Enthesis Healing Is Dependent on Scaffold Interphase Morphology—Results from a Rodent Patellar Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biphasic Silk Fibroin Scaffolds
2.2. Rat Patellar Defect Model
2.3. Mechanical Testing
2.4. Micro-Computed Tomography (μ-CT) Analysis
2.5. Histology
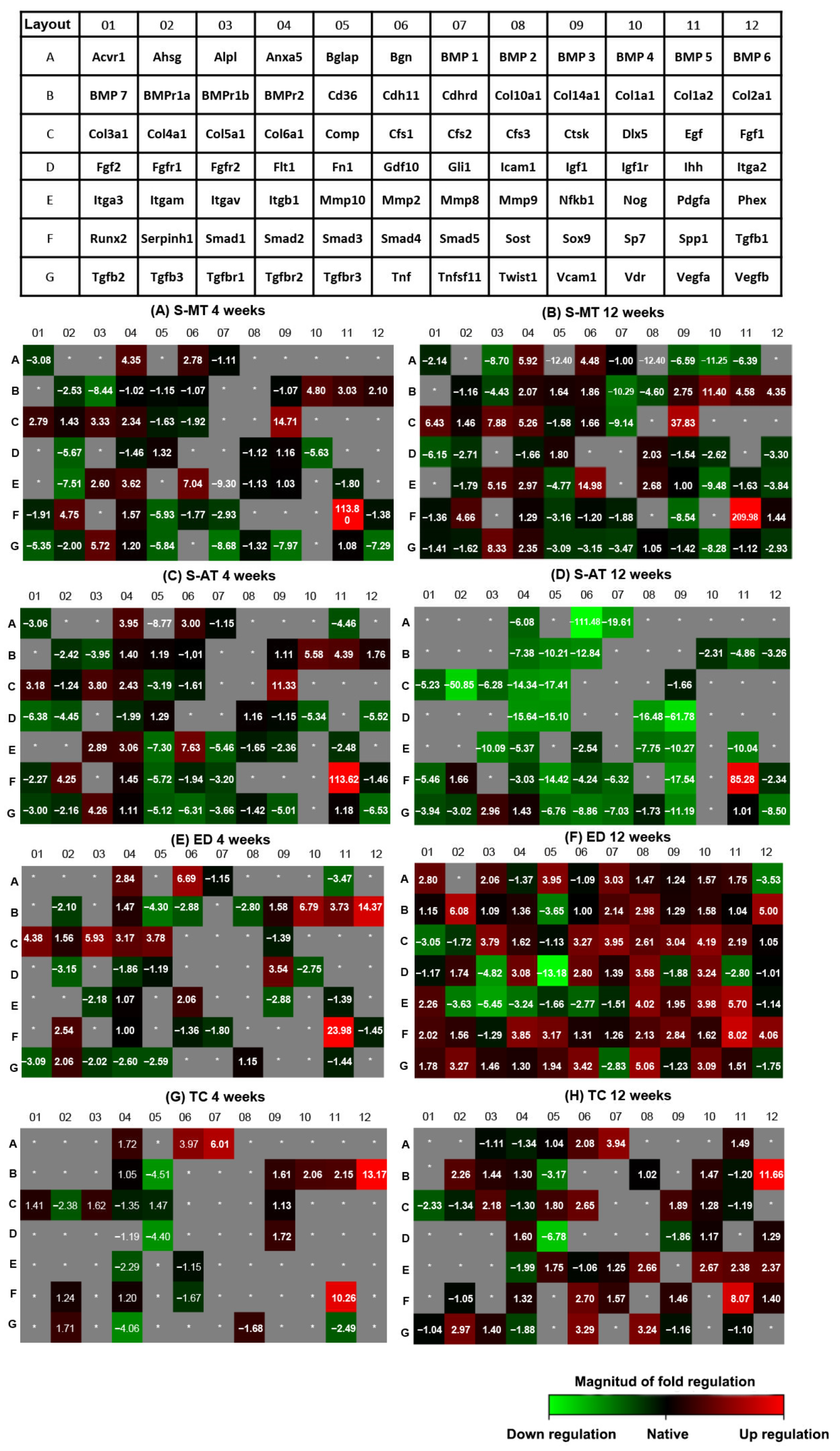
2.6. Gene Expression; qPCR Array
2.7. Statistical Analysis
3. Results
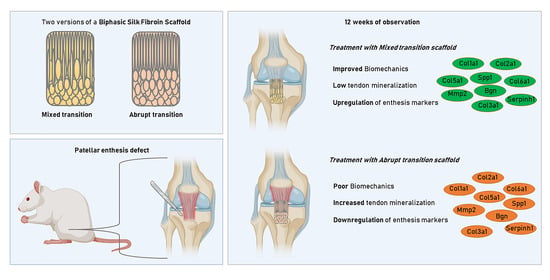
3.1. Rat Patellar Injury and Scaffold Treatment
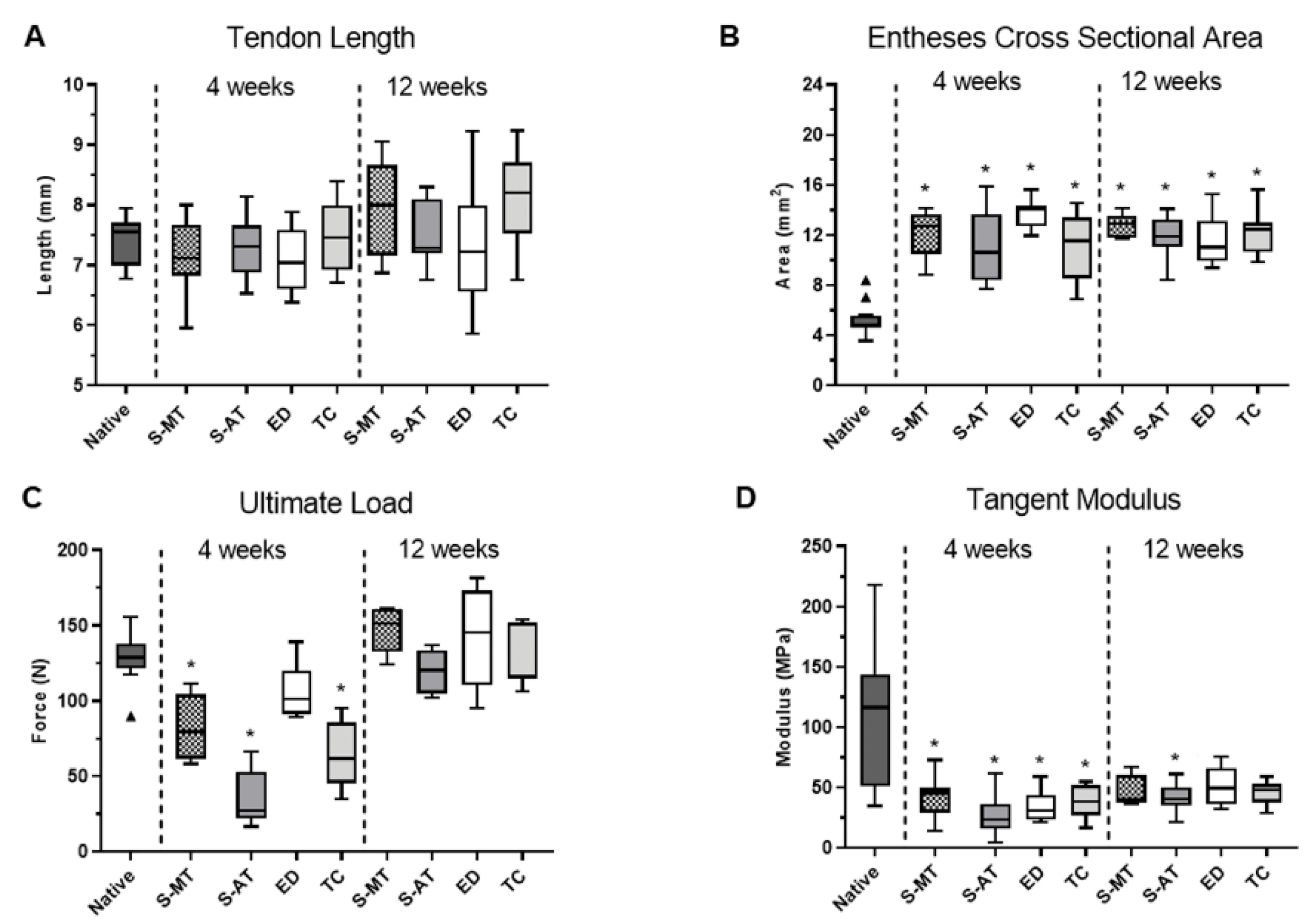
3.2. Mechanical Testing
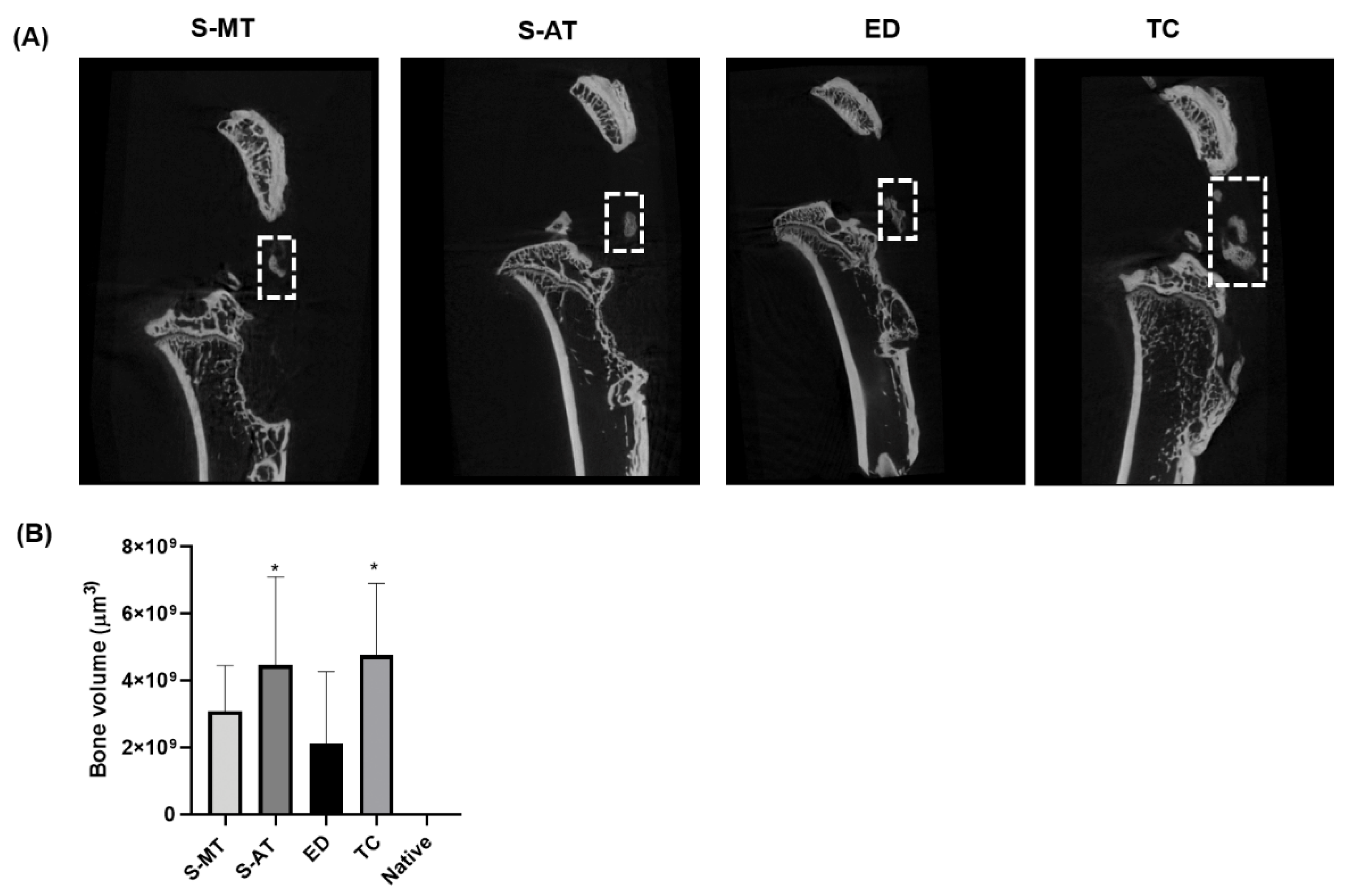
3.3. Micro-Computed Tomography (μ-CT) Analysis
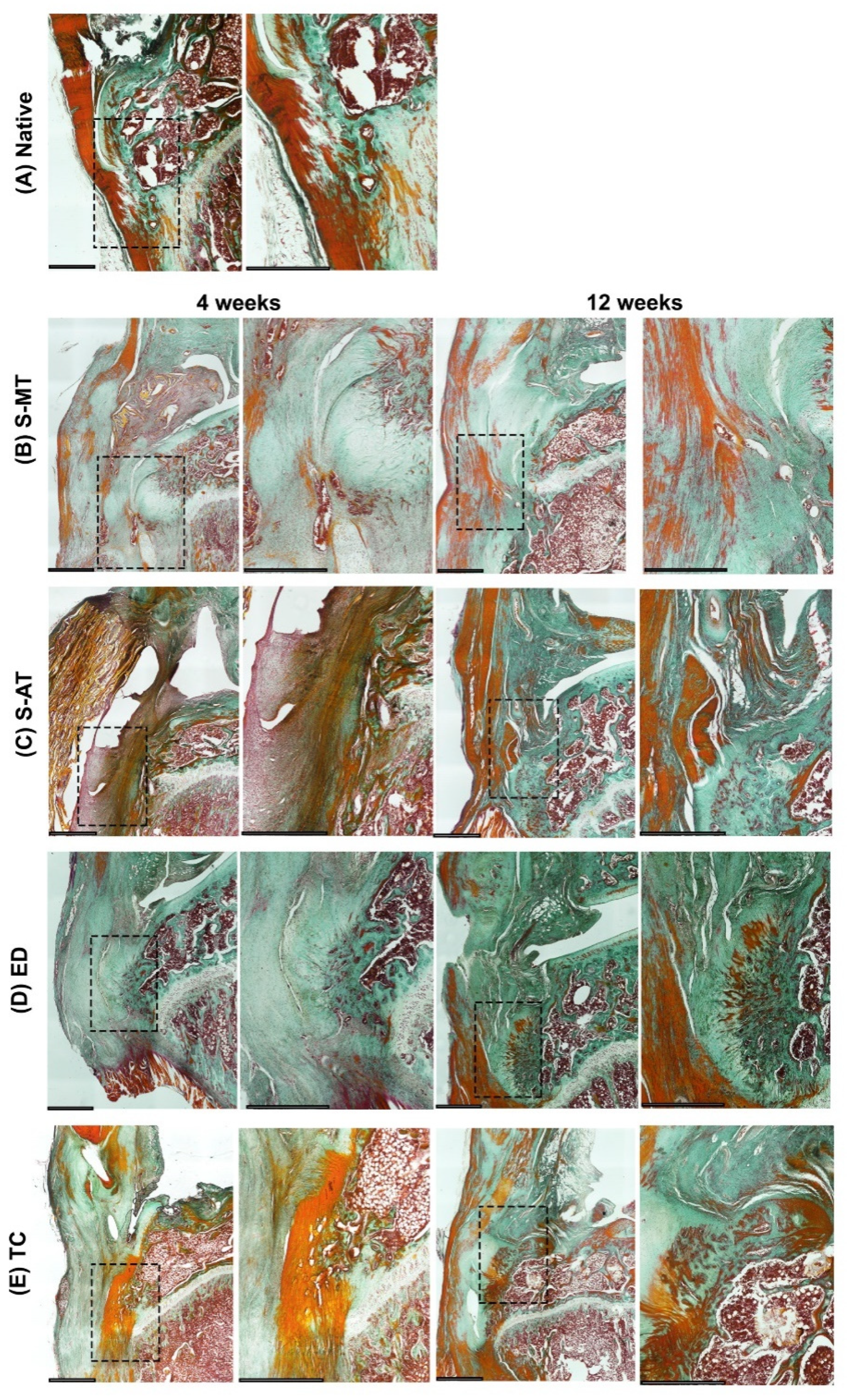
3.4. Histology
3.5. Gene Expression; qPCR Array
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 4 Weeks | 12 Weeks | ||||||
| S-MT Scaffold-Treated Group | S-AT Scaffold-Treated Group | S-MT Scaffold-Treated Group | S-AT Scaffold-Treated Group | ||||
| Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation |
| Ssp1 | 113.8 | Spp1 | 113.6 | Ssp1 | 209.98 | Spp1 | 85.28 |
| Ctsk | 14.71 | Ctsk | 11.3 | Ctsk | 37.83 | Tgfbr1 | 2.96 |
| Mmp2 | 7.04 | Mmp2 | 7.63 | Mmp2 | 14.98 | Serpinh1 | 1.66 |
| Col1a1 | 4.8 | Col1a1 | 5.58 | Col1a1 | 11.4 | ||
| Serpinh1 | 4.75 | Bgn | 4.48 | Col5a1 | 7.88 | ||
| Anxa 5 | 4.35 | Col1a2 | 4.39 | Col3a1 | 6.43 | ||
| Col5a1 | 3.33 | Anxa 5 | 3.95 | Col6a1 | 5.26 | ||
| Col1a2 | 3.03 | Col5a1 | 3.8 | Col1a2 | 4.58 | ||
| Col3a1 | 2.79 | Col3a1 | 3.18 | Bgn | 4.48 | ||
| Bgn | 2.78 | Smad1 | 2.89 | Col2a1 | 4.35 | ||
| 4 Weeks | 12 Weeks | ||||||
| ED Control Group | TC Control Group | ED Control Group | TC Control Group | ||||
| Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation |
| Ssp1 | 23.98 | Col2 | 13.17 | Ssp1 | 8.02 | Col2a1 | 11.66 |
| Col2a1 | 14.37 | Spp1 | 10.26 | Col2a1 | 6.08 | Spp1 | 8.07 |
| Col1a1 | 6.79 | Bmp1 | 6.01 | Pdgfa | 5.7 | Bmp1 | 3.94 |
| Bgn | 6.69 | Bgn | 3.97 | Twist1 | 5.06 | Tnf | 3.29 |
| Col5a1 | 5.93 | Col1a2 | 2.15 | Col2a1 | 5.00 | Twist1 | 3.24 |
| Col3a1 | 4.38 | Col1a1 | 2.06 | Dlx5 | 4.19 | Tgfb3 | 2.97 |
| Comp | 3.78 | Anax5 | 1.72 | Tgfb1 | 4.06 | Smad4 | 2.7 |
| Col1a2 | 3.73 | Tgfb2 | 1.71 | Nog | 3.98 | Nog | 2.67 |
| Igf1 | 3.54 | Col5a1 | 1.62 | Bglap | 3.95 | Mmp9 | 2.66 |
| Col6a1 | 3.17 | Ctsk | 1.61 | Cfs2 | 3.95 | Cfs1 | 2.65 |
| 4 Weeks | 12 Weeks | ||||||
| S-MT Scaffold Treated Group | S-AT Scaffold Treated Group | S-MT Scaffold Treated Group | S-AT Scaffold Treated Group | ||||
| Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation |
| Tnfsf11 | −8.68 | Mmp10 | −7.30 | Bmp4 | −11.25 | Bgn | −111.4 |
| Bmpr1 | −8.44 | Vgefb | −6.53 | Cdhrd | −10.29 | Col4a1 | −50.85 |
| Vcam1 | −7.97 | Fgf2 | −6.38 | Nog | −9.48 | Igf1 | −61.78 |
| Itgam | −7.51 | Tnf | −6.31 | Cfs2 | −9.14 | Bmp1 | −19.61 |
| Vegfb | −7.29 | Smad4 | −5.72 | Alpl | −8.70 | Sox9 | −17.54 |
| Smad3 | −5.93 | Itga2 | −5.52 | Vdr | −8.28 | Comp | −17.41 |
| Tgfbr3 | −5.84 | Mmp8 | −5.46 | Bmp3 | −6.59 | Icam1 | −16.48 |
| Fgfr1 | −5.67 | Igf1r | −5.34 | Bmp5 | −6.39 | Flt1 | −15.64 |
| Igf1r | −5.63 | Tgfbr3 | −5.12 | Fgf2 | −6.15 | Smad3 | −14.42 |
| Tgfb2 | −5.35 | Vcam1 | −5.01 | Col10a1 | −4.60 | Col6a1 | −14.34 |
| 4 Weeks | 12 Weeks | ||||||
| ED Control Group | TC Control Group | ED Control Group | TC Control Group | ||||
| Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation | Gene Symbol | Fold Regulation |
| Cd36 | −4.30 | Cd36 | −4.51 | Fn1 | −13.18 | Fn1 | −6.78 |
| Bmp5 | −3.47 | Fn1 | −4.40 | Itgav | −5.45 | Cd36 | −3.17 |
| Fgfr1 | −3.15 | Tgfbr2 | −4.06 | Fgfr2 | −4.82 | Col3a1 | −2.33 |
| Tgfb2 | −3.09 | Vegfa | −2.49 | Cd36 | −3.65 | Itgb1 | −1.99 |
| Nfkb1 | −2.88 | Col4a1 | −2.38 | Itgam | −3.63 | Tgbr2 | −1.88 |
| Cdh11 | −2.88 | Itgb1 | −2.29 | Bmp6 | −3.53 | Igf1 | −1.86 |
| Col10a1 | −2.80 | Twist1 | −1.68 | Itgb1 | −3.24 | Col4a1 | −1.34 |
| Igf1r | −2.75 | Smad4 | −1.67 | Col3a1 | −3.05 | Anxa5 | −1.34 |
| Tgfbr2 | −2.60 | Col6a1 | −1.35 | Tnfsf11 | −2.83 | Vcam1 | −1.16 |
| Tgfbr3 | −2.59 | Mmp2 | −1.15 | Ihh | −2.80 | Vegfa | −1.10 |
References
- Rossetti, L.; Kuntz, L.A.; Kunold, E.; Schock, J.; Muller, K.W.; Grabmayr, H.; Stolberg-Stolberg, J.; Pfeiffer, F.; Sieber, S.A.; Burgkart, R.; et al. The microstructure and micromechanics of the tendon-bone insertion. Nat. Mater. 2017, 16, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Font Tellado, S.; Balmayor, E.R.; Van Griensven, M. Strategies to engineer tendon/ligament-to-bone interface: Biomaterials, cells and growth factors. Adv. Drug Deliv. Rev. 2015, 94, 126–1400. [Google Scholar] [CrossRef] [PubMed]
- Derwin, K.A.; Galatz, L.M.; Ratcliffe, A.; Thomopoulos, S. Enthesis Repair: Challenges and Opportunities for Effective Tendon-to-Bone Healing. J. Bone Jt. Surg. Am. Vol. 2018, 100, e109. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Thomopoulos, S. Functional Attachment of Soft Tissues to Bone: Development, Healing, and Tissue Engineering. Annu. Rev. Biomed. Eng. 2013, 15, 201–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawata, K.; Minamizaki, T.F.; Yamashita, Y.; Yamashita, Y.F.; Teshima, R.; Teshima, R. Development of the attachment zones in the rat anterior cruciate ligament: Changes in the distributions of proliferating cells and fibrillar collagens during postnatal growth. J. Orthop. Res. 2002, 20, 1339–1344. [Google Scholar] [CrossRef]
- Bunker, D.L.; Ilie, V.; Ilie, V.; Nicklin, S. Tendon to bone healing and its implications for surgery. Muscles Ligaments Tendons J. 2014, 4, 343–350. [Google Scholar] [CrossRef]
- Klinger, H.M.; Buchhorn Gh Fau Heidrich, G.; Heidrich GFau Kahl, E.; Kahl EFau Baums, M.H.; Baums, M.H. Biomechanical evaluation of rotator cuff repairs in a sheep model: Suture anchors using arthroscopic Mason-Allen stitches compared with transosseous sutures using traditional modified Mason-Allen stitches. Clin. Biomech. 2008, 23, 291–298. [Google Scholar] [CrossRef]
- Moffat, K.L.; Wang, I.N.E.; Rodeo, S.A.; Lu, H.H. Orthopedic Interface Tissue Engineering for the Biological Fixation of Soft Tissue Grafts. Clin. Sports Med. 2009, 28, 157–176. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, M.; Lustig, S.; Huten, D. Extensor tendon ruptures after total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2016, 102, S21–S31. [Google Scholar] [CrossRef]
- O’Brien, E.J.; Frank, C.B.; Shrive, N.G.; Shrive, N.F.; Hallgrímsson, B.; Hart, D.A. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int. J. Exp. Pathol. 2012, 93, 319–331. [Google Scholar] [CrossRef]
- Zhang, K.; Asai, S.; Hast, M.W.; Liu, M.; Usami, Y.; Iwamoto, M.; Soslowsky, L.J.; Enomoto-Iwamoto, M. Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model. Matrix. Biol. 2016, 52–54, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Kumai, T.; Higashiyama, I.; Shinohara, Y.; Matsuda, T.; Takakura, Y. Repair process after fibrocartilaginous enthesis drilling: Histological study in a rabbit model. J. Orthop. Sci. 2009, 14, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Heisterbach, P.; Jaquiéry, C.; Dürselen, L.; Todorov, A.; Martin, I.; Evans, C.; Müller, S. Improved tendon healing using bFGF, BMP-12 and TGFβ1 in a rat model. Eur. Cells Mater. 2018, 35, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Ateschrang, A.; Gratzer CFau Weise, K.; Weise, K. Incidence and effect of calcifications after open-augmented Achilles tendon repair. Arch. Orthop. Trauma Surg. 2008, 128, 1087–1092. [Google Scholar] [CrossRef]
- Järvelä, T.; Paakkala TFau Kannus, P.; Kannus PFau Toivanen, J.; Toivanen JFau Järvinen, M.; Järvinen, M. Ultrasonographic and power Doppler evaluation of the patellar tendon ten years after harvesting its central third for reconstruction of the anterior cruciate ligament: Comparison of patients without or with anterior knee pain. Am. J. Sports Med. 2004, 32, 39–46. [Google Scholar] [CrossRef]
- Su, W.; Wang, Z.; Jiang, J.; Liu, X.; Zhao, J.; Zhang, Z. Promoting tendon to bone integration using graphene oxide-doped electrospun poly(lactic-co-glycolic acid) nanofibrous membrane. Int. J. Nanomed. 2019, 14, 1835–1847. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Caldwell, J.M.; Doty, S.B.; Levine, W.N.; Rodeo, S.; Soslowsky, L.J.; Thomopoulos, S.; Lu, H.H. Integrating soft and hard tissues via interface tissue engineering. J. Orthop. Res. 2018, 36, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Teh, T.K.; Toh, S.L.; Goh, J.C.H.; Goh, J.C. Aligned hybrid silk scaffold for enhanced differentiation of mesenchymal stem cells into ligament fibroblasts. Tissue Eng. Part C Methods 2011, 17, 687–703. [Google Scholar] [CrossRef]
- Spalazzi, J.P.; Dagher, E.; Doty, S.B.; Guo, X.E.; Rodeo, S.A.; Lu, H.H. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J. Biomed. Mater. Res. A 2008, 86, 1–12. [Google Scholar] [CrossRef]
- Spalazzi, J.P.; Vyner, M.C.; Jacobs, M.T.; Jacobs Mt Moffat, K.L.; Moffat Kl Lu, H.H.; Lu, H.H. Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage markers. Clin. Orthop. Relat. Res. 2008, 466, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Jeon, O.; Song, S.J.; Kang, S.W.; Putnam, A.J.; Kim, B.S. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials 2007, 28, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Siew, R.; Herring, S.W.; Lin, A.S.; Guldberg, R.; Stayton, P.S. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials 2010, 31, 6772–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Font Tellado, S.; Bonani, W.; Balmayor, E.R.; Foehr, P.; Motta, A.; Migliaresi, C.; van Griensven, M. Fabrication and Characterization of Biphasic Silk Fibroin Scaffolds for Tendon/Ligament-to-Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 859–872. [Google Scholar] [CrossRef]
- Spalazzi, J.P.; Doty, S.B.; Moffat, K.L.; Moffat, K.L.; Levine, W.N.; Lu, H.H. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006, 12, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Harley, B.A. Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Adv. Healthc. Mater. 2014, 3, 1086–1096. [Google Scholar] [CrossRef] [Green Version]
- Qu, D.; Mosher, C.Z.; Boushell, M.K.; Lu, H.H. Engineering complex orthopaedic tissues via strategic biomimicry. Ann. Biomed. Eng. 2015, 43, 697–717. [Google Scholar] [CrossRef] [Green Version]
- Criscenti, G.; Longoni, A.; Di Luca, A.; De Maria, C.; van Blitterswijk, C.A.; Vozzi, G.; Moroni, L. Triphasic scaffolds for the regeneration of the bone–ligament interface. Biofabrication 2016, 8, 015009. [Google Scholar] [CrossRef]
- Li, T.; Tian, L.; Liao, S.; Ding, X.; Irvine, S.A.; Ramakrishna, S. Fabrication, mechanical property and in vitro evaluation of poly (L-lactic acid-co-ε-caprolactone) core-shell nanofiber scaffold for tissue engineering. J. Mech. Behav. Biomed. Mater. 2019, 98, 48–57. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, S.; Zhao, D.; Li, Y.; Cheong, S.S.; Han, D.; Li, Q. Three-dimensional printed multiphasic scaffolds with stratified cell-laden gelatin methacrylate hydrogels for biomimetic tendon-to-bone interface engineering. J. Orthop. Transl. 2020, 23, 89–100. [Google Scholar] [CrossRef]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef] [Green Version]
- Chainani, A.; Hippensteel, K.J.; Kishan, A.; Garrigues, N.W.; Ruch, D.S.; Guilak, F.; Little, D. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. Part A 2013, 19, 2594–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotticchia, A.; Musson, D.; Lenardi, C.; Demirci, E.; Liu, Y. Anisotropic cytocompatible electrospun scaffold for tendon tissue engineering elicits limited inflammatory response in vitro. J. Biomater. Appl. 2018, 33, 127–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerurkar, N.L.; Sen, S.; Baker, B.M.; Elliott, D.M.; Mauck, R.L. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomater. 2011, 7, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipner, J.; Shen, H.; Cavinatto, L.; Liu, W.; Havlioglu, N.; Xia, Y.; Galatz, L.M.; Thomopoulos, S. In Vivo Evaluation of Adipose-Derived Stromal Cells Delivered with a Nanofiber Scaffold for Tendon-to-Bone Repair. Tissue Eng. Part A 2015, 21, 2766–2774. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, E.; Zeugolis, D. Design of Multilayer Collagen-Based Scaffolds for the Tendon to Bone Integration. Orthop. Proc. 2020, 102-B, 34. [Google Scholar]
- Font Tellado, S.; Chiera, S.; Bonani, W.; Poh, P.S.P.; Migliaresi, C.; Motta, A.; Balmayor, E.R.; van Griensven, M. Heparin functionalization increases retention of TGF-β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018, 72, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, E.; Korntner, S.; Zeugolis, D.I. Multilayer Collagen-Based Scaffold as Delivery Vehicles of Bioactive Molecules for the Bone-to-Tendon Interface Regeneration. Orthop. Proc. 2018, 100-B, 97. [Google Scholar]
- Zheng, C.; Lu, H.; Tang, Y.; Wang, Z.; Ma, H.; Li, H.; Chen, H.; Chen, Y.; Chen, C. Autologous Freeze-Dried, Platelet-Rich Plasma Carrying Icariin Enhances Bone-Tendon Healing in a Rabbit Model. Am. J. Sports Med. 2019, 47, 1964–1974. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Saeb, M.R.; Zarrintaj, P.; Kundu, S.C.; Khademhosseini, A. Silk fibroin scaffolds for common cartilage injuries: Possibilities for future clinical applications. Eur. Polym. J. 2019, 115, 251–267. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk Fibroin in Tissue Engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Mozdzen, L.C.; Thorpe, S.D.; Screen, H.R.C.; Harley, B.A.C. The Effect of Gradations in Mineral Content, Matrix Alignment, and Applied Strain on Human Mesenchymal Stem Cell Morphology within Collagen Biomaterials. Adv. Healthc. Mater. 2016, 5, 1731–1739. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.B.; Park Sh Fau Gil, E.S.; Gil Es Fau Kaplan, D.L.; Kaplan, D.L. Multilayered silk scaffolds for meniscus tissue engineering. Biomaterials 2011, 32, 639–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quirk, N.P.; Lopez De Padilla, C.; De La Vega, R.E.; Coenen, M.J.; Tovar, A.; Evans, C.H.; Müller, S.A. Effects of freeze-thaw on the biomechanical and structural properties of the rat Achilles tendon. J. Biomech. 2018, 81, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wieloch, P.; Buchmann, G.; Roth, W.; Rickert, M. A cryo-jaw designed for in vitro tensile testing of the healing Achilles tendons in rats. J. Biomech. 2004, 37, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Yee Lui, P.P.; Wong, Y.M.; Rui, Y.F.; Lee, Y.W.; Chan, L.S.; Chan, K.M. Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy. J. Orthop. Res. 2011, 29, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, D.; Wang, H.; Tan, J. Heterotopic ossification of tendon and ligament. J. Cell. Mol. Med. 2020, 24, 5428–5437. [Google Scholar] [CrossRef] [Green Version]
- Holwein, C.; von Bibra, B.; Jungmann, P.M.; Karampinos, D.C.; Wörtler, K.; Scheibel, M.; Imhoff, A.B.; Buchmann, S. No healing improvement after rotator cuff reconstruction augmented with an autologous periosteal flap. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 3212–3221. [Google Scholar] [CrossRef]
- Sensini, A.; Massafra, G.; Gotti, C.; Zucchelli, A.; Cristofolini, L. Tissue Engineering for the Insertions of Tendons and Ligaments: An Overview of Electrospun Biomaterials and Structures. Front. Bioeng. Biotechnol. 2021, 9, 98. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Butler, D.L.; Dyment, N.A.; Cagle, P.J., Jr.; Proctor, C.S.; Ratcliffe, S.S.; Flatow, E.L. Scaffolds for tendon and ligament repair and regeneration. Ann. Biomed. Eng. 2015, 43, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Li, X.; Lipner, J.; Manning, C.N.; Schwartz, A.G.; Thomopoulos, S.; Xia, Y. Aligned-to-random nanofiber scaffolds for mimicking the structure of the tendon-to-bone insertion site. Nanoscale 2010, 2, 923–926. [Google Scholar] [CrossRef] [Green Version]
- Nowlin, J.; Bismi, M.A.; Delpech, B.; Dumas, P.; Zhou, Y.; Tan, G.Z. Engineering the hard-soft tissue interface with random-to-aligned nanofiber scaffolds. Nanobiomedicine 2018, 5, 1849543518803538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calejo, I.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. Enthesis Tissue Engineering: Biological Requirements Meet at the Interface. Tissue Eng. Part B Rev. 2019, 25, 330–356. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, J.; Durant, T.J.; Dwyer, C.R.; Russell, R.P.; Weinreb, J.H.; Alaee, F.; Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. The enthesis: A review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014, 4, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Peffers, M.J.; Thorpe, C.T.; Collins, J.A.; Eong, R.; Wei, T.K.J.; Screen, H.R.C.; Clegg, P.D. Proteomic Analysis Reveals Age-related Changes in Tendon Matrix Composition, with Age- and Injury-specific Matrix Fragmentation. J. Biol. Chem. 2014, 289, 25867–25878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Widmer, C.; Gebauer, J.M.; Brunstein, E.; Rosenbaum, S.; Zaucke, F.; Drögemüller, C.; Leeb, T.; Baumann, U. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. USA 2012, 109, 13243. [Google Scholar] [CrossRef] [Green Version]
- Oshiro, W.; Lou JFau Xing, X.; Xing XFau Tu, Y.; Tu YFau Manske, P.R.; Manske, P.R. Flexor tendon healing in the rat: A histologic and gene expression study. J. Hand Surg. Am. 2003, 28, 814–823. [Google Scholar] [CrossRef]
- Del Buono, A.; Oliva, F.; Osti, L.; Maffulli, N. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013, 3, 51–57. [Google Scholar] [CrossRef]
- Juneja, S.C.; Schwarz, E.M.; O’Keefe, R.J.; Awad, H.A. Cellular and Molecular Factors in Flexor Tendon Repair and Adhesions: A Histological and Gene Expression Analysis. Connect. Tissue Res. 2013, 54, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, A.D.; Pinnow, E.L.; Maeda, A.; Brown, A.C.; McCartney-Francis, N.; Kram, V.; Owens, R.T.; Robey, P.G.; Holmbeck, K.; de Castro, L.F.; et al. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix Biol. 2014, 35, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kram, V.; Shainer, R.; Jani, P.; Meester, J.A.N.; Loeys, B.; Young, M.F. Biglycan in the Skeleton. J. Histochem. Cytochem. 2020, 68, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chaudhuri, A.; Bera, A.K. Role of BMP1/Tolloid like Proteases in Bone Morphogenesis and Tissue Remodeling. In Proteases in Physiology and Pathology; Chakraborti, S., Dhalla, N., Eds.; Springer: Singapore, 2017; pp. 77–88. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peniche Silva, C.J.; Müller, S.A.; Quirk, N.; Poh, P.S.P.; Mayer, C.; Motta, A.; Migliaresi, C.; Coenen, M.J.; Evans, C.H.; Balmayor, E.R.; et al. Enthesis Healing Is Dependent on Scaffold Interphase Morphology—Results from a Rodent Patellar Model. Cells 2022, 11, 1752. https://doi.org/10.3390/cells11111752
Peniche Silva CJ, Müller SA, Quirk N, Poh PSP, Mayer C, Motta A, Migliaresi C, Coenen MJ, Evans CH, Balmayor ER, et al. Enthesis Healing Is Dependent on Scaffold Interphase Morphology—Results from a Rodent Patellar Model. Cells. 2022; 11(11):1752. https://doi.org/10.3390/cells11111752
Chicago/Turabian StylePeniche Silva, Carlos J., Sebastian A. Müller, Nicholas Quirk, Patrina S. P. Poh, Carla Mayer, Antonella Motta, Claudio Migliaresi, Michael J. Coenen, Christopher H. Evans, Elizabeth R. Balmayor, and et al. 2022. "Enthesis Healing Is Dependent on Scaffold Interphase Morphology—Results from a Rodent Patellar Model" Cells 11, no. 11: 1752. https://doi.org/10.3390/cells11111752
APA StylePeniche Silva, C. J., Müller, S. A., Quirk, N., Poh, P. S. P., Mayer, C., Motta, A., Migliaresi, C., Coenen, M. J., Evans, C. H., Balmayor, E. R., & van Griensven, M. (2022). Enthesis Healing Is Dependent on Scaffold Interphase Morphology—Results from a Rodent Patellar Model. Cells, 11(11), 1752. https://doi.org/10.3390/cells11111752