Divergence between Neuronal and Oligodendroglial Cell Fate, in Postnatal Brain Neural Stem Cells, Leads to Divergent Properties in Polymorphic In Vitro Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents, Substrates and Functional Antibodies for Cell Cultures
2.3. NSC Cultures
2.4. Coating of Coverslips
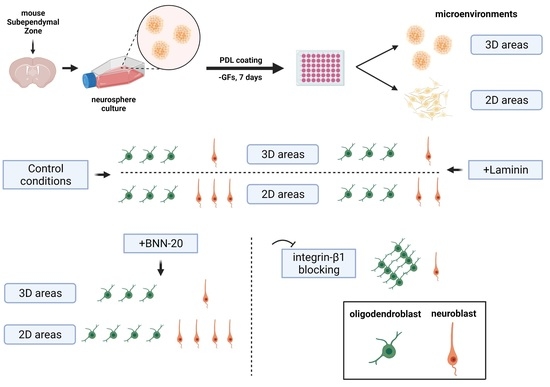
2.5. Polymorphic Neurosphere Assay
2.6. Laminin, Integrin-β1 Blocking and BNN-20 Experiments; Time-Lapse Imaging
2.7. Immunocytochemistry and Antibodies
2.8. Imaging, Cell counts and Statistical Analysis
3. Results
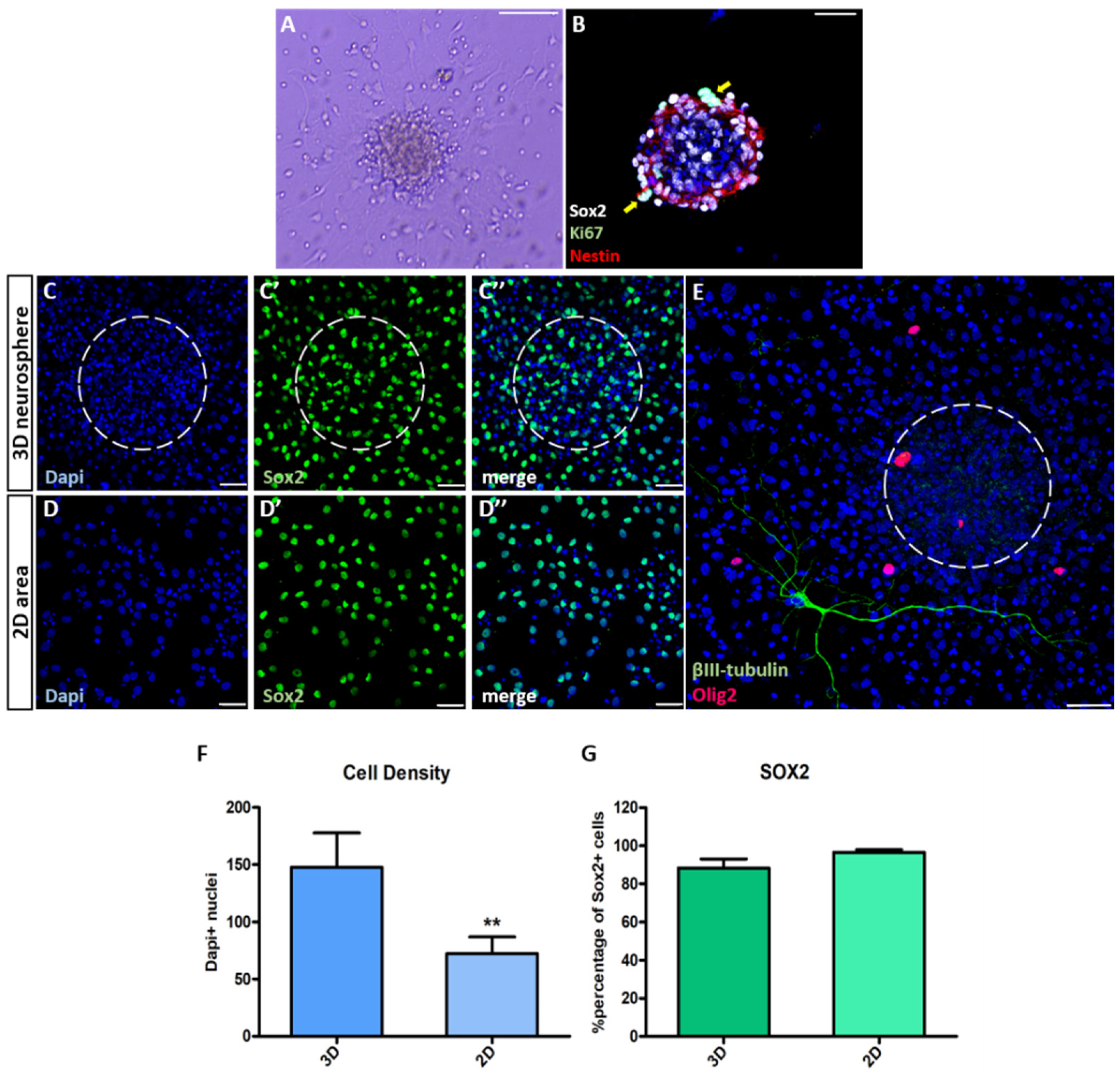
3.1. Characterization of NSC Polymorphic Assays
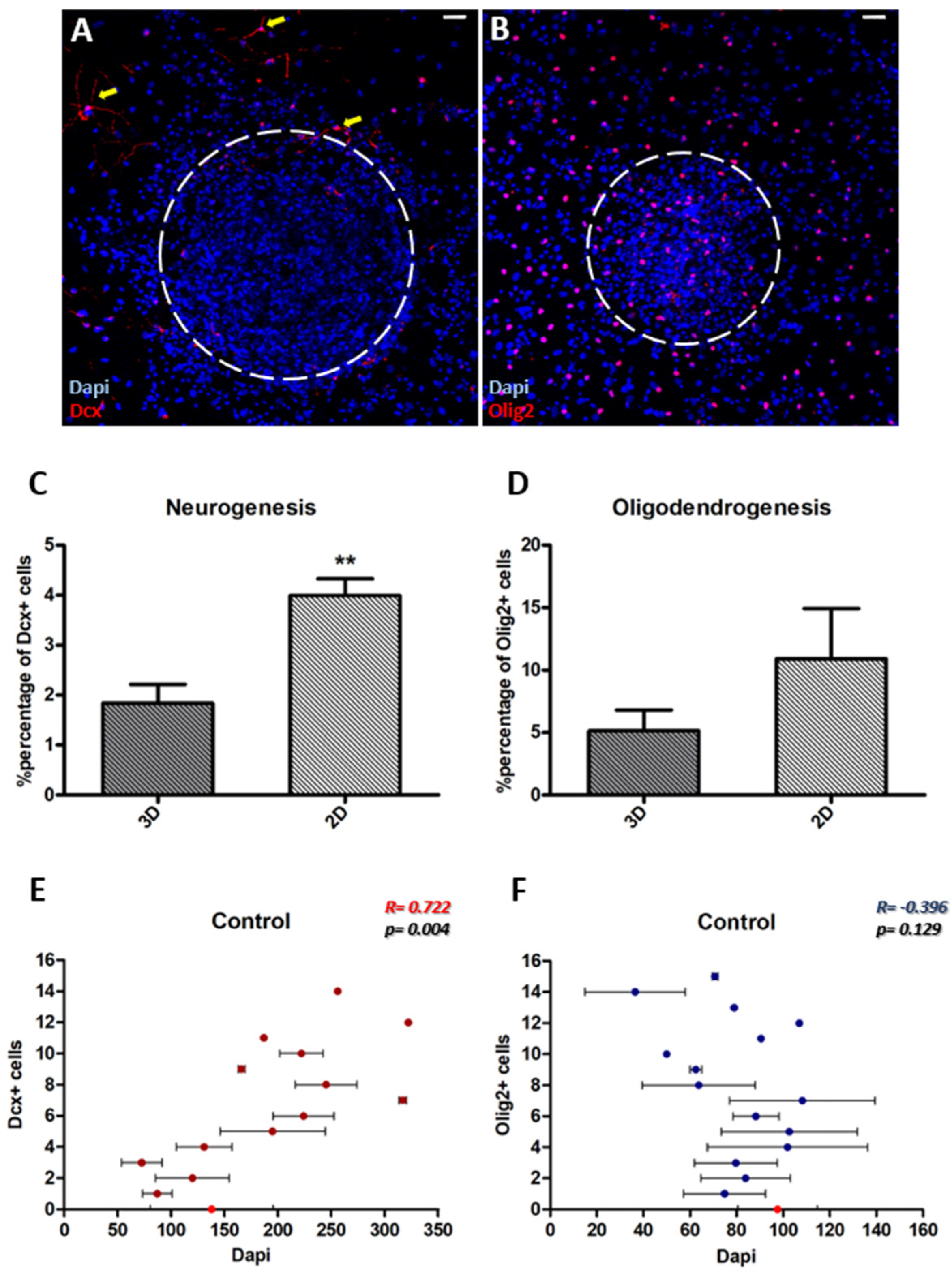
3.2. Neurogenesis Is More Dependent on the Cytoarchitecture of the Microenvironment Than Oligodendrogenesis
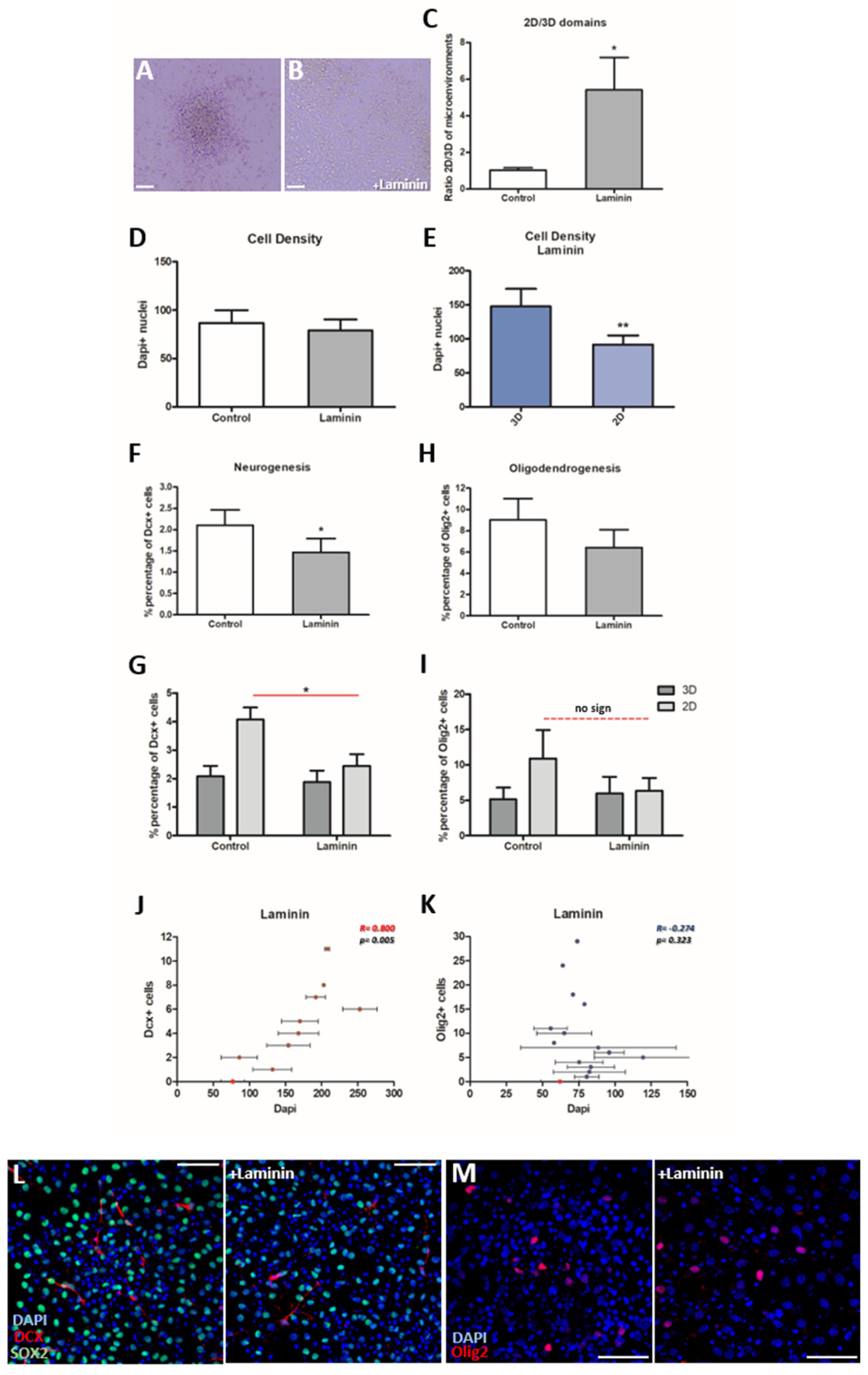
3.3. The addition of Laminin Affects the Cytoarchitecture of the Cultures
3.4. Neurogenesis Is Significantly Decreased in the Presence of Laminin, While Oligodendrogenesis Is Not Affected
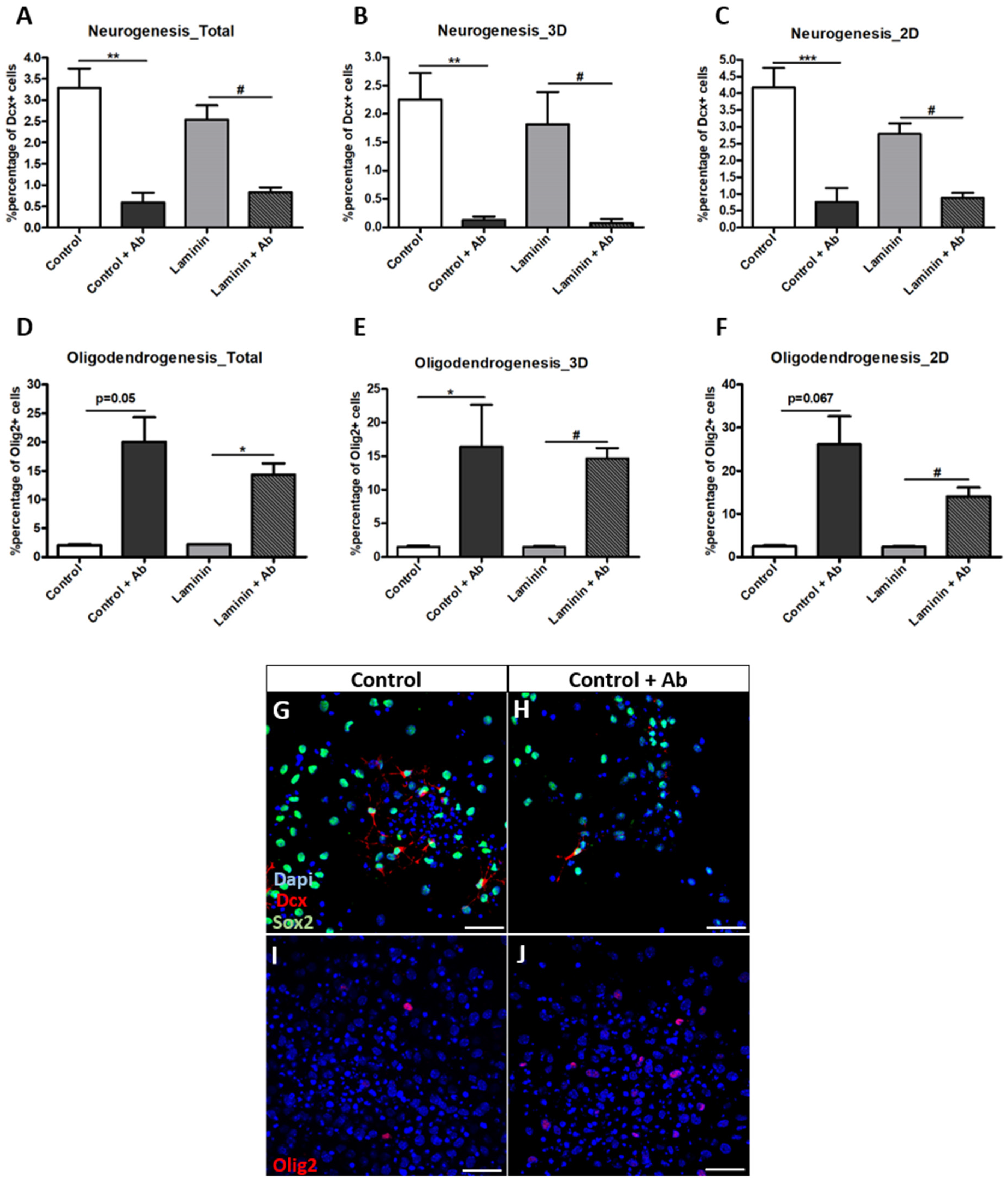
3.5. Neurogenesis and Oligodendrogenesis Are Differentially Affected by Blocking Integrin-Β1 Function
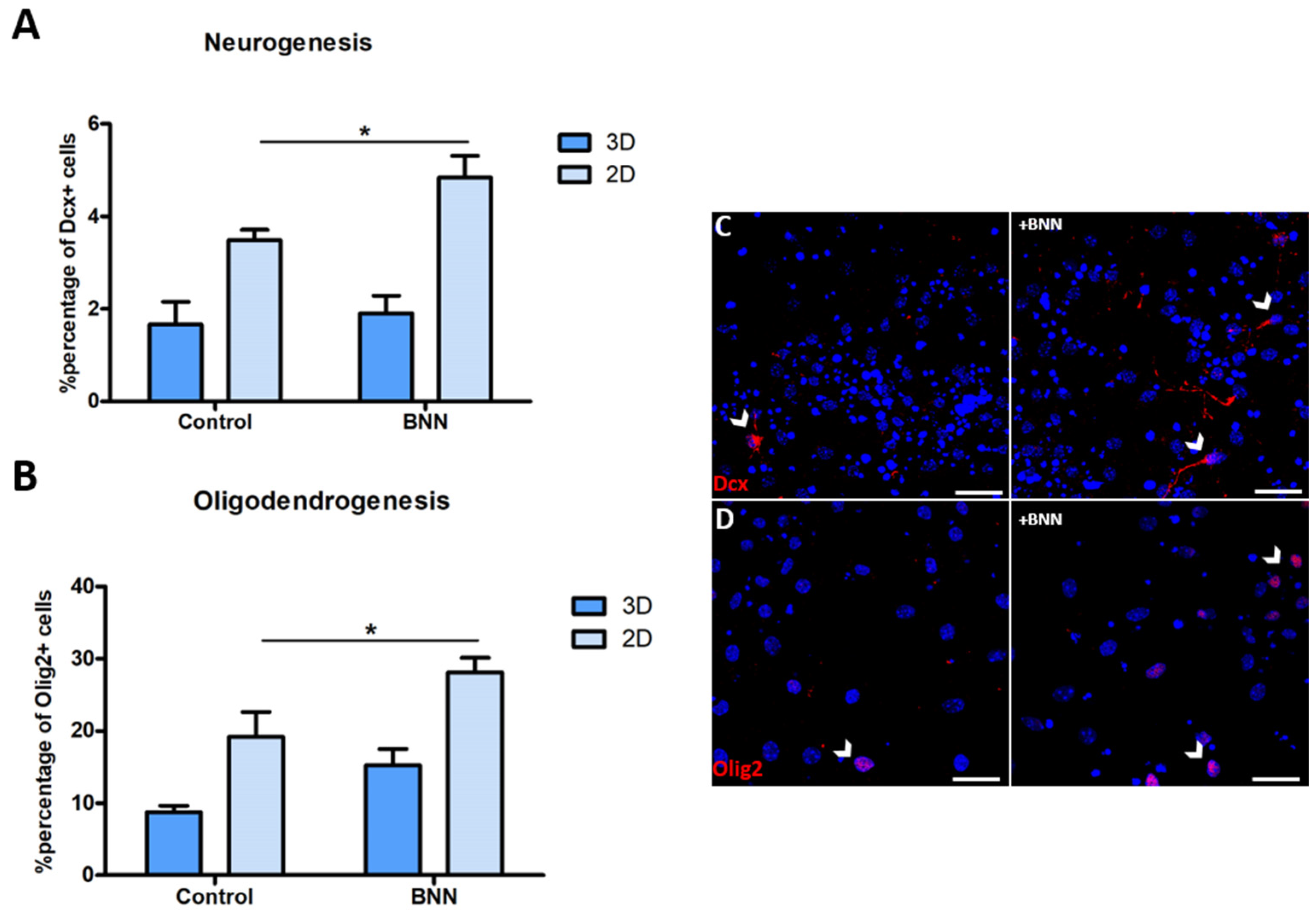
3.6. BNN-20 Affects Neurogenesis and Oligodendrogenesis Only in the 2D Microenvironments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazanis, I. Neurogenesis in the Adult Mammalian Brain: How Much Do We Need, How Much Do We Have? In Neurogenesis and Neural Plasticity; Belzung, C., Wigmore, P., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2012; Volume 15, pp. 3–29. ISBN 978-3-642-36231-6. [Google Scholar]
- Ruddy, R.M.; Morshead, C.M. Home Sweet Home: The Neural Stem Cell Niche throughout Development and after Injury. Cell Tissue Res 2018, 371, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Lledo, P.-M.; Saghatelyan, A. Integrating New Neurons into the Adult Olfactory Bulb: Joining the Network, Life–Death Decisions, and the Effects of Sensory Experience. Trends Neurosci. 2005, 28, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Mouret, A.; Lepousez, G.; Gras, J.; Gabellec, M.-M.; Lledo, P.-M. Turnover of Newborn Olfactory Bulb Neurons Optimizes Olfaction. J. Neurosci. 2009, 29, 12302–12314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etxeberria, A.; Mangin, J.-M.; Aguirre, A.; Gallo, V. Adult-Born SVZ Progenitors Receive Transient Synapses during Remyelination in Corpus Callosum. Nat. Neurosci. 2010, 13, 287–289. [Google Scholar] [CrossRef] [Green Version]
- Kazanis, I.; Evans, K.A.; Andreopoulou, E.; Dimitriou, C.; Koutsakis, C.; Karadottir, R.T.; Franklin, R.J.M. Subependymal Zone-Derived Oligodendroblasts Respond to Focal Demyelination but Fail to Generate Myelin in Young and Aged Mice. Stem Cell Rep. 2017, 8, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Calzolari, F.; Michel, J.; Baumgart, E.V.; Theis, F.; Götz, M.; Ninkovic, J. Fast Clonal Expansion and Limited Neural Stem Cell Self-Renewal in the Adult Subependymal Zone. Nat. Neurosci. 2015, 18, 490–492. [Google Scholar] [CrossRef]
- Obernier, K.; Cebrian-Silla, A.; Thomson, M.; Parraguez, J.I.; Anderson, R.; Guinto, C.; Rodas Rodriguez, J.; Garcia-Verdugo, J.-M.; Alvarez-Buylla, A. Adult Neurogenesis Is Sustained by Symmetric Self-Renewal and Differentiation. Cell Stem Cell 2018, 22, 221–234.e8. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.-H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.-M.; et al. Corridors of Migrating Neurons in the Human Brain and Their Decline during Infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Shook, B.A.; Manz, D.H.; Peters, J.J.; Kang, S.; Conover, J.C. Spatiotemporal Changes to the Subventricular Zone Stem Cell Pool through Aging. J. Neurosci. 2012, 32, 6947–6956. [Google Scholar] [CrossRef] [Green Version]
- Capilla-Gonzalez, V.; Herranz-Pérez, V.; García-Verdugo, J.M. The Aged Brain: Genesis and Fate of Residual Progenitor Cells in the Subventricular Zone. Front. Cell. Neurosci. 2015, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Franklin, R.J.M.; ffrench-Constant, C. Regenerating CNS Myelin—From Mechanisms to Experimental Medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culver, J.C.; Vadakkan, T.J.; Dickinson, M.E. A Specialized Microvascular Domain in the Mouse Neural Stem Cell Niche. PLoS ONE 2013, 8, e53546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercier, F.; Kitasako, J.T.; Hatton, G.I. Anatomy of the Brain Neurogenic Zones Revisited: Fractones and the Fibroblast/Macrophage Network. J. Comp. Neurol. 2002, 451, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Kazanis, I.; Lathia, J.D.; Vadakkan, T.J.; Raborn, E.; Wan, R.; Mughal, M.R.; Eckley, D.M.; Sasaki, T.; Patton, B.; Mattson, M.P.; et al. Quiescence and Activation of Stem and Precursor Cell Populations in the Subependymal Zone of the Mammalian Brain Are Associated with Distinct Cellular and Extracellular Matrix Signals. J. Neurosci. 2010, 30, 9771–9781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjell, J.; Fischer-Sternjak, J.; Thompson, A.J.; Friess, C.; Sticco, M.J.; Salinas, F.; Cox, J.; Martinelli, D.C.; Ninkovic, J.; Franze, K.; et al. Defining the Adult Neural Stem Cell Niche Proteome Identifies Key Regulators of Adult Neurogenesis. Cell Stem Cell 2020, 26, 277–293.e8. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Yao, Y. Laminin Regulates Oligodendrocyte Development and Myelination. Glia 2022, 70, 414–429. [Google Scholar] [CrossRef]
- Hall, P.E.; Lathia, J.D.; Caldwell, M.A.; ffrench-Constant, C. Laminin Enhances the Growth of Human Neural Stem Cells in Defined Culture Media. BMC Neurosci. 2008, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, L.F.; Tomaselli, K.J. Extracellular Matrix Molecules and Their Receptors: Functions in Neural Development. Annu. Rev. Neurosci. 1991, 14, 531–570. [Google Scholar] [CrossRef]
- Götz, M.; Nakafuku, M.; Petrik, D. Neurogenesis in the Developing and Adult Brain—Similarities and Key Differences. Cold Spring Harb. Perspect. Biol. 2016, 8, a018853. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular–Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte Progenitors Balance Growth with Self-Repulsion to Achieve Homeostasis in the Adult Brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, K.; Lo, E.H. An Oligovascular Niche: Cerebral Endothelial Cells Promote the Survival and Proliferation of Oligodendrocyte Precursor Cells. J. Neurosci. 2009, 29, 4351–4355. [Google Scholar] [CrossRef]
- Arai, K.; Lo, E.H. Wiring and Plumbing: Oligodendrocyte Precursors and Angiogenesis in the Oligovascular Niche. J. Cereb. Blood Flow Metab. 2021, 41, 2132–2133. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Neumann, B.; Hill, M.F.E.; Weber, I.P.; Viscomi, C.; Zhao, C.; Young, A.; Agley, C.C.; Thompson, A.J.; Gonzalez, G.A.; et al. Niche Stiffness Underlies the Ageing of Central Nervous System Progenitor Cells. Nature 2019, 573, 130–134. [Google Scholar] [CrossRef]
- Azari, H.; Rahman, M.; Sharififar, S.; Reynolds, B.A. Isolation and Expansion of the Adult Mouse Neural Stem Cells Using the Neurosphere Assay. JoVE 2010, 45, e2393. [Google Scholar] [CrossRef] [Green Version]
- Azari, H.; Louis, S.A.; Sharififar, S.; Vedam-Mai, V.; Reynolds, B.A. Neural-Colony Forming Cell Assay: An Assay To Discriminate Bona Fide Neural Stem Cells from Neural Progenitor Cells. JoVE 2011, 6, e2639. [Google Scholar] [CrossRef]
- Soares, R.; Ribeiro, F.F.; Lourenço, D.M.; Rodrigues, R.S.; Moreira, J.B.; Sebastião, A.M.; Morais, V.A.; Xapelli, S. Isolation and Expansion of Neurospheres from Postnatal (P1–3) Mouse Neurogenic Niches. JoVE 2020, 159, e60822. [Google Scholar] [CrossRef]
- Azari, H.; Reynolds, B.A. In Vitro Models for Neurogenesis. Cold Spring Harb Perspect Biol 2016, 8, a021279. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.K.M.A.; Isaksen, T.J.; Yamashita, T. Protocol for Mouse Adult Neural Stem Cell Isolation and Culture. STAR Protoc. 2021, 2, 100522. [Google Scholar] [CrossRef]
- Galiakberova, A.A.; Dashinimaev, E.B. Neural Stem Cells and Methods for Their Generation From Induced Pluripotent Stem Cells in Vitro. Front. Cell Dev. Biol. 2020, 8, 815. [Google Scholar] [CrossRef]
- Mourtzi, T.; Dimitrakopoulos, D.; Kakogiannis, D.; Salodimitris, C.; Botsakis, K.; Meri, D.K.; Anesti, M.; Dimopoulou, A.; Charalampopoulos, I.; Gravanis, A.; et al. Characterization of Substantia Nigra Neurogenesis in Homeostasis and Dopaminergic Degeneration: Beneficial Effects of the Microneurotrophin BNN-20. Stem Cell Res. 2021, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tavakoli, T.; Derby, E.; Serebryakova, Y.; Rao, M.S.; Mattson, M.P. Cell-Extracellular Matrix Interactions Regulate Neural Differentiation of Human Embryonic Stem Cells. BMC Dev. Biol. 2008, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Scuderi, S.; Altobelli, G.G.; Cimini, V.; Coppola, G.; Vaccarino, F.M. Cell-to-Cell Adhesion and Neurogenesis in Human Cortical Development: A Study Comparing 2D Monolayers with 3D Organoid Cultures. Stem Cell Rep. 2021, 16, 264–280. [Google Scholar] [CrossRef]
- Conover, J.C.; Todd, K.L. Development and Aging of a Brain Neural Stem Cell Niche. Exp. Gerontol. 2017, 94, 9–13. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, X.; An, J.; Zhang, Z.; Chen, X.; Tan, J.; Zhang, P.; Lu, H.; Liu, Y. Cell-Cell Connection Enhances Proliferation and Neuronal Differentiation of Rat Embryonic Neural Stem/Progenitor Cells. Front. Cell. Neurosci. 2017, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Kokovay, E.; Goderie, S.; Wang, Y.; Lotz, S.; Lin, G.; Sun, Y.; Roysam, B.; Shen, Q.; Temple, S. Adult SVZ Lineage Cells Home to and Leave the Vascular Niche via Differential Responses to SDF1/CXCR4 Signaling. Cell Stem Cell 2010, 7, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Hack, M.A.; Saghatelyan, A.; de Chevigny, A.; Pfeifer, A.; Ashery-Padan, R.; Lledo, P.-M.; Götz, M. Neuronal Fate Determinants of Adult Olfactory Bulb Neurogenesis. Nat. Neurosci. 2005, 8, 865–872. [Google Scholar] [CrossRef]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Gotz, M. Progeny of Olig2-Expressing Progenitors in the Gray and White Matter of the Adult Mouse Cerebral Cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef]
- Long, K.R.; Huttner, W.B. How the Extracellular Matrix Shapes Neural Development. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, L.A.; Rebaza, L.M.; Derzic, S.; Schwartz, P.H.; Monuki, E.S. Regulation of Human Neural Precursor Cells by Laminin and Integrins. J. Neurosci. Res. 2006, 83, 845–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Marziali, L.N.; Arenas, E.; Feltri, M.L.; ffrench-Constant, C. Laminin A2 Controls Mouse and Human Stem Cell Behaviour during Midbrain Dopaminergic Neuron Development. Development 2019, 146, dev172668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relucio, J.; Menezes, M.J.; Miyagoe-Suzuki, Y.; Takeda, S.; Colognato, H. Laminin Regulates Postnatal Oligodendrocyte Production by Promoting Oligodendrocyte Progenitor Survival in the Subventricular Zone. Glia 2012, 60, 1451–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Hyodo, M.; Hayashi, C.; Mabuchi, Y.; Sekimoto, K.; Onchi, C.; Sekiguchi, K.; Akazawa, C. Laminin A2, A4, and A5 Chains Positively Regulate Migration and Survival of Oligodendrocyte Precursor Cells. Sci. Rep. 2019, 9, 19882. [Google Scholar] [CrossRef]
- Buttery, P.C.; ffrench-Constant, C. Laminin-2/Integrin Interactions Enhance Myelin Membrane Formation by Oligodendrocytes. Mol. Cell. Neurosci. 1999, 14, 199–212. [Google Scholar] [CrossRef]
- Relucio, J.; Tzvetanova, I.D.; Ao, W.; Lindquist, S.; Colognato, H. Laminin Alters Fyn Regulatory Mechanisms and Promotes Oligodendrocyte Development. J. Neurosci. 2009, 29, 11794–11806. [Google Scholar] [CrossRef]
- Stabenfeldt, S.E.; Munglani, G.; García, A.J.; LaPlaca, M.C. Biomimetic Microenvironment Modulates Neural Stem Cell Survival, Migration, and Differentiation. Tissue Eng. Part A 2010, 16, 3747–3758. [Google Scholar] [CrossRef] [Green Version]
- Nakaji-Hirabayashi, T.; Fujimoto, K.; Yoshikawa, C.; Kitano, H. Functional Surfaces for Efficient Differentiation of Neural Stem/Progenitor Cells into Dopaminergic Neurons: Functional Surfaces for Efficient Differentiation to Dopamine Neurons. J. Biomed. Mater. Res. 2019, 107, 860–871. [Google Scholar] [CrossRef]
- Campos, L.S.; Leone, D.P.; Relvas, J.B.; Brakebusch, C.; Fässler, R.; Suter, U.; ffrench-Constant, C. Β1 Integrins Activate a MAPK Signalling Pathway in Neural Stem Cells That Contributes to Their Maintenance. Development 2004, 131, 3433–3444. [Google Scholar] [CrossRef] [Green Version]
- Loulier, K.; Lathia, J.D.; Marthiens, V.; Relucio, J.; Mughal, M.R.; Tang, S.-C.; Coksaygan, T.; Hall, P.E.; Chigurupati, S.; Patton, B.; et al. Β1 Integrin Maintains Integrity of the Embryonic Neocortical Stem Cell Niche. PLoS Biol. 2009, 7, e1000176. [Google Scholar] [CrossRef] [Green Version]
- Belvindrah, R.; Hankel, S.; Walker, J.; Patton, B.L.; Muller, U. 1 Integrins Control the Formation of Cell Chains in the Adult Rostral Migratory Stream. J. Neurosci. 2007, 27, 2704–2717. [Google Scholar] [CrossRef]
- Leone, D.P.; Relvas, J.B.; Campos, L.S.; Hemmi, S.; Brakebusch, C.; Fässler, R.; ffrench-Constant, C.; Suter, U. Regulation of Neural Progenitor Proliferation and Survival by Β1 Integrins. J. Cell Sci. 2005, 118, 2589–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooker, S.M.; Bond, A.M.; Peng, C.-Y.; Kessler, J.A. Β1-Integrin Restricts Astrocytic Differentiation of Adult Hippocampal Neural Stem Cells: Β1-Integrin Regulates Hippocampal NSC Fate. Glia 2016, 64, 1235–1251. [Google Scholar] [CrossRef]
- Yoneda, A.; Couchman, J.R. Regulation of Cytoskeletal Organization by Syndecan Transmembrane Proteoglycans. Matrix Biol. 2003, 22, 25–33. [Google Scholar] [CrossRef]
- Mouthon, M.-A.; Morizur, L.; Dutour, L.; Pineau, D.; Kortulewski, T.; Boussin, F.D. Syndecan-1 Stimulates Adult Neurogenesis in the Mouse Ventricular-Subventricular Zone after Injury. iScience 2020, 23, 101784. [Google Scholar] [CrossRef]
- Benninger, Y.; Colognato, H.; Thurnherr, T.; Franklin, R.J.M.; Leone, D.P.; Atanasoski, S.; Nave, K.-A.; ffrench-Constant, C.; Suter, U.; Relvas, J.B. 1-Integrin Signaling Mediates Premyelinating Oligodendrocyte Survival But Is Not Required for CNS Myelination and Remyelination. J. Neurosci. 2006, 26, 7665–7673. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; North, H.A.; Sahni, V.; Jeong, S.J.; Mcguire, T.L.; Berns, E.J.; Stupp, S.I.; Kessler, J.A. Β1-Integrin and Integrin Linked Kinase Regulate Astrocytic Differentiation of Neural Stem Cells. PLoS ONE 2014, 9, e104335. [Google Scholar] [CrossRef] [Green Version]
- McClenahan, F.K.; Sharma, H.; Shan, X.; Eyermann, C.; Colognato, H. Dystroglycan Suppresses Notch to Regulate Stem Cell Niche Structure and Function in the Developing Postnatal Subventricular Zone. Dev. Cell 2016, 38, 548–566. [Google Scholar] [CrossRef] [Green Version]
| Microenvironment Factors | Neurogenesis | Oligodendrogenesis |
|---|---|---|
| 1. Levels of cell fate commitment | ||
| 2.28% ± 0.34 Dcx+ cells | 9.30% ± 2.20 Olig2+ cells | |
| 2. Cytoarchitecture | ||
| 2a. 3D vs. 2D |
| No dependence |
| 2b. Cell density restrictions | Appears in wider range of cell densities (on average 192 ± 21 cells per opt. field) |
|
| 2c. Correlation to cell density | Significantly Positive (r = 0.722) → proportional | No correlation (r = −0.396) |
| 3. Laminin (niche ECM: leads to predominance of 2D areas) | ||
| 3a. Dependence on laminin | Significant reduction in 2D areas | No dependence |
| 3b. Cell density restrictions |
|
|
| 3c. Correlation to cell density | Significantly Positive (r = 0.800) → proportional | No correlation (r = −0.274) |
| 4. Integrin-β1 (effects of antibody-mediated blocking) | ||
| Significantly reduced (3D & 2D) irrespective of substrate |
| |
| 5. Microneurotrophin BNN-20 (BDNF-mimicking) | ||
| Significantly increased in 2D areas | Significantly increased in 2D areas | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anesti, M.; Magkafa, S.; Prantikou, E.; Kazanis, I. Divergence between Neuronal and Oligodendroglial Cell Fate, in Postnatal Brain Neural Stem Cells, Leads to Divergent Properties in Polymorphic In Vitro Assays. Cells 2022, 11, 1743. https://doi.org/10.3390/cells11111743
Anesti M, Magkafa S, Prantikou E, Kazanis I. Divergence between Neuronal and Oligodendroglial Cell Fate, in Postnatal Brain Neural Stem Cells, Leads to Divergent Properties in Polymorphic In Vitro Assays. Cells. 2022; 11(11):1743. https://doi.org/10.3390/cells11111743
Chicago/Turabian StyleAnesti, Maria, Stavroula Magkafa, Efstathia Prantikou, and Ilias Kazanis. 2022. "Divergence between Neuronal and Oligodendroglial Cell Fate, in Postnatal Brain Neural Stem Cells, Leads to Divergent Properties in Polymorphic In Vitro Assays" Cells 11, no. 11: 1743. https://doi.org/10.3390/cells11111743
APA StyleAnesti, M., Magkafa, S., Prantikou, E., & Kazanis, I. (2022). Divergence between Neuronal and Oligodendroglial Cell Fate, in Postnatal Brain Neural Stem Cells, Leads to Divergent Properties in Polymorphic In Vitro Assays. Cells, 11(11), 1743. https://doi.org/10.3390/cells11111743