Progesterone Signaling in Endometrial Epithelial Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organoid Culture
2.2. RNA and Chromatin Analyses
2.3. Western Blot
3. Results
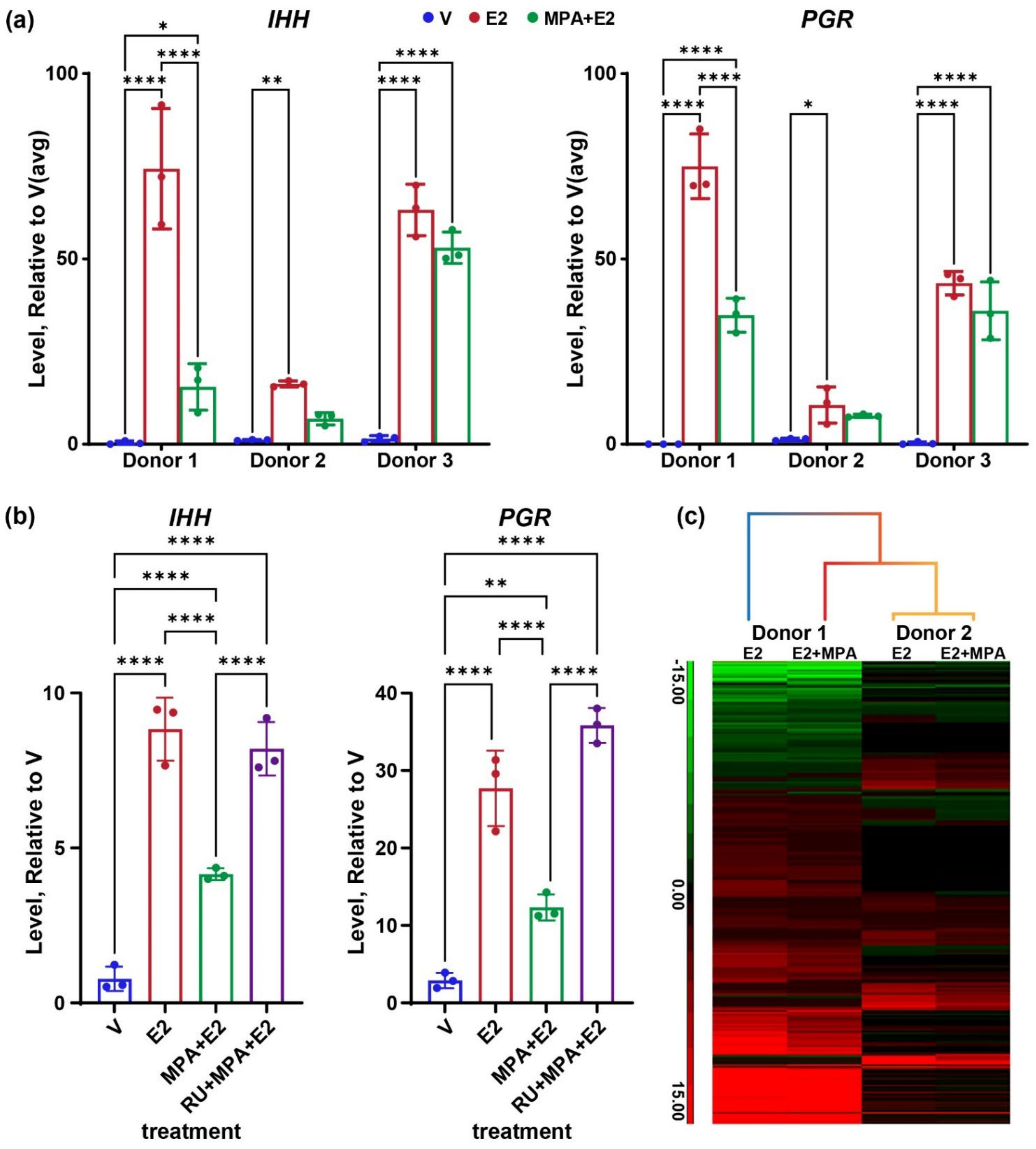
3.1. Impact of Progesterone on Endometrial Organoid Genes
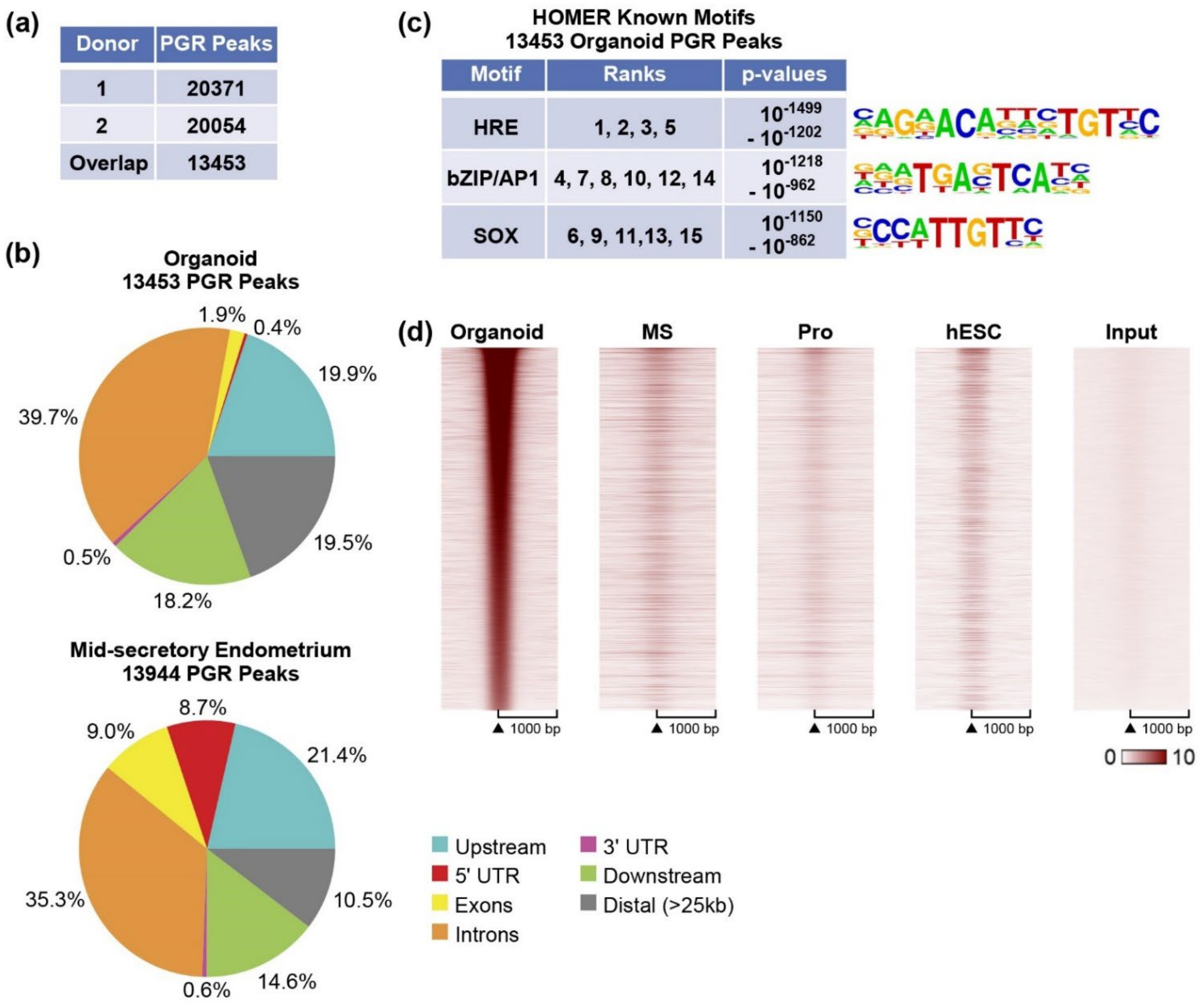
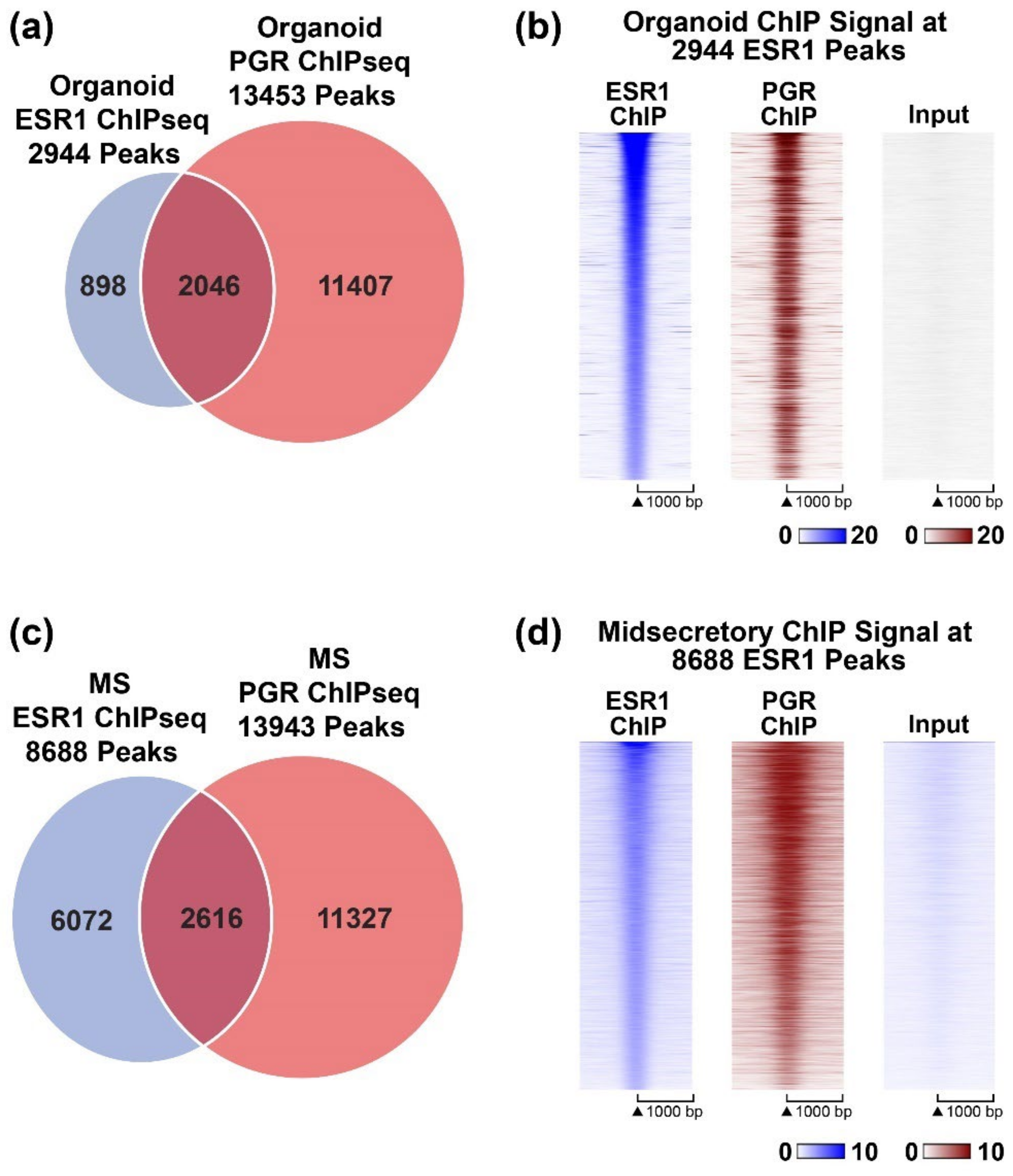
3.2. Progesterone Receptor Cistrome of Organoids
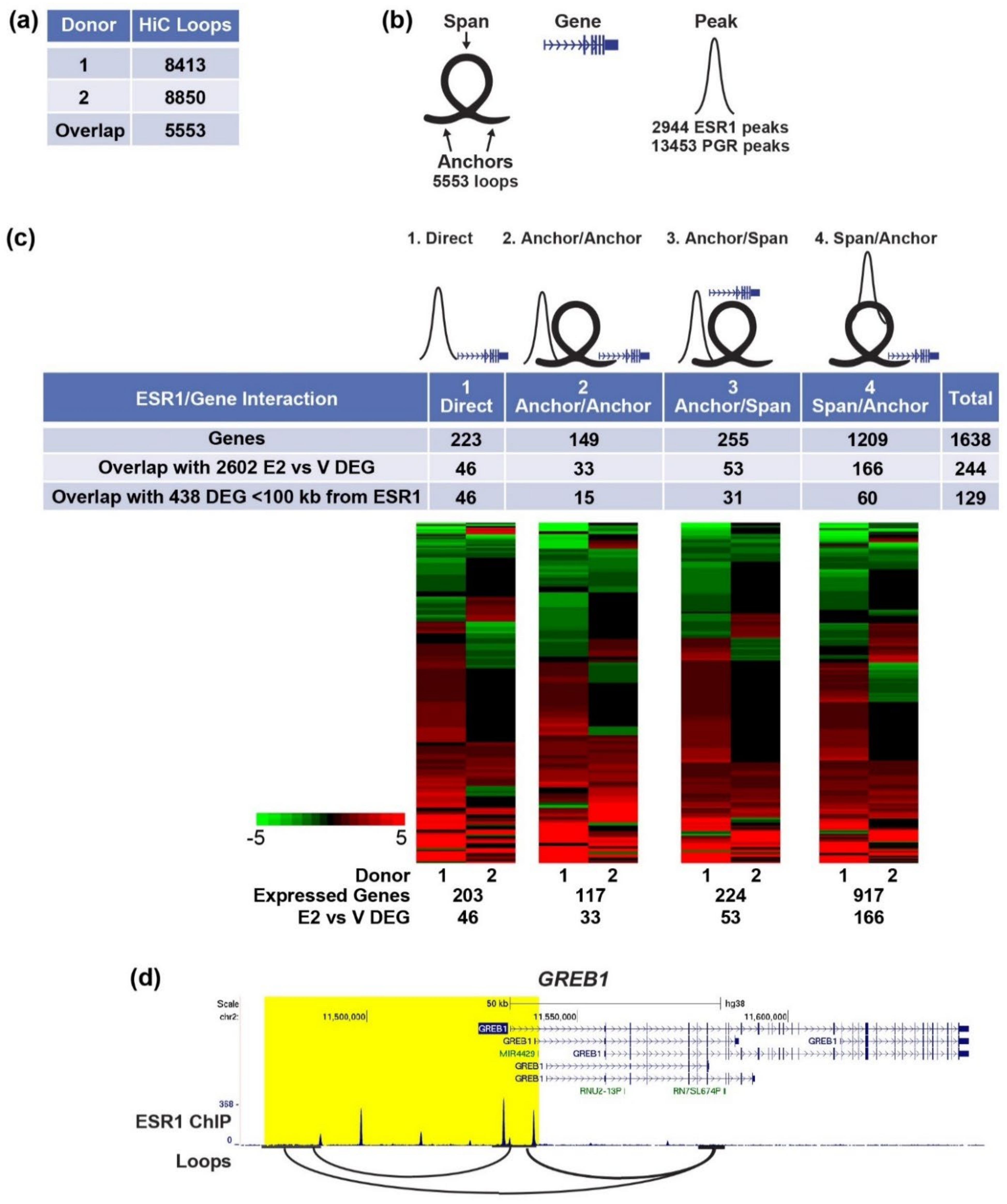
3.3. Chromatin Structure of Epithelial Cells
3.3.1. Enhancer-Gene Interactions in Organoids
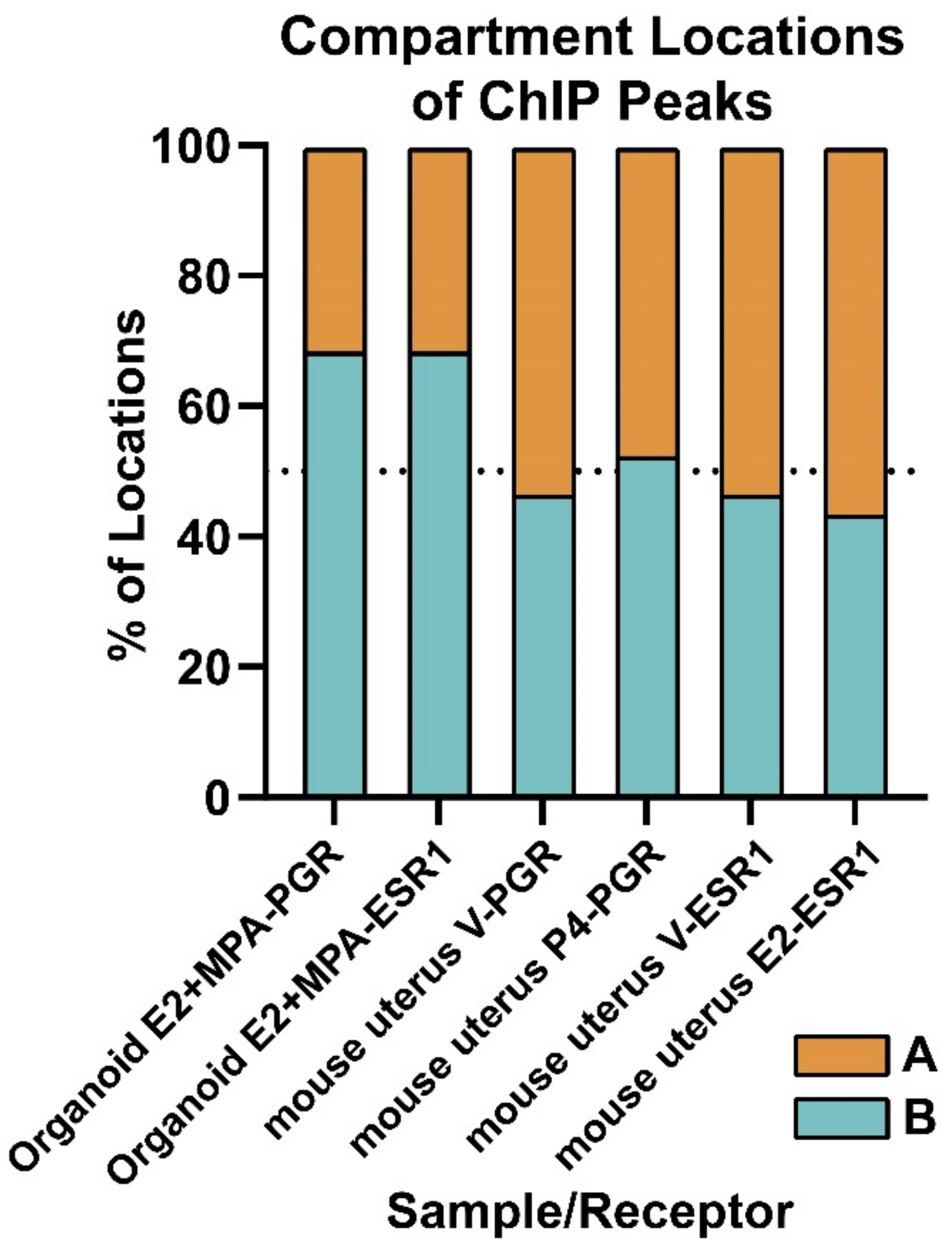
3.3.2. Compartment Localization of PGR and ESR1 Peaks
3.4. Chromatin Landscape of IHH and PGR Likely Mediates Their Hormone Regulation
4. Discussion
4.1. Progesterone Attenuates Estrogen Gene Response of Endometrial Epithelial Organoids
4.2. Localization of PGR and ESR1 in the B (Inactive) Compartment of Organoid Chromatin
4.3. Chromatin Structure Reveals Enhancer-Gene Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawkins, S.M.; Matzuk, M.M. The menstrual cycle: Basic biology. Ann. N. Y. Acad. Sci. 2008, 1135, 10–18. [Google Scholar] [CrossRef]
- Mazur, E.C.; Large, M.J.; DeMayo, F.J. Chapter 24—Human Oviduct and Endometrium: Changes over the Menstrual Cycle. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1077–1097. [Google Scholar] [CrossRef]
- Zeleznik, A.J.; Plant, T.M. Chapter 28—Control of the Menstrual Cycle. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1307–1361. [Google Scholar] [CrossRef]
- Hobeika, E.; Armouti, M.; Kala, H.S.; Stocco, C. Ovarian Hormones; Academic Press Ltd.: San Diego, CA, USA; Elsevier Science Ltd.: London, UK, 2020; pp. 565–583. [Google Scholar] [CrossRef]
- Binder, A.K.; Winuthayanon, W.; Hewitt, S.C.; Couse, J.F.; Korach, K.S. Steroid Receptors in the Uterus and Ovary. In Knobil and Neill’s Physiology of Reproduction; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1099–1193. [Google Scholar] [CrossRef]
- DeMayo, F.J.; Lydon, J.P. 90 years of progesterone: New insights into progesterone receptor signaling in the endometrium required for embryo implantation. J. Mol. Endocrinol. 2020, 65, T1–T14. [Google Scholar] [CrossRef]
- Wu, S.P.; Li, R.; DeMayo, F.J. Progesterone Receptor Regulation of Uterine Adaptation for Pregnancy. Trends Endocrinol. Metab. TEM 2018, 29, 481–491. [Google Scholar] [CrossRef]
- Vasquez, Y.M.; Wang, X.; Wetendorf, M.; Franco, H.L.; Mo, Q.; Wang, T.; Lanz, R.B.; Young, S.L.; Lessey, B.A.; Spencer, T.E.; et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018, 14, e1007787. [Google Scholar] [CrossRef]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.A., Jr.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266–2278. [Google Scholar] [CrossRef] [Green Version]
- Franco, H.L.; Rubel, C.A.; Large, M.J.; Wetendorf, M.; Fernandez-Valdivia, R.; Jeong, J.W.; Spencer, T.E.; Behringer, R.R.; Lydon, J.P.; Demayo, F.J. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012, 26, 1218–1227. [Google Scholar] [CrossRef] [Green Version]
- Gebril, M.; Hirota, Y.; Aikawa, S.; Fukui, Y.; Kaku, T.; Matsuo, M.; Hirata, T.; Akaeda, S.; Hiraoka, T.; Shimizu-Hirota, R.; et al. Uterine Epithelial Progesterone Receptor Governs Uterine Receptivity Through Epithelial Cell Differentiation. Endocrinology 2020, 161, bqaa195. [Google Scholar] [CrossRef]
- Wetendorf, M.; Wu, S.P.; Wang, X.; Creighton, C.J.; Wang, T.; Lanz, R.B.; Blok, L.; Tsai, S.Y.; Tsai, M.J.; Lydon, J.P.; et al. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachment. Biol. Reprod. 2017, 96, 313–326. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Huang, Z.; Balaji, J.; Kim, T.H.; Wang, T.; Zhou, L.; Deleon, A.; Cook, M.E.; Marbrey, M.W.; et al. The role of epithelial progesterone receptor isoforms in embryo implantation. iScience 2021, 24, 103487. [Google Scholar] [CrossRef]
- Kim, J.J.; Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef]
- Beato, M.; Wright, R.H.G.; Le Dily, F. 90 years of progesterone molecular mechanisms of progesterone receptor action on the breast cancer genome. J. Mol. Endocrinol. 2020, 65, T65–T79. [Google Scholar] [CrossRef]
- Rubel, C.A.; Lanz, R.B.; Kommagani, R.; Franco, H.L.; Lydon, J.P.; DeMayo, F.J. Research resource: Genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol. Endocrinol. 2012, 26, 1428–1442. [Google Scholar] [CrossRef] [Green Version]
- Chi, R.P.A.; Wang, T.Y.; Adams, N.; Wu, S.P.; Young, S.L.; Spencer, T.E.; DeMayo, F. Human Endometrial Transcriptome and Progesterone Receptor Cistrome Reveal Important Pathways and Epithelial Regulators. J. Clin. Endocrinol. Metab. 2020, 105, 21. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wang, T.; Wu, S.P.; Jeong, J.W.; Kim, T.H.; Young, S.L.; Lessey, B.A.; Lanz, R.B.; Lydon, J.P.; et al. SOX17 regulates uterine epithelial-stromal cross-talk acting via a distal enhancer upstream of Ihh. Nat. Commun. 2018, 9, 4421. [Google Scholar] [CrossRef]
- Le Dily, F.; Beato, M. Signaling by Steroid Hormones in the 3D Nuclear Space. Int. J. Mol. Sci. 2018, 19, 306. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Grimm, S.A.; Wu, S.P.; DeMayo, F.J.; Korach, K.S. Estrogen receptor alpha (ERalpha)-binding super-enhancers drive key mediators that control uterine estrogen responses in mice. J. Biol. Chem. 2020, 295, 8387–8400. [Google Scholar] [CrossRef]
- La Greca, A.; Bellora, N.; Le Dily, F.; Jara, R.; Nacht, A.S.; Quilez Oliete, J.; Villanueva, J.L.; Vidal, E.; Merino, G.; Fresno, C.; et al. Chromatin topology defines estradiol-primed progesterone receptor and PAX2 binding in endometrial cancer cells. eLife 2022, 11, e66034. [Google Scholar] [CrossRef]
- Heremans, R.; Jan, Z.; Timmerman, D.; Vankelecom, H. Organoids of the Female Reproductive Tract: Innovative Tools to Study Desired to Unwelcome Processes. Front. Cell. Dev. Biol. 2021, 9, 661472. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Tryfonos, M.; Lucas, E.S. Organoids to model the endometrium: Implantation and beyond. Reprod. Fertil. 2021, 2, R85–R101. [Google Scholar] [CrossRef]
- Alzamil, L.; Nikolakopoulou, K.; Turco, M.Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell. Death Differ. 2021, 28, 35–51. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Wu, S.-P.; Wang, T.; Ray, M.; Brolinson, M.; Young, S.L.; Spencer, T.E.; DeCherney, A.; DeMayo, F.J. The estrogen receptor α cistrome in human endometrium and epithelial organoids. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Deroo, B.J.; Hansen, K.; Collins, J.; Grissom, S.; Afshari, C.A.; Korach, K.S. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol. Endocrinol. 2003, 17, 2070–2083. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.C.; Lierz, S.L.; Garcia, M.; Hamilton, K.J.; Gruzdev, A.; Grimm, S.A.; Lydon, J.P.; Demayo, F.J.; Korach, K.S. A distal super enhancer mediates estrogen-dependent mouse uterine-specific gene transcription of Igf1 (insulin-like growth factor 1). J. Biol. Chem. 2019, 294, 9746–9759. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Wingett, S.; Ewels, P.; Furlan-Magaril, M.; Nagano, T.; Schoenfelder, S.; Fraser, P.; Andrews, S. HiCUP: Pipeline for mapping and processing Hi-C data. F1000 Res. 2015, 4, 1310. [Google Scholar] [CrossRef] [Green Version]
- Durand, N.C.; Shamim, M.S.; Machol, I.; Rao, S.S.; Huntley, M.H.; Lander, E.S.; Aiden, E.L. Juicer Provides a One-Click System for Analyzing Loop-Resolution Hi-C Experiments. Cell. Syst. 2016, 3, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010, 38, 576–589. [Google Scholar] [CrossRef] [Green Version]
- Lerdrup, M.; Johansen, J.V.; Agrawal-Singh, S.; Hansen, K. An interactive environment for agile analysis and visualization of ChIP-sequencing data. Nat. Struct. Mol. Biol. 2016, 23, 349–357. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Loganantharaj, R.; Schroeder, B.; Fargo, D.; Li, L. PAVIS: A tool for Peak Annotation and Visualization. Bioinformatics 2013, 29, 3097–3099. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, M.; Huber, W.; Pages, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef]
- Mazur, E.C.; Vasquez, Y.M.; Li, X.; Kommagani, R.; Jiang, L.; Chen, R.; Lanz, R.B.; Kovanci, E.; Gibbons, W.E.; DeMayo, F.J. Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology 2015, 156, 2239–2253. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Li, L.; Grimm, S.A.; Chen, Y.; Liu, L.; Li, Y.; Bushel, P.R.; Fargo, D.; Korach, K.S. Research resource: Whole-genome estrogen receptor alpha binding in mouse uterine tissue revealed by ChIP-seq. Mol. Endocrinol. 2012, 26, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Fukui, Y.; Hirota, Y.; Matsuo, M.; Gebril, M.; Akaeda, S.; Hiraoka, T.; Osuga, Y. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod. Med. Biol. 2019, 18, 234–240. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Handfield, L.F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef]
- Haider, S.; Gamperl, M.; Burkard, T.R.; Kunihs, V.; Kaindl, U.; Junttila, S.; Fiala, C.; Schmidt, K.; Mendjan, S.; Knofler, M.; et al. Estrogen Signaling Drives Ciliogenesis in Human Endometrial Organoids. Endocrinology 2019, 160, 2282–2297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewitt, S.C.; Wu, S.-p.; Wang, T.; Young, S.L.; Spencer, T.E.; DeMayo, F.J. Progesterone Signaling in Endometrial Epithelial Organoids. Cells 2022, 11, 1760. https://doi.org/10.3390/cells11111760
Hewitt SC, Wu S-p, Wang T, Young SL, Spencer TE, DeMayo FJ. Progesterone Signaling in Endometrial Epithelial Organoids. Cells. 2022; 11(11):1760. https://doi.org/10.3390/cells11111760
Chicago/Turabian StyleHewitt, Sylvia C., San-pin Wu, Tianyuan Wang, Steven L. Young, Thomas E. Spencer, and Francesco J. DeMayo. 2022. "Progesterone Signaling in Endometrial Epithelial Organoids" Cells 11, no. 11: 1760. https://doi.org/10.3390/cells11111760
APA StyleHewitt, S. C., Wu, S.-p., Wang, T., Young, S. L., Spencer, T. E., & DeMayo, F. J. (2022). Progesterone Signaling in Endometrial Epithelial Organoids. Cells, 11(11), 1760. https://doi.org/10.3390/cells11111760





