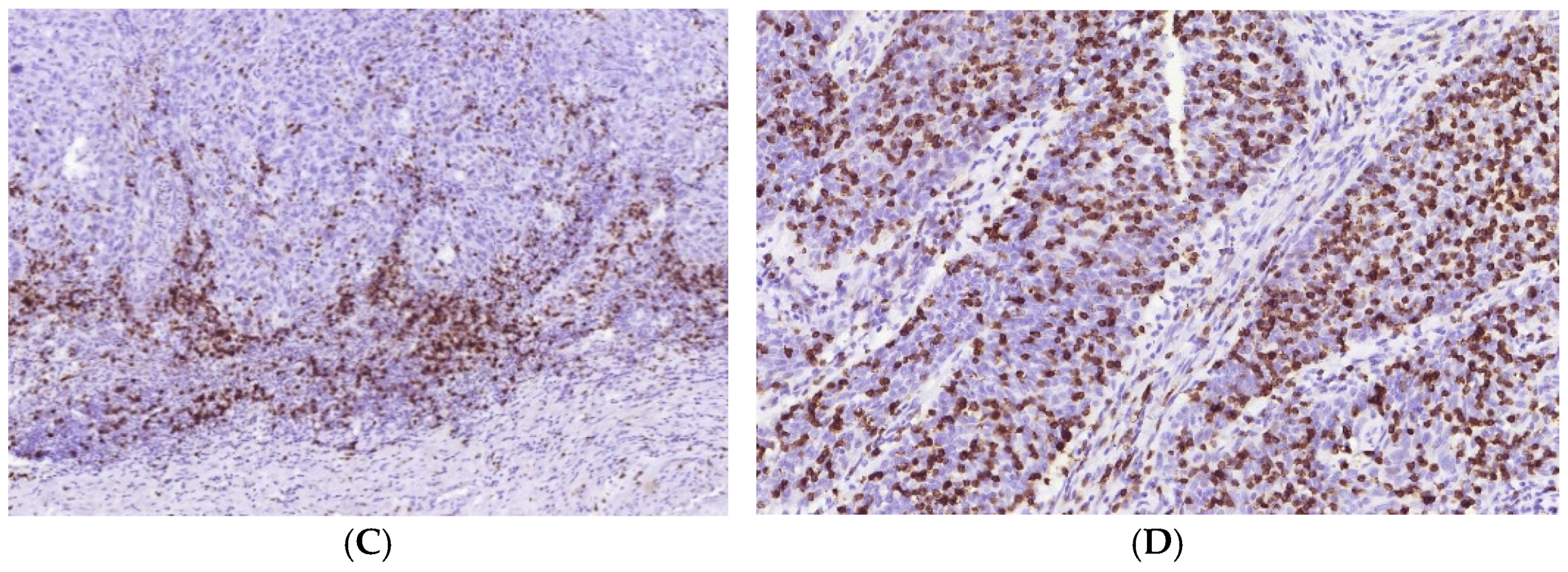
3.1. Immune Cell Count and Distribution in MIUCs
3.2. Immune Cycle Patterns in MIUCs
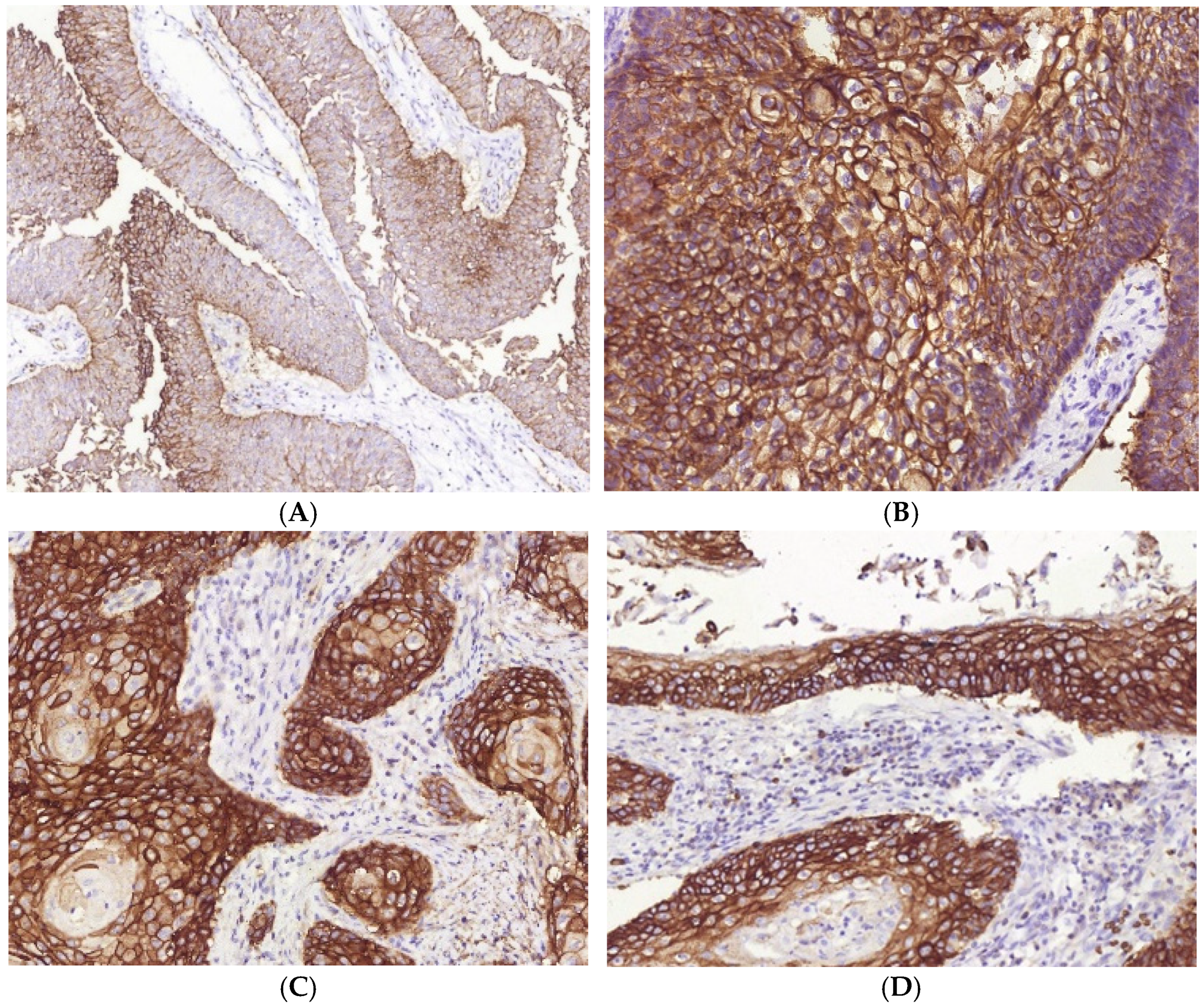
3.3. Immunophenotype and PD-L1 Expression
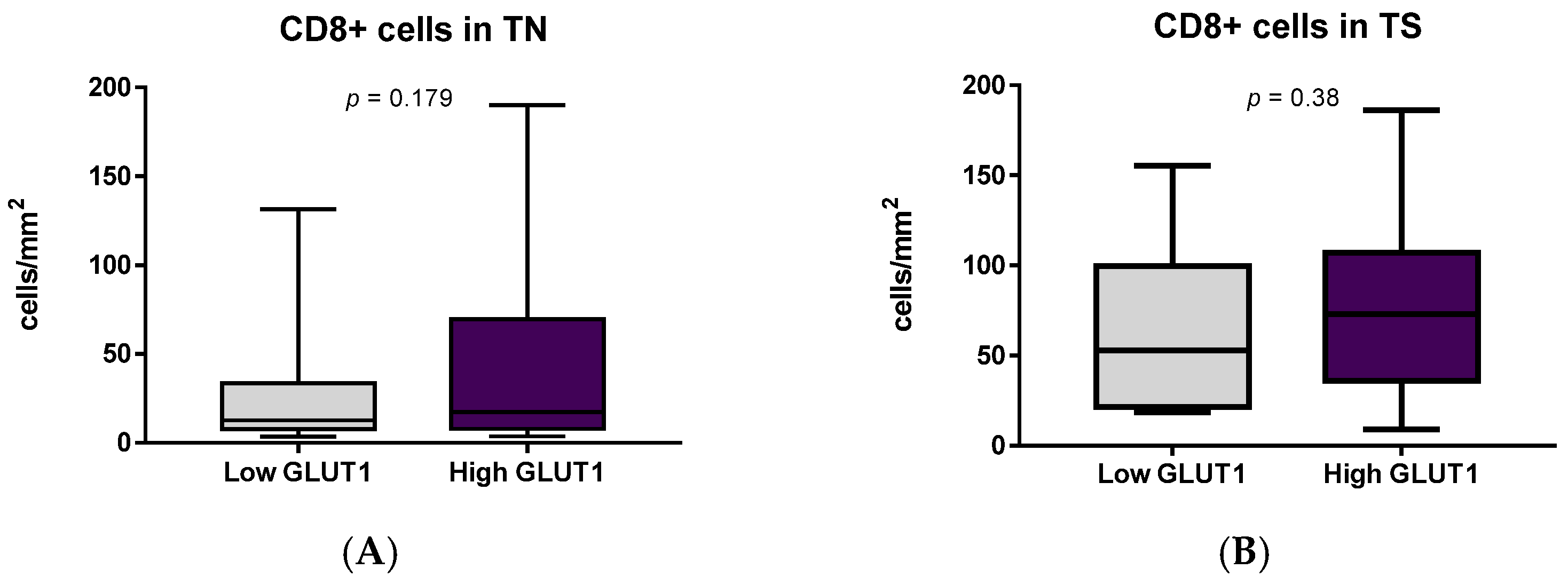
3.4. Immune Cells and Hypoxia in MIUCs
3.5. Prognostic Value of the Immune Cells, PD-L1 Expression and GLUT1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Limitations of the Study
References
- Audenet, F.; Attalla, K.; Sfakianos, J.P. The evolution of bladder cancer genomics: What have we learned and how can we use it? Urol. Oncol. 2018, 36, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, W.; Yang, X.; Wu, C.; Cheng, F. Traditional Classification and Novel Subtyping Systems for Bladder Cancer. Front. Oncol. 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Mar, N.; Dayyani, F. Management of Urothelial Bladder Cancer in Clinical Practice: Real-World Answers to Difficult Questions. J. Oncol. Pract. 2019, 15, 421–428. [Google Scholar] [CrossRef]
- Stadler, W.M. Management of Urothelial Bladder Cancer: Predicting the Future. J. Oncol. Pract. 2019, 15, 429–430. [Google Scholar] [CrossRef]
- Sjödahl, G.; Eriksson, P.; Liedberg, F.; Höglund, M. Molecular classification of urothelial carcinoma: Global mRNA classification versus tumour-cell phenotype classification. J. Pathol. 2017, 242, 113–125. [Google Scholar] [CrossRef]
- Tan, T.Z.; Rouanne, M.; Tan, K.T.; Huang, R.Y.J.; Thiery, J.P. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors. Eur. Urol. 2019, 75, 423–432. [Google Scholar] [CrossRef]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.G.; Winters, B.; Douglas, J.; Van Kessel, K.E.; Fransen van de Putte, E.E.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Minoli, M.; Kiener, M.; Thalmann, G.N.; Kruithof-de Julio, M.; Seiler, R. Evolution of Urothelial Bladder Cancer in the Context of Molecular Classifications. Int. J. Mol. Sci. 2020, 21, 5670. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl, G.; Lauss, M.; Lövgren, K.; Chebil, G.; Gudjonsson, S.; Veerla, S.; Patschan, O.; Aine, M.; Fernö, M.; Ringnér, M.; et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 3377–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavallee, E.; Sfakianos, J.P.; Mulholland, D.J. Tumor Heterogeneity and Consequences for Bladder Cancer Treatment. Cancers 2021, 13, 5297. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, D.; Kitano, S.; Tsuyukubo, T.; Takenouchi, K.; Nakayama, T.; Onagi, H.; Sakaguchi, A.; Yamashita, M.; Mizugaki, H.; Maekawa, S.; et al. Pretreatment tumour immune microenvironment predicts clinical response and prognosis of muscle-invasive bladder cancer in the neoadjuvant chemotherapy setting. Br. J. Cancer 2021, 126, 606–614. [Google Scholar] [CrossRef]
- Mashukov, A.; Shapochka, D.; Seleznov, O.; Kobyliak, N.; Sulaieva, O.; Falalyeyeva, T.; Kirkilevsky, S.; Yarema, R. Histological differentiation impacts the tumor immune microenvironment in gastric carcinoma: Relation to the immune cycle. World J. Gastroenterol. 2021, 27, 5259–5271. [Google Scholar] [CrossRef]
- Cao, R.; Yuan, L.; Ma, B.; Wang, G.; Tian, Y. Tumour microenvironment (TME) characterization identified prognosis and immunotherapy response in muscle-invasive bladder cancer (MIBC). Cancer Immunol. Immunother. CII 2021, 70, 1–18. [Google Scholar] [CrossRef]
- Kang, Z.; Li, W.; Yu, Y.-H.; Che, M.; Yang, M.-L.; Len, J.-J.; Wu, Y.-R.; Yang, J.-F. Identification of Immune-Related Genes Associated with Bladder Cancer Based on Immunological Characteristics and Their Correlation with the Prognosis. Front. Genet. 2021, 12, 763590. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; He, D.; Cheng, Y.; Zhu, Y.; Xiao, M.; Lan, H.; Wang, Z.; Cao, K. Analysis of Tumor Microenvironment Characteristics in Bladder Cancer: Implications for Immune Checkpoint Inhibitor Therapy. Front. Immunol. 2021, 12, 672158. [Google Scholar] [CrossRef]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.; Ye, Z.; Wu, J.; Zhou, R.; Fan, X.; Wang, G.; Huang, Y.; Wu, J.; Sun, H.; Wang, M.; et al. Macrophage correlates with immunophenotype and predicts anti-PD-L1 response of urothelial cancer. Theranostics 2020, 10, 7002–7014. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, R.; He, D.; Zhang, Y.; Chen, H.; Zhu, Y.; Cheng, Y.X.; Liu, R.; Zhu, R.; Gong, L.; et al. CD8 + T effector and immune checkpoint signatures predict prognosis and responsiveness to immunotherapy in bladder cancer. Oncogene 2021, 40, 6223–6234. [Google Scholar] [CrossRef] [PubMed]
- Zabolotnyi, D.; Kizim, Y.; Zabolotna, D.; Sulaieva, O.; Kizim, V. Laryngopharyngeal Reflux Alters Macrophage Polarization in Human Papilloma Virus-Negative Squamous Cell Carcinoma of the Larynx in Males. Clin. Exp. Otorhinolaryngol. 2021, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Crispen, P.L.; Kusmartsev, S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol. Immunother. CII 2020, 69, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, M.; Enting, D. Immune Responses in Bladder Cancer-Role of Immune Cell Populations, Prognostic Factors and Therapeutic Implications. Front. Oncol. 2019, 9, 1270. [Google Scholar] [CrossRef] [Green Version]
- Abou Khouzam, R.; Zaarour, R.F.; Brodaczewska, K.; Azakir, B.; Venkatesh, G.H.; Thiery, J.; Terry, S.; Chouaib, S. The Effect of Hypoxia and Hypoxia-Associated Pathways in the Regulation of Antitumor Response: Friends or Foes? Front. Immunol. 2022, 13, 828875. [Google Scholar] [CrossRef]
- Eslami, M.; Sadrifar, S.; Karbalaei, M.; Keikha, M.; Kobyliak, N.M.; Yousefi, B. Importance of the Microbiota Inhibitory Mechanism on the Warburg Effect in Colorectal Cancer Cells. J. Gastrointest. Cancer 2020, 51, 738–747. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Boström, P.J.; Thoms, J.; Sykes, J.; Ahmed, O.; Evans, A.; Van Rhijn, B.W.G.; Mirtti, T.; Stakhovskyi, O.; Laato, M.; Margel, D.; et al. Hypoxia Marker GLUT-1 (Glucose Transporter 1) is an Independent Prognostic Factor for Survival in Bladder Cancer Patients Treated with Radical Cystectomy. Bladder Cancer 2016, 2, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yaguchi, H.; Ohkura, N.; Kitabayashi, I.; Nagamura, Y.; Nomura, T.; Miyachi, Y.; Tsukada, T.; Sakaguchi, S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature 2007, 446, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzadra, R.; Sun, C.; Jae, L.T.; Gomez-Eerland, R.; De Vries, E.; Wu, W.; Logtenberg, M.E.W.; Slagter, M.; Rozeman, E.A.; Hofland, I.; et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 2017, 549, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Reis, H.; Tschirdewahn, S.; Szarvas, T.; Rübben, H.; Schmid, K.W.; Grabellus, F. Expression of GLUT1 is associated with increasing grade of malignancy in non-invasive and invasive urothelial carcinomas of the bladder. Oncol. Lett. 2011, 2, 1149–1153. [Google Scholar] [CrossRef] [Green Version]
- Rademakers, S.E.; Lok, J.; van der Kogel, A.J.; Bussink, J.; Kaanders, J.H.A.M. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer 2011, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.W.; Cho, H.; Chung, J.Y.; Conway, C.; Ylaya, K.; Kim, J.H.; Hewitt, S.M. Prognostic assessment of hypoxia and metabolic markers in cervical cancer using automated digital image analysis of immunohistochemistry. J. Transl. Med. 2013, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Sulaieva, O.; Chernenko, O.; Selesnov, O.; Nechay, O.; Maievskyi, O.; Falalyeyeva, T.; Kobyliak, N.; Tsyryuk, O.; Penchuk, Y.; Shapochka, D. Mechanisms of the Impact of Hashimoto Thyroiditis on Papillary Thyroid Carcinoma Progression: Relationship with the Tumor Immune Microenvironment. Endocrinol. Metab. 2020, 35, 443–455. [Google Scholar] [CrossRef]
- Nakad, R.; Schumacher, B. DNA Damage Response and Immune Defense: Links and Mechanisms. Front. Genet. 2016, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.; Wang, J.; Lu, D.; Xu, X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Sign. Transduct. Target. Ther. 2021, 6, 75. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chuang, H.W.; Kuo, W.T.; Lin, B.S.; Chang, Y.C. Current Development and Application of Anaerobic Glycolytic Enzymes in Urothelial Cancer. Int. J. Mol. Sci. 2021, 22, 10612. [Google Scholar] [CrossRef] [PubMed]
- Reis, H.; Serrette, R.; Posada, J.; Lu, V.; Chen, Y.B.; Gopalan, A.; Fine, S.W.; Tickoo, S.K.; Sirintrapun, S.J.; Iyer, G.; et al. PD-L1 Expression in Urothelial Carcinoma with Predominant or Pure Variant Histology: Concordance Among 3 Commonly Used and Commercially Available Antibodies. Am. J. Surg. Pathol. 2019, 43, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Tyryshkin, K.; Graham, C.H.; Koti, M.; Siemens, D.R. Comprehensive immune transcriptomic analysis in bladder cancer reveals subtype specific immune gene expression patterns of prognostic relevance. Oncotarget 2017, 8, 70982–71001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Markers | Main Target | Antibody Characteristics | Cellular Localization | Rationale |
|---|---|---|---|---|
| CD8 | T-cell | Dako; Clone C8/144B | Membranous | The CD8 antigen is found on most cytotoxic T-lymphocytes and acts primarily as a coreceptor for MHC class I molecule. CD8 contributes to efficient cell–cell interactions in antitumor immunity. |
| FoxP3 | T-cell | Cell Marque, Clone EP340 | Nuclear | FOXP3 is a member of the forkhead/winged-helix family of transcriptional regulators that is crucial for the development and suppressive function of T-regulatory cells (Treg) [32]. |
| CD68 | Macrophage | Dako, Clone KP1 | Cytoplasmic | CD68 is considered a pan-macrophage marker. It is a member of the lysosomal/endosomal-associated membrane glycoprotein localized to lysosomes and plays a role in the phagocytic activities of tissue macrophages. CD68 is also a member of the scavenger receptor family, acting to clear cellular debris and mediate the recruitment and activation of macrophages. |
| CD163 | M2-macrophages | Cell Marque, Clone MRQ-26 | Cytoplasmic | CD163 is a member of the scavenger receptor superfamily and a marker of alternatively activated or anti-inflammatory macrophages. CD163 is involved in the clearance of hemoglobin/haptoglobin complexes, contributing to tissue protection from free hemoglobin-mediated oxidative damage, and additionally acts as an innate immune sensor. Its role was shown in defining bladder cancer prognosis and sensitivity to therapy. |
| PD-L1 | Immune checkpoint | Dako, 22C3 | Membranous | PD-L1 (CD274) is a ligand that binds with the PD1 receptor, reducing T-cell effector response and cytokine production, providing an immune escape for tumor cells [33] and facilitating tumor survival [34]. |
| GLUT1 | Hypoxic conditions | Cell Marque, polyclonal | Membranous | GLUT1 is energy-independent glucose transporter 1, or Solute Carrier Family 2 Member 1 (SLC2A1), with a high affinity for glucose. There is no GLUT1 expression in normal bladder mucosa, however it is upregulated in urothelial carcinomas [35] and is considered to be a metabolic marker of prolonged hypoxia [36]. |
| Characteristics | Value | % |
|---|---|---|
| Total number of the enrolled patients | 42 | 100 |
| Men | 42 | 100 |
| Age | 56.8 ± 1.7 (53.4–60.2) | |
| Under 55 years | 13 | 30.9 |
| Over 55 years | 29 | 69.1 |
| PFS | 20 | 47.6 |
| Survived | 29 | 69 |
| Died | 13 | 31 |
| Pathological features | ||
| pT staging | ||
| pT2 | 30 | 71.4 |
| pT3 | 12 | 28.6 |
| CIS presence | 14 | 33.3 |
| Lymphovascular invasion | 22 | 52.4 |
| Variant histology | 26 | 61.9 |
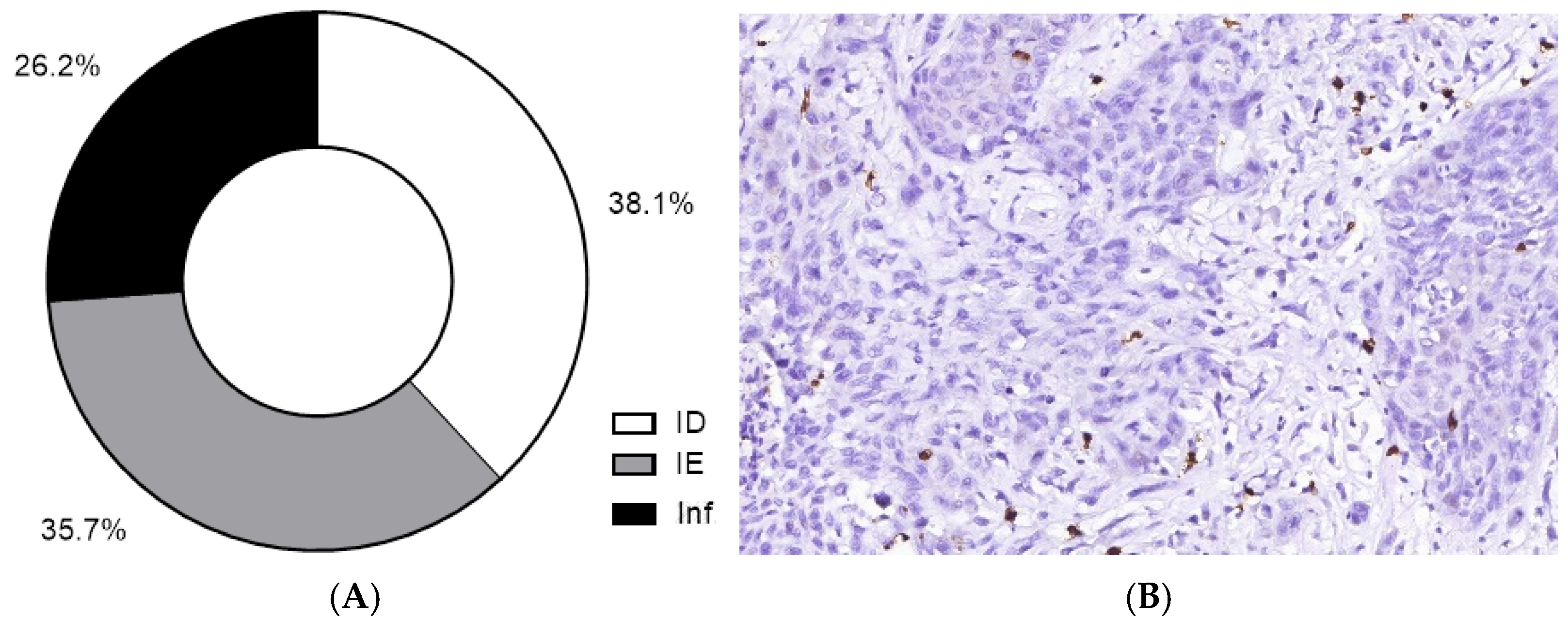
| TIME type | ||
| ID | 16 | 38.1 |
| IE | 15 | 35.7 |
| Inflamed | 11 | 26.2 |
| Hazard Ratio | 95% CI | p | |
|---|---|---|---|
| pT3 | 3.91 | 0.96–10.14 | 0.049 |
| LVI | 6.19 | 2.06–18.56 | 0.0017 |
| CIS | 5.48 | 1.838–16.34 | 0.0024 |
| Variant histology | 8.295 | 2.437–28.23 | 0.0023 |
| TLS | 0.487 | 0.1597–1.485 | 0.242 |
| TIME ID and IE vs. Inf | 0.57 | 0.1874–1.762 | 0.295 |
| CD8+ | 1.74 | 0.5675–5.336 | 0.122 |
| CD68+ | 1.42 | 0.439–4.632 | 0.338 |
| CD163+ | 3.388 | 1.13–10.16 | 0.035 |
| PMN infiltration | 8.358 | 2.675–26.12 | 0.001 |
| PD-L1 | 1.544 | 0.466–5.117 | 0.286 |
| GLUT1 low/high | 1.243 | 0.398–3.882 | 0.708 |
| Histological Subtype | Number of Cases | % |
|---|---|---|
| Papillary-invasive carcinoma | 9 | 21% |
| Urothelial-invasive carcinoma (UIC) | 7 | 17% |
| UIC + squamous differentiation | 14 | 33% |
| UIC + micropapillary differentiation | 2 | 5% |
| UIC + poorly differentiated (basaloid) | 2 | 5% |
| UIC + sarcomatoid (mesenchymal-like) | 4 | 10% |
| UIC + 2 and more differential patterns | 4 | 10% |
| Outcome | |||
|---|---|---|---|
| Good Prognosis | Poor Prognosis | ||
| Mean ± SEM 95% CI | Mean ± SEM 95% CI | p | |
| ID | 10 | 6 | 0.4930 |
| IE | 10 | 5 | |
| Inf | 9 | 2 | |
| CD8+ cells in tumor clusters | 30.2 ± 6.42 17.1–43.4 | 40.1 ± 15.0 7.4–72.8 | 0.479 |
| CD8+ cells in peritumoral stroma | 78.1 ± 10.22 57.1–98.9 | 79.7 ± 15.7 45.6–113.9 | 0.929 |
| CD163+ cells in tumor clusters | 44.5 ± 6.40 31.4–57.7 | 98.4 ± 10.7 74.9–121.8 | <0.001 |
| CD163+ cells in tumor stroma | 102.7 ± 8.45 85.3–120.0 | 156.7 ± 14.1 125.9–187.6 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stakhovskyi, O.; Kobyliak, N.; Voylenko, O.; Stakhovskyi, E.; Ponomarchuk, R.; Sulaieva, O. Immune Microenvironment of Muscular-Invasive Urothelial Carcinoma: The Link to Tumor Immune Cycle and Prognosis. Cells 2022, 11, 1802. https://doi.org/10.3390/cells11111802
Stakhovskyi O, Kobyliak N, Voylenko O, Stakhovskyi E, Ponomarchuk R, Sulaieva O. Immune Microenvironment of Muscular-Invasive Urothelial Carcinoma: The Link to Tumor Immune Cycle and Prognosis. Cells. 2022; 11(11):1802. https://doi.org/10.3390/cells11111802
Chicago/Turabian StyleStakhovskyi, Oleksandr, Nazarii Kobyliak, Oleg Voylenko, Eduard Stakhovskyi, Roman Ponomarchuk, and Oksana Sulaieva. 2022. "Immune Microenvironment of Muscular-Invasive Urothelial Carcinoma: The Link to Tumor Immune Cycle and Prognosis" Cells 11, no. 11: 1802. https://doi.org/10.3390/cells11111802
APA StyleStakhovskyi, O., Kobyliak, N., Voylenko, O., Stakhovskyi, E., Ponomarchuk, R., & Sulaieva, O. (2022). Immune Microenvironment of Muscular-Invasive Urothelial Carcinoma: The Link to Tumor Immune Cycle and Prognosis. Cells, 11(11), 1802. https://doi.org/10.3390/cells11111802






