Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
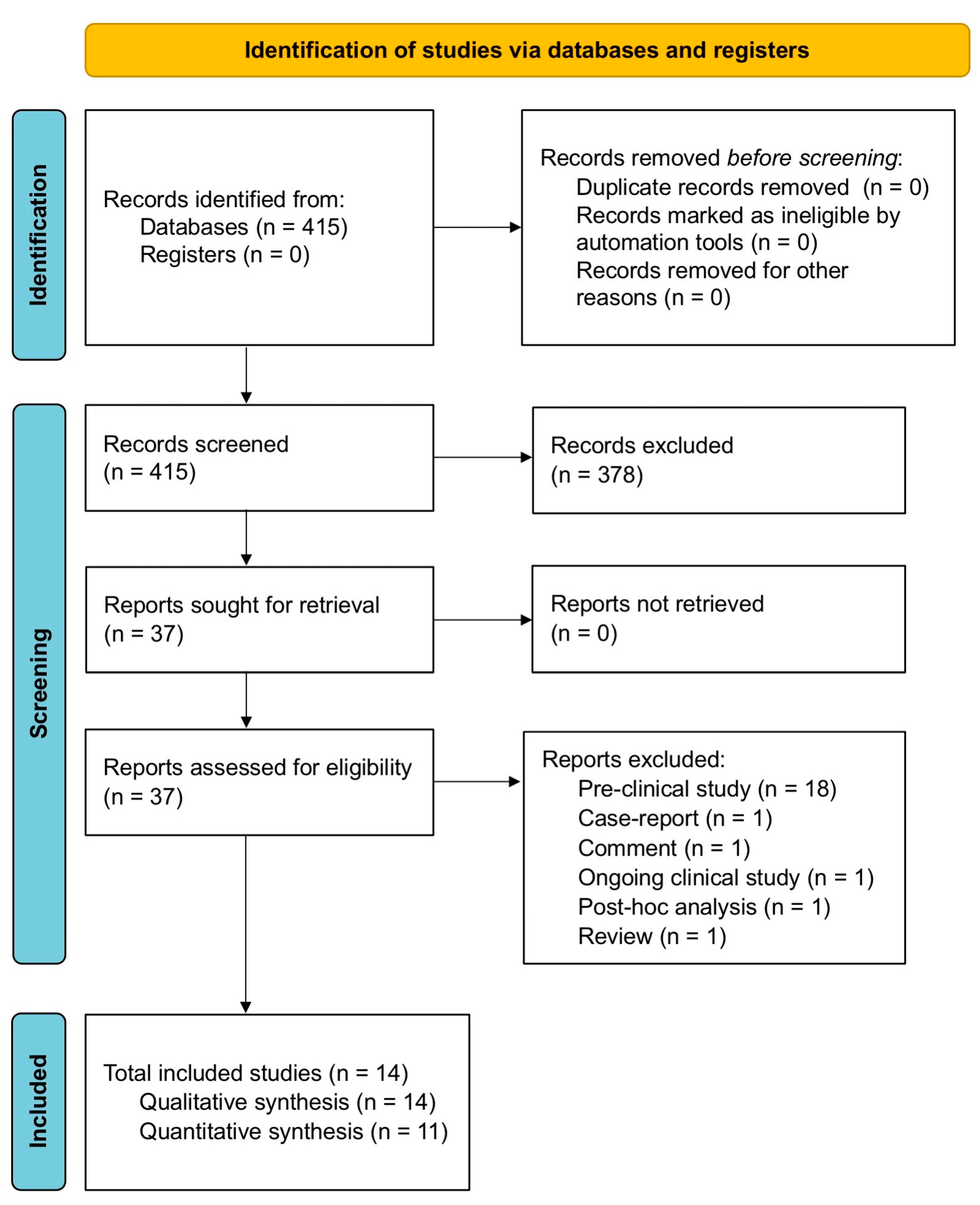
2.1. Search Strategy and Study Eligibility
2.2. Study Selection
2.3. Data Extraction
2.4. Endpoints
2.5. Data Synthesis and Analysis
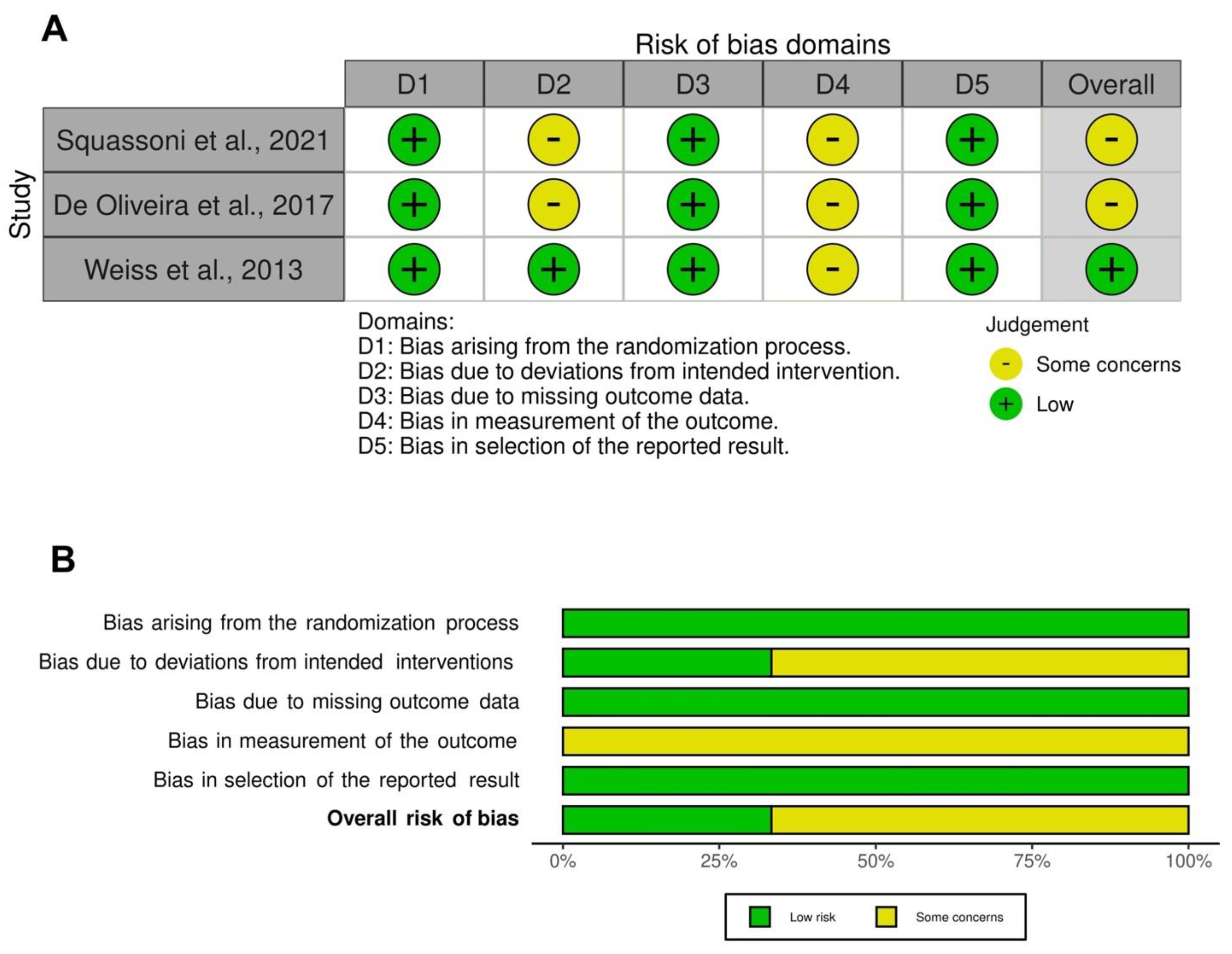
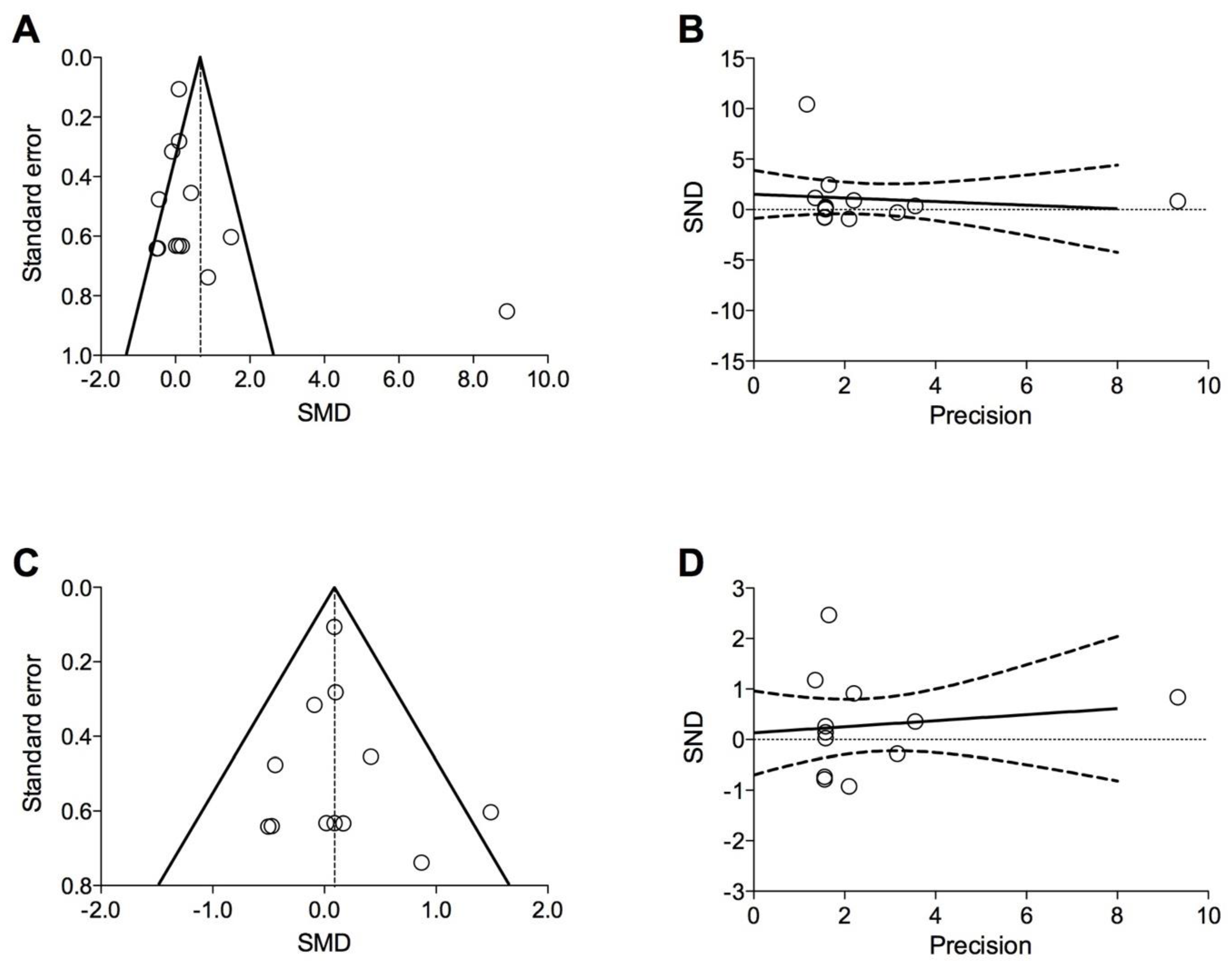
2.6. Quality of Studies, Risk Bias, and Evidence Profile
2.7. Software and Statistical Significance
3. Results
3.1. Study Characteristics
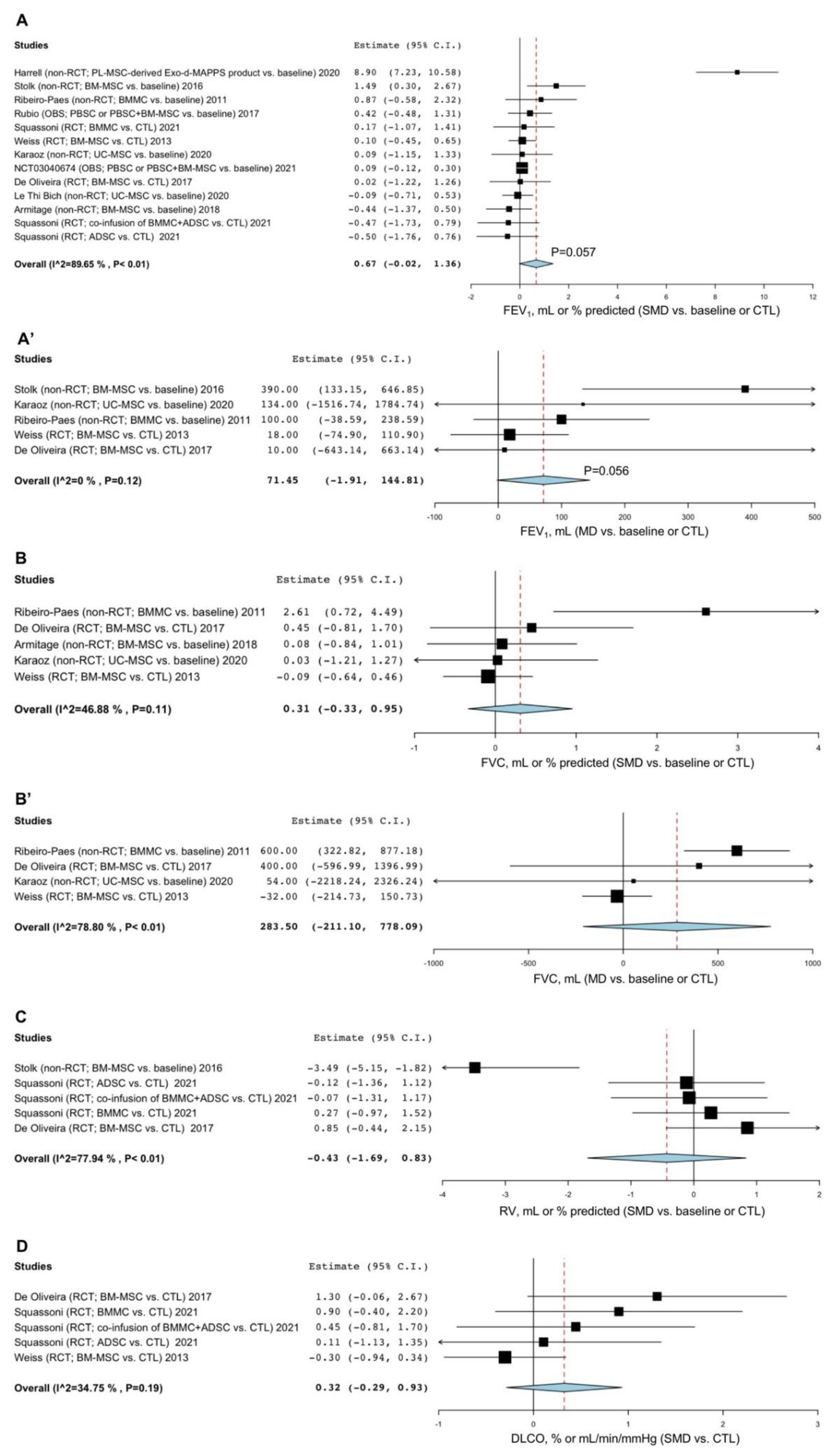
3.2. Pairwise Meta-Analysis
3.2.1. Lung Function
3.2.2. AECOPD
3.2.3. Hospitalization Due to AECOPD
3.2.4. Exercise Capacity
3.2.5. Blood Gas Analysis
3.3. Risk of Bias and Quality of Evidence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of Chronic Obstructive Pulmonary Disease; 2022 Report. Available online: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf (accessed on 5 April 2022).
- World Health Organization. WHO—The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 5 April 2022).
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic Triad in COPD: Oxidative Stress, Protease-Antiprotease Imbalance, and Inflammation. Int. J. Chron. Obs. Pulmon. Dis. 2011, 6, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New Insights into the Immunology of Chronic Obstructive Pulmonary Disease. Lancet 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Barnes, P.J. Immunology of Asthma and Chronic Obstructive Pulmonary Disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Pathology, Pathogenesis, and Pathophysiology. BMJ 2006, 332, 1202–1204. [Google Scholar] [CrossRef] [Green Version]
- Houghton, A.M. Matrix Metalloproteinases in Destructive Lung Disease. Matrix Biol. 2015, 44, 167–174. [Google Scholar] [CrossRef]
- Rogliani, P.; Ora, J.; Matera, M.G.; Cazzola, M.; Calzetta, L. The Safety of Dual Bronchodilation on Cardiovascular Serious Adverse Events in COPD. Expert Opin. Drug Saf. 2018, 17, 589–596. [Google Scholar] [CrossRef]
- Calzetta, L.; Coppola, A.; Ritondo, B.L.; Matino, M.; Chetta, A.; Rogliani, P. The Impact of Muscarinic Receptor Antagonists on Airway Inflammation: A Systematic Review. Int. J. COPD. 2021, 16, 257–279. [Google Scholar] [CrossRef]
- Celli, B.R.; Anderson, J.A.; Cowans, N.J.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Morris, A.N.; Quasny, H.; Yates, J.; Vestbo, J. Pharmacotherapy and Lung Function Decline in Patients with Chronic Obstructive Pulmonary Disease. A Systematic Review. Am. J. Respir. Crit. Care Med. 2021, 203, 689–698. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, J.; Li, S.; Fukunaga, A.; Yodoi, J.; Tian, H. Progress in the Mechanism and Targeted Drug Therapy for COPD. Signal Transduct. Target. Ther. 2020, 5, 1–20. [Google Scholar] [CrossRef]
- Lo Bello, F.; Hansbro, P.M.; Donovan, C.; Coppolino, I.; Mumby, S.; Adcock, I.M.; Caramori, G. New Drugs under Development for COPD. Expert Opin. Emerg. Drugs 2020, 25, 419–431. [Google Scholar] [CrossRef]
- Sun, Z.; Li, F.; Zhou, X.; Chung, K.F.; Wang, W.; Wang, J. Stem Cell Therapies for Chronic Obstructive Pulmonary Disease: Current Status of Pre-Clinical Studies and Clinical Trials. J. Thorac. Dis. 2018, 10, 1084–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X. Stem Cell-Based Disease Modeling and Cell Therapy. Cells 2020, 9, 2193. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.A.; DuTreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 Receptor Antagonist Mediates the Antiinflammatory and Antifibrotic Effect of Mesenchymal Stem Cells during Lung Injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Woods, C.R.; Mora, A.L.; Joodi, R.; Brigham, K.L.; Iyer, S.; Rojas, M. Prevention of Endotoxin-Induced Systemic Response by Bone Marrow-Derived Mesenchymal Stem Cells in Mice. Am. J. Physiol. Cell. Mol. Physiol. 2007, 293, L131–L141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Xu, J.; Qu, J.; Zhang, J.; Sai, Y.; Chen, C.; Wu, L.; Yu, L. Intrapleural Delivery of MSCs Attenuates Acute Lung Injury by Paracrine/Endocrine Mechanism. J. Cell. Mol. Med. 2012, 16, 2745–2753. [Google Scholar] [CrossRef]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal Stem Cells Enhance Recovery and Repair Following Ventilator-Induced Lung Injury in the Rat. Thorax 2012, 67, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.A.; Abreu, S.C.; Cruz, F.F.; Teixeira, A.C.; Lopes-Pacheco, M.; Bandeira, E.; Olsen, P.C.; Diaz, B.L.; Takyia, C.M.; Freitas, I.P.R.G. Effects of Different Mesenchymal Stromal Cell Sources and Delivery Routes in Experimental Emphysema. Respir. Res. 2014, 15, 118. [Google Scholar] [CrossRef] [Green Version]
- Mohammadipoor, A.; Antebi, B.; Batchinsky, A.I.; Cancio, L.C. Therapeutic Potential of Products Derived from Mesenchymal Stem/Stromal Cells in Pulmonary Disease. Respir. Res. 2018, 19, 218. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inf. Decis Mak 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassberg, M.K.; Csete, I.; Simonet, E.; Elliot, S.J. Stem Cell Therapy for COPD: Hope and Exploitation. Chest 2021, 160, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Pedder, H.; Sarri, G.; Keeney, E.; Nunes, V.; Dias, S. Data Extraction for Complex Meta-Analysis (DECiMAL) Guide. Syst. Rev. 2016, 5, 212. [Google Scholar] [CrossRef] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Gianinazzi, M.E.; Rueegg, C.S.; Zimmerman, K.; Kuehni, C.E.; Michel, G.; Swiss Paediatric Oncology, G. Intra-Rater and Inter-Rater Reliability of a Medical Record Abstraction Study on Transition of Care after Childhood Cancer. PLoS ONE 2015, 10, e0124290. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009; p. 421. [Google Scholar]
- DeCoster, J. DeCoster, J. Meta-Analysis Notes; University of Alabama: Tuscaloosa, AL, USA, 2004. [Google Scholar]
- Turner, J.R.; Durham, T.A. Meta-methodology: Conducting and Reporting Meta-analyses. J. Clin. Hypertens. 2014, 16, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-Acetylcysteine on Chronic Bronchitis or COPD Exacerbations: A Meta-Analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankey, S.S.; Weissfeld, L.A.; Fine, M.J.; Kapoor, W. An Assessment of the Use of the Continuity Correction for Sparse Data in Meta-Analysis. Commun. Stat. Part B Simul. Comput. 1996, 25, 1031–1056. [Google Scholar] [CrossRef]
- Wells, G.A.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 April 2022).
- Rogliani, P.; Beasley, R.; Cazzola, M.; Calzetta, L. SMART for the Treatment of Asthma: A Network Meta-Analysis of Real-World Evidence. Respiratory Medicine. Respir. Med. 2021, 188, 106611. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis. Section 13.3.5.2 Funnel Plots. Available online: https://training.cochrane.org/handbook/current/chapter-13#section-13-3-5-2 (accessed on 5 April 2022).
- Sterne, J.A.C.; Gavaghan, D.; Egger, M. Publication and Related Bias in Meta-Analysis: Power of Statistical Tests and Prevalence in the Literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M. Funnel Plots for Detecting Bias in Meta-Analysis: Guidelines on Choice of Axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, L.A. Robvis: An R Package and Web Application for Visualising Risk-of-Bias Assessments. Available online: https://github.com/mcguinlu/robvis (accessed on 5 April 2022).
- Harrell, C.R.; Miloradovic, D.; Sadikot, R.; Fellabaum, C.; Markovic, B.S.; Miloradovic, D.; Acovic, A.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular and Cellular Mechanisms Responsible for Beneficial Effects of Mesenchymal Stem Cell-Derived Product “Exo-d-MAPPS” in Attenuation of Chronic Airway Inflammation. Anal. Cell. Pathol. 2020, 2020, 3153891. [Google Scholar] [CrossRef] [Green Version]
- Le Thi Bich, P.; Nguyen Thi, H.; Dang Ngo Chau, H.; Phan Van, T.; Do, Q.; Dong Khac, H.; Le Van, D.; Nguyen Huy, L.; Mai Cong, K.; Ta Ba, T.; et al. Allogeneic Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation for Treating Chronic Obstructive Pulmonary Disease: A Pilot Clinical Study. Stem Cell Res. Ther. 2020, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Armitage, J.D.; Tan, D.B.A.; Sturm, M.; Moodley, Y.P. Transcriptional Profiling of Circulating Mononuclear Cells from Patients with Chronic Obstructive Pulmonary Disease Receiving Mesenchymal Stromal Cell Infusions. Stem Cells Transl. Med. 2021, 10, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Comella, K.; Blas, J.A.P.; Ichim, T.; Lopez, J.; Limon, J.; Moreno, R.C. Autologous Stromal Vascular Fraction in the Intravenous Treatment of End-Stage Chronic Obstructive Pulmonary Disease: A Phase I Trial of Safety and Tolerability. J. Clin. Med. Res. 2017, 9, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stessuk, T.; Ruiz, M.A.; Greco, O.T.; Bilaqui, A.; Ribeiro-Paes, M.J.d.O.; Ribeiro-Paes, J.T. Phase I Clinical Trial of Cell Therapy in Patients with Advanced Chronic Obstructive Pulmonary Disease: Follow-up of up to 3 Years. Rev. Bras. Hematol. Hemoter. 2013, 35, 352–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCT03040674. An Observational Outcomes Study for Autologous Cell Therapy among Patients with COPD and Interstitial Lung Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT03040674 (accessed on 18 March 2022).
- Armitage, J.; Tan, D.B.A.; Troedson, R.; Young, P.; Lam, K.V.; Shaw, K.; Sturm, M.; Weiss, D.J.; Moodley, Y.P. Mesenchymal Stromal Cell Infusion Modulates Systemic Immunological Responses in Stable COPD Patients: A Phase I Pilot Study. Eur. Respir. J. 2018, 51, 1702369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolk, J.; Broekman, W.; Mauad, T.; Zwaginga, J.J.; Roelofs, H.; Fibbe, W.E.; Oostendorp, J.; Bajema, I.; Versteegh, M.I.M.; Taube, C.; et al. A Phase I Study for Intravenous Autologous Mesenchymal Stromal Cell Administration to Patients with Severe Emphysema. QJM 2016, 109, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Paes, J.T.; Bilaqui, A.; Greco, O.T.; Ruiz, M.A.; Marcelino, M.Y.; Stessuk, T.; de Faria, C.A.; Lago, M.R. Unicentric Study of Cell Therapy in Chronic Obstructive Pulmonary Disease/Pulmonary Emphysema. Int. J. COPD 2011, 6, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaoz, E.; Kalemci, S.; Ece, F. Improving Effects of Mesenchymal Stem Cells on Symptoms of Chronic Obstructive Pulmonary Disease. Bratisl. Lek. Listy 2020, 121, 188–191. [Google Scholar] [CrossRef]
- Squassoni, S.D.; Sekiya, E.J.; Fiss, E.; Lapa, M.S.; Cayetano, D.d.S.; Nascimento, F.; Alves, A.; Machado, N.C.; Escaramboni, B.; Lívero, F.A.d.R.; et al. Autologous Infusion of Bone Marrow and Mesenchymal Stromal Cells in Patients with Chronic Obstructive Pulmonary Disease: Phase I Randomized Clinical Trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 3561–3574. [Google Scholar] [CrossRef]
- De Oliveira, H.G.; Cruz, F.F.; Antunes, M.A.; De Macedo Neto, A.V.; Oliveira, G.A.; Svartman, F.M.; Borgonovo, T.; Rebelatto, C.L.K.; Weiss, D.J.; Brofman, P.R.S.; et al. Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. Stem Cells Transl. Med. 2017, 6, 962–969. [Google Scholar] [CrossRef]
- Weiss, D.J.; Casaburi, R.; Flannery, R.; LeRoux-Williams, M.; Tashkin, D.P. A Placebo-Controlled, Randomized Trial of Mesenchymal Stem Cells in COPD. Chest 2013, 143, 1590–1598. [Google Scholar] [CrossRef] [Green Version]
- NCT03044431. Autologous Stem Cell Treatment for Chronic Lung Disease Study. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03044431 (accessed on 18 February 2022).
- Liu, X.; Fang, Q.; Kim, H. Preclinical Studies of Mesenchymal Stem Cell (MSC) Administration in Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0157099. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, Y.S.; Hong, S.H.; Oh, Y.M. Therapeutic Effects of Adipose-Derived Stem Cells Pretreated with Pioglitazone in an Emphysema Mouse Model. Exp. Mol. Med. 2016, 48, e266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekman, W.; Khedoe, P.P.S.J.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal Stromal Cells: A Novel Therapy for the Treatment of Chronic Obstructive Pulmonary Disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Tonin, F.S.; Rotta, I.; Mendes, A.M.; Pontarolo, R. Network Meta-Analysis: A Technique to Gather Evidence from Direct andindirect Comparisons. Pharm. Pract. 2017, 15, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kebriaei, P.; Isola, L.; Bahceci, E.; Holland, K.; Rowley, S.; McGuirk, J.; Devetten, M.; Jansen, J.; Herzig, R.; Schuster, M. Adult Human Mesenchymal Stem Cells Added to Corticosteroid Therapy for the Treatment of Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2009, 15, 804–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S. A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study of Intravenous Adult Human Mesenchymal Stem Cells (Prochymal) after Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Shigemura, N.; Okumura, M.; Mizuno, S.; Imanishi, Y.; Nakamura, T.; Sawa, Y. Autologous Transplantation of Adipose Tissue-Derived Stromal Cells Ameliorates Pulmonary Emphysema. Am. J. Transplant. 2006, 6, 2592–2600. [Google Scholar] [CrossRef]
- Cho, R.J.; Kim, Y.S.; Kim, J.Y.; Oh, Y.M. Human Adipose-Derived Mesenchymal Stem Cell Spheroids Improve Recovery in a Mouse Model of Elastase-Induced Emphysema. BMB Rep. 2017, 50, 79–84. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.Y.; Shin, D.M.; Huh, J.W.; Lee, S.W.; Oh, Y.M. Tracking Intravenous Adipose-Derived Mesenchymal Stem Cells in a Model of Elastase-Induced Emphysema. Tuberc. Respir. Dis. 2014, 77, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Kim, J.Y.; Huh, J.W.; Lee, S.W.; Choi, S.J.; Oh, Y.M. The Therapeutic Effects of Optimal Dose of Mesenchymal Stem Cells in a Murine Model of an Elastase Induced-Emphysema. Tuberc. Respir. Dis. 2015, 78, 239–245. [Google Scholar] [CrossRef]
- Chen, Y.B.; Lan, Y.W.; Chen, L.G.; Huang, T.T.; Choo, K.B.; Cheng, W.T.K.; Lee, H.S.; Chong, K.Y. Mesenchymal Stem Cell-Based HSP70 Promoter-Driven VEGFA Induction by Resveratrol Alleviates Elastase-Induced Emphysema in a Mouse Model. Cell Stress Chaperones 2015, 20, 979–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, W.; Song, L.; Li, X.M.; Wang, D.; Guo, X.J.; Xu, W.G. Mesenchymal Stem Cells Alleviate Airway Inflammation and Emphysema in COPD through Down-Regulation of Cyclooxygenase-2 via P38 and ERK MAPK Pathways. Sci. Rep. 2015, 5, 8733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.W.; Kim, S.Y.; Lee, J.H.; Lee, J.S.; van Ta, Q.; Kim, M.; Oh, Y.M.; Lee, Y.S.; Lee, S.D. Bone Marrow Cells Repair Cigarette Smoke-Induced Emphysema in Rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L255–L266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibboel, J.; Keijzer, R.; Reiss, I.; De Jongste, J.C.; Post, M. Intravenous and Intratracheal Mesenchymal Stromal Cell Injection in a Mouse Model of Pulmonary Emphysema. COPD 2014, 11, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Gu, C.; Xu, W.; Yan, J.; Xia, Y.; Ma, Y.; Chen, C.; He, X.; Tao, H. Therapeutic Effects of Amniotic Fluid-Derived Mesenchymal Stromal Cells on Lung Injury in Rats with Emphysema. Respir. Res. 2014, 15, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingenito, E.P.; Tsai, L.; Murthy, S.; Tyagi, S.; Mazan, M.; Hoffman, A. Autologous Lung-Derived Mesenchymal Stem Cell Transplantation in Experimental Emphysema. Cell Transplant. 2012, 21, 175–189. [Google Scholar] [CrossRef]
- Ostanin, A.A.; Petrovskii, Y.L.; Shevela, E.Y.; Chernykh, E.R. Multiplex Analysis of Cytokines, Chemokines, Growth Factors, MMP-9 and TIMP-1 Produced by Human Bone Marrow, Adipose Tissue, and Placental Mesenchymal Stromal Cells. Bull. Exp. Biol. Med. 2011, 151, 133–141. [Google Scholar] [CrossRef]
- Ricciardi, M.; Malpeli, G.; Bifari, F.; Bassi, G.; Pacelli, L.; Kamdje, A.H.N.; Chilosi, M.; Krampera, M. Comparison of Epithelial Differentiation and Immune Regulatory Properties of Mesenchymal Stromal Cells Derived from Human Lung and Bone Marrow. PLoS ONE 2012, 7, e35639. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, M.; Wollert, K.C.; Meyer, G.P.; Menke, A.; Arseniev, L.; Hertenstein, B.; Ganser, A.; Knapp, W.H.; Drexler, H. Monitoring of Bone Marrow Cell Homing into the Infarcted Human Myocardium. Circulation 2005, 111, 2198–2202. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Francos, S.; Eiro, N.; Costa, L.A.; Escudero-Cernuda, S.; Fernández-Sánchez, M.L.; Vizoso, F.J. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef]
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitbach, M.; Bostani, T.; Roell, W.; Xia, Y.; Dewald, O.; Nygren, J.M.; Fries, J.W.U.; Tiemann, K.; Bohlen, H.; Hescheler, J.; et al. Potential Risks of Bone Marrow Cell Transplantation into Infarcted Hearts. Blood 2007, 110, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, Y.S.; Park, J.S.; Tkebuchava, T.; Luedeman, C.; Losordo, D.W. Unexpected Severe Calcification after Transplantation of Bone Marrow Cells in Acute Myocardial Infarction. Circulation 2004, 109, 3154–3157. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The Therapeutic Potential of the Mesenchymal Stem Cell Secretome in Ischaemic Stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1276–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toraldo, D.M.; Toraldo, S.; Conte, L. The Clinical Use of Stem Cell Research in Chronic Obstructive Pulmonary Disease: A Critical Analysis of Current Policies. J. Clin. Med. Res. 2018, 10, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daley, G.Q.; Hyun, I.; Apperley, J.F.; Barker, R.A.; Benvenisty, N.; Bredenoord, A.L.; Breuer, C.K.; Caulfield, T.; Cedars, M.I.; Frey-Vasconcells, J. Setting Global Standards for Stem Cell Research and Clinical Translation: The 2016 ISSCR Guidelines. Stem Cell Rep. 2016, 6, 787–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzetta, L.; Ritondo, B.L.; Coppola, A.; Matera, M.G.; Di Daniele, T.; Rogliani, P. Factors Influencing the Efficacy of COVID-19 Vaccines: A Quantitative Synthesis of Phase III Trials. Vaccines 2021, 9, 341. [Google Scholar] [CrossRef]
- Ciapponi, A.; Belizán, J.M.; Piaggio, G.; Yaya, S. There Is Life beyond the Statistical Significance. Reprod. Health 2021, 18, 80. [Google Scholar] [CrossRef]
- Stang, A.; Poole, C.; Kuss, O. The Ongoing Tyranny of Statistical Significance Testing in Biomedical Research. Eur. J. Epidemiol. 2010, 25, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Herpel, L.B.; Kanner, R.E.; Lee, S.M.; Fessler, H.E.; Sciurba, F.C.; Connett, J.E.; Wise, R.A. Variability of Spirometry in Chronic Obstructive Pulmonary Disease: Results from Two Clinical Trials. Am. J. Respir. Crit. Care Med. 2006, 173, 1106–1113. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.Y.; Meijer, K.; Delbressine, J.M.; Willems, P.J.; Franssen, F.M.E.; Wouters, E.F.M.; Spruit, M.A. Reproducibility and Validity of the 6-Minute Walk Test Using the Gait Real-Time Analysis Interactive Lab in Patients with COPD and Healthy Elderly. PLoS ONE 2016, 11, e0162444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandes, N.A.; Wouters, E.F.M.; Meijer, K.; Annegarn, J.; Pitta, F.; Spruit, M.A. Reproducibility of 6-Minute Walking Test in Patients with COPD. Eur. Respir. J. 2011, 38, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, W.; Liu, Y.; Liu, D.; Kesten, S.; Tashkin, D.P.; Celli, B.R.; Decramer, M. Quality and Reproducibility of Spirometry in COPD Patients in a Randomized Trial (UPLIFT®). Respir. Med. 2013, 107, 1409–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eapen, M.S.; Myers, S.; Walters, E.H.; Sohal, S.S. Airway Inflammation in Chronic Obstructive Pulmonary Disease (COPD): A True Paradox. Expert Rev. Respir. Med. 2017, 11, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Górska, K.; Maskey-Warzȩchowska, M.; Krenke, R. Airway Inflammation in Chronic Obstructive Pulmonary Disease. Curr. Opin. Pulm. Med. 2010, 16, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.C.; Lopes-Pacheco, M.; Weiss, D.J.; Rocco, P.R.M. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Perspectives. Front. Cell Dev. Biol. 2021, 9, 97. [Google Scholar] [CrossRef]

| Author, Year, and References | Trial Number Identifier | Study Characteristics | Study Duration (Weeks) | Number of Analyzed Patients | Types of Stem Cell-Based Treatment or Derived Products | Regimen and Route of Administration | Patients’ Characteristics | Age (Years) | Male (%) | Pre-Bronchodilator FEV1 (% Predicted) | Current Smokers (%) | Smoking History (Pack-Years) | Investigated Outcomes | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Armitage et al., 2021 [48] | ANZCTR12614000731695 | Phase I, monocentric, non-randomized, non-controlled, open-label, pilot study | 4 | 9 | Allogeneic BM-MSC (2 × 106 cells/kg) | Two IV infusions, 1 week apart (1st infusion composed of radiolabelled cells, the 2nd infusion used unlabelled cells) | Stable COPD | 70.0 | 44.0 | 37.0 | 0.0 | 32.0 | # | NC |
| Squassoni et al., 2021 [56] | NCT02412332 | Phase I, monocentric, randomized, open-controlled, parallel-group study | 52 | 20 | BMMC (1 × 108 cells/30 mL), ADSC (1 × 108 cells/30 mL), or co-administration of BMMC and ADSC (5 × 107 and 5 × 107 cells/30 mL) | Single IV infusion | Moderate-to-severe COPD (GOLD grade III; FEV1 > 30% and ≤50% predicted; no tobacco use for ≥6 months) | 62.4 | 25.0 | NA | NA | NA | Lung function and blood gas analysis | 3 |
| NA, 2021 [51] | NCT03040674 | Observational, monocentric, prospective, cohort study | 12 | 175 | Autologous PBSC or co-administration of PBSC and BM-MSC | Single infusion in peripheral circulation | COPD and ILD (no active infection, no history of cancer within past 5 years) | ≥16.0 | 64.6 | 34.2 | 0.0 | NA | Lung function | NC |
| Harrell et al., 2020 [46] | NA | Monocentric, non-randomized, non-controlled, open-label study | 3 | 30 | PL-MSC-derived Exo-d-MAPPS product (0.5 mL), containing a high concentration of immunosuppressive factors including soluble TNF receptors I and II, IL−1 receptor antagonist, and sRAGE | One inhalation per week | COPD (post-bronchodilator FEV1 ≥ 30% and <80% predicted and FEV1/FVC < 0.7) | 50.0–75.0 | 66.6 | NA | NA | ≥10.0 | Lung function and exercise capacity | NC |
| Le Thi Bich et al., 2020 [47] | ISRCTN70443938 | Monocentric, non-randomized, non-controlled, open-label, pilot study | 26 | 20 | Allogeneic UC-MSC (1.5 × 106 cells/kg) | Single IV infusion | Moderate-to-severe COPD (GOLD stage C and D; post-bronchodilator FEV1 between 30% and 70% predicted and FEV1/FVC < 0.7) | 67.0 | 100.0 | NA | 0.0 | 17.5 | Lung function, number of exacerbations and exercise capacity | NC |
| Karaoz et al., 2020 [55] | NA | Phase I/II, monocentric, non-randomized, non-controlled, open-label study | 6 | 5 | UC-MSC (1–2 × 106 cells/kg) | Four IV infusions at 2-week intervals | COPD | 56.0 | 100.0 | NA | 0.0 | NA | Lung function and exercise capacity | NC |
| Armitage et al., 2018 [52] | ANZCTR12614000731695 | Phase I, monocentric, non-randomized, non-controlled, open-label, pilot study | 4 | 9 | Allogeneic BM-MSC (2 × 106 cells/kg) | Two IV infusions, 1 week apart (1st infusion composed of radiolabelled cells, the 2nd infusion used unlabelled cells) | Mild-to-very-severe stable COPD (GOLD stage I, II, III, IV; no exacerbations for ≥3 months) | 70.0 | 44.0 | 37.0 | 0.0 | 32.0 | Lung function and hospitalization due to AECOPD | NC |
| Comella et al., 2017 [49] | NCT02041000 | Phase I, non-randomized, open-label study | 52 | 12 | Autologous ADSC administered as SVF (1.5–3 × 108 cells) | IV infusion | Severe COPD (GOLD stage III or IV; post-bronchodilator FEV1 ≤ 49% predicted and FEV1/FVC < 0.7; no active infection and/or malignancy; no current use of tobacco) | 69.0 | 50.0 | NA | 0.0 | NA | # | NC |
| De Oliveira et al., 2017 [57] | NCT01872624 | Phase I, prospective, monocentric, randomized, patient-blinded, PCB (vehicle)-controlled, parallel-group study | 12 | 10 | Allogeneic BM-MSC (1 × 108 cells/30 mL) +EBV | Bronchoscopical infusion in region where EBV were to be placed | Severe heterogenous pulmonary emphysema (GOLD stage III, IV; post-bronchodilator FEV1 < 45% predicted and FEV1/FVC < 0.7; no tobacco use for ≥6 months; mMRC Dyspnea Scale stage ≥ 2) | 60.5 | 50.0 | NA | NA | 62.9 | Lung function, hospitalization due to AECOPD, exercise capacity, and blood gas analysis | 3 |
| Rubio et al., 2017 [59] | NCT03044431 | Observational, monocentric, prospective, cohort study | 26 | 5 | Autologous PBSC or co-administration of PBSC and BM-MSC | Single infusion in peripheral circulation | COPD and ILD (no active infection, no history of cancer within past 5 years) | ≥16.0 | 54.1 | 36.9 (only COPD patients) | 0.0 | NA | Lung function | NC |
| Stolk et al., 2016 [53] | NCT01306513 | Phase I, monocentric, prospective, non-randomized, non-controlled, open-label study | 12 | 7 | Autologous BM-MSC (1–2 × 106 cells/kg) +LVRS | Two IV infusions, 1 week apart following LVRS | Severe pulmonary emphysema in both upper lung lobes (FEV1 ≤ 40% predicted; no tobacco use for ≥6 months) | 52.4 | 28.6 | 31.4 | NA | NA | Lung function | NC |
| Stessuk et al., 2013 [50] | NCT01110252 | Follow-up of a previous Phase I study [54] | Up to 156 | 3 | Autologous BMMC (30 mL of approximately 1 × 108 cells/kg) | Single IV infusion | Severe COPD with advanced pulmonary emphysema (limited life expectancy, ineffective clinical treatments; smoking cessation for ≥6 months; mMRC Dyspnea Scale Stage > 3) | 65.8 | 100.0 | NA | NA | NA | # | NC |
| Weiss et al., 2013 [58] | NCT00683722 | Phase II, multicenter, prospective, randomized, double-blind, PCB (vehicle)-controlled study | 104 | 62 | Allogeneic BM-MSC (Prochymal™, 100 × 106 cells) | Four monthly IV infusions | Moderate-to-severe COPD (GOLD stage II, III; post-bronchodilator FEV1 > 30% and <70% predicted and FEV1/FVC < 0.7) | 66.1 | 58.0 | NA | 27.1 | 21.5 | Lung function and hospitalization due to AECOPD | 4 |
| Ribeiro-Paes et al., 2011 [54] | NCT01110252 | Phase I, monocentric, non-randomized, non-controlled, open-label study | 52 | 4 | Autologous BMMC (30 mL diluted in physiological serum at 5% albumin) | Single IV infusion | Severe COPD with advanced pulmonary emphysema (limited life expectancy, ineffective clinical treatments; smoking cessation for ≥6 months; mMRC Dyspnea Scale Stage > 3) | 65.8 | 100.0 | NA | NA | NA | Lung function and blood gas analysis | NC |
| Trial Number Identifier | Trial Status | Trial Phase | Number of Enrolled Patients | Condition | Type of Stem Cell-Based Treatment (Dose) | Regimen and Route of Administration | Follow Up |
|---|---|---|---|---|---|---|---|
| NCT02348060 | Unknown recruitment status | Observational study | 100 | COPD | Adipose-derived SVF containing ADSC (NA) | NA | 1 year |
| NCT02645305 | Unknown | Phase I/II | 20 | Moderate to severe COPD | Adipose-derived SVF containing ADSC + platelet-rich plasma (NA) | NA | 1 year |
| NCT03500731 | Recruiting | Phase I/II | 8 | IPF, emphysema or COPD | CD3/CD19 negative hematopoietic stem cells (NA) | NA | Up to 2 years |
| NCT03655795 | Unknown | Phase I | 20 | COPD | Bronchial basal cells | NA | 1 year |
| NCT01758055 | Unknown | Phase I | 12 | Moderate to severe emphysema | Autologous BM-MSC (0.6 × 108 cells) | Single dose, endobronchial | 1 year |
| NCT04433104 | Recruiting | Phase I/II | 40 | Moderate to severe COPD | UC-MSC (1 × 106 cells/kg) | Two doses, the 2nd will be performed 3 months after the first transplantation, IV | 1 year |
| NCT04047810 | Active, not recruiting | Phase I | 15 | Advanced COPD | MSC (0.5–2 × 106 cells/kg) | Single dose, IV | 1 day |
| NCT04206007 | Recruiting | Phase I | 9 | Moderate COPD | Ex vivo cultured human umbilical cord tissue-derived mesenchymal stem cells, named UMC119-06 (NA) | Single dose, IV | ≈ 4 months |
| NCT04018729 | Unknown status | Phase II/III | 34 | Severe emphysema | EV + BM-MSC (NA) | Single dose, endoscopic administration | 6 months |
| NCT02946658 | Active, not recruiting | Phase I/II | 100 | COPD | Adipose-derived SVF containing ADSC (NA) | Single dose, IV | 1 year |
| NCT05147688 | Recruiting | Phase I | 20 | COPD | UC-MSC (1 × 108 cells) | Single dose, IV | 4 year |
| NCT03899298 | Active, not recruiting | Phase I | 5000 | COPD, among others | Amniotic stem cells and UC-MSC (NA) + nebulizer | IV + nebulizer inhalation | Up to 10 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzetta, L.; Aiello, M.; Frizzelli, A.; Camardelli, F.; Cazzola, M.; Rogliani, P.; Chetta, A. Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis. Cells 2022, 11, 1797. https://doi.org/10.3390/cells11111797
Calzetta L, Aiello M, Frizzelli A, Camardelli F, Cazzola M, Rogliani P, Chetta A. Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis. Cells. 2022; 11(11):1797. https://doi.org/10.3390/cells11111797
Chicago/Turabian StyleCalzetta, Luigino, Marina Aiello, Annalisa Frizzelli, Francesca Camardelli, Mario Cazzola, Paola Rogliani, and Alfredo Chetta. 2022. "Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis" Cells 11, no. 11: 1797. https://doi.org/10.3390/cells11111797
APA StyleCalzetta, L., Aiello, M., Frizzelli, A., Camardelli, F., Cazzola, M., Rogliani, P., & Chetta, A. (2022). Stem Cell-Based Regenerative Therapy and Derived Products in COPD: A Systematic Review and Meta-Analysis. Cells, 11(11), 1797. https://doi.org/10.3390/cells11111797








