Transcribed Ultraconserved Regions in Cancer
Abstract
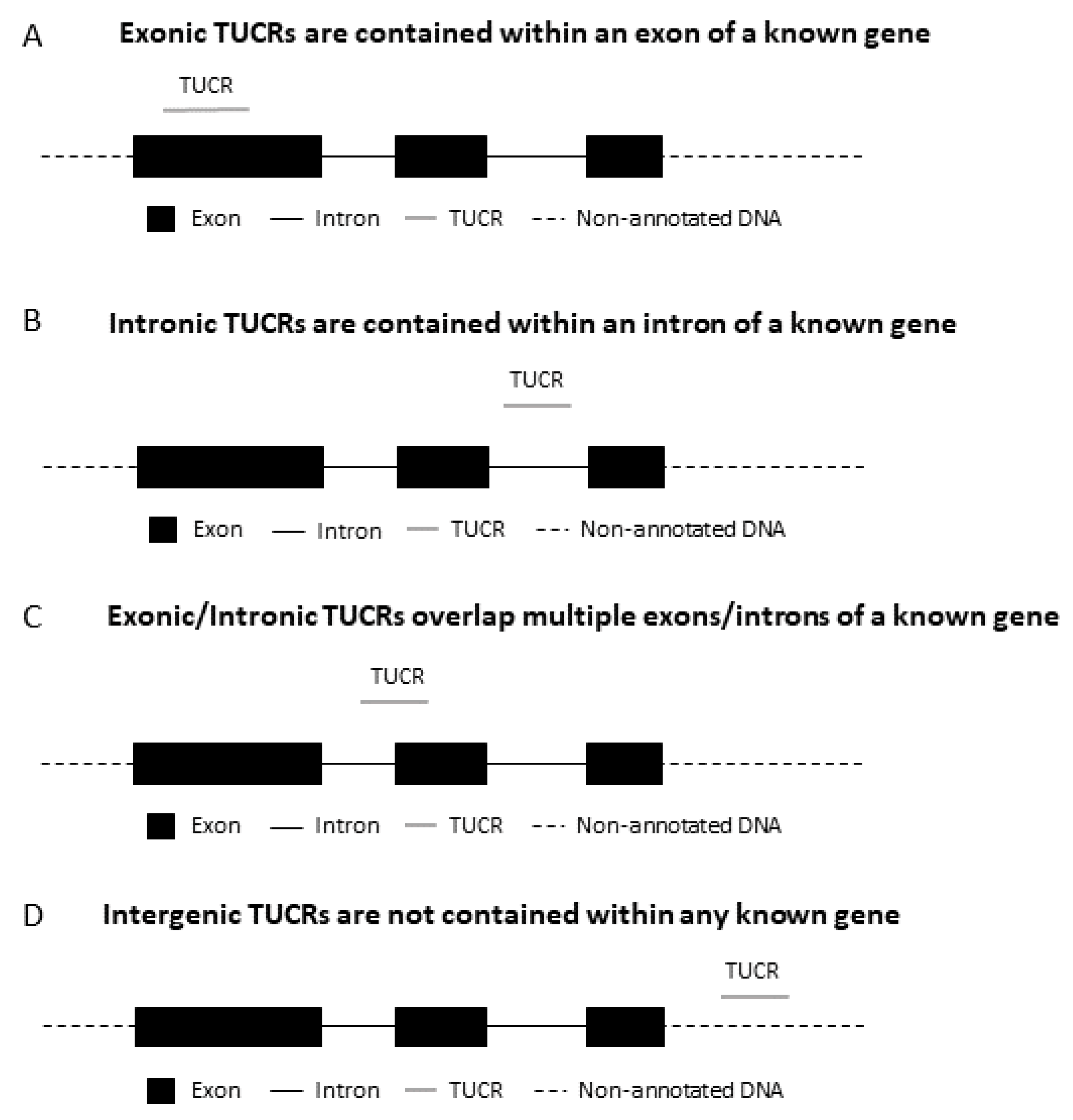
1. Introduction
2. Expression and Deregulation of TUCRs in Cancer
3. Functions and Mechanisms of Action of TUCRs in Cancer
4. Translational and Clinical Perspectives Associated with TUCRs
4.1. Caveats and Considerations in the Study of TUCRs
4.2. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braicu, C.; Zimta, A.A.; Harangus, A.; Iurca, I.; Irimie, A.; Coza, O.; Berindan-Neagoe, I. The Function of Non-Coding RNAs in Lung Cancer Tumorigenesis. Cancers 2019, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Catana, C.S.; Pichler, M.; Giannelli, G.; Mader, R.M.; Berindan-Neagoe, I. Non-coding RNAs, the Trojan horse in two-way communication between tumor and stroma in colorectal and hepatocellular carcinoma. Oncotarget 2017, 8, 29519–29534. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Zimta, A.A.; Ciocan, C.; Mehterov, N.; Dudea, D.; Braicu, C.; Berindan-Neagoe, I. The Unforeseen Non-Coding RNAs in Head and Neck Cancer. Genes 2018, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Vincent, K.; Pichler, M.; Fodde, R.; Berindan-Neagoe, I.; Slack, F.J.; Calin, G.A. Junk DNA and the long non-coding RNA twist in cancer genetics. Oncogene 2015, 34, 5003–5011. [Google Scholar] [CrossRef] [PubMed]
- Liz, J.; Esteller, M. lncRNAs and microRNAs with a role in cancer development. Biochim. Biophys. Acta 2016, 1859, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Honma, R.; Sekino, Y.; Goto, K.; Sentani, K.; Ishikawa, A.; Oue, N.; Yasui, W. Non-coding RNAs are promising targets for stem cell-based cancer therapy. Noncoding RNA Res. 2017, 2, 83–87. [Google Scholar] [CrossRef]
- Bejerano, G.; Pheasant, M.; Makunin, I.; Stephen, S.; Kent, W.J.; Mattick, J.S.; Haussler, D. Ultraconserved elements in the human genome. Science 2004, 304, 1321–1325. [Google Scholar] [CrossRef]
- Calin, G.A.; Liu, C.G.; Ferracin, M.; Hyslop, T.; Spizzo, R.; Sevignani, C.; Fabbri, M.; Cimmino, A.; Lee, E.J.; Wojcik, S.E.; et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell 2007, 12, 215–229. [Google Scholar] [CrossRef]
- Edwards, J.K.; Pasqualini, R.; Arap, W.; Calin, G.A. MicroRNAs and ultraconserved genes as diagnostic markers and therapeutic targets in cancer and cardiovascular diseases. J. Cardiovasc. Transl. Res. 2010, 3, 271–279. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Fabris, L.; Calin, G.A. Understanding the Genomic Ultraconservations: T-UCRs and Cancer. Int. Rev. Cell Mol. Biol. 2017, 333, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Pereira Zambalde, E.; Mathias, C.; Rodrigues, A.C.; de Souza Fonseca Ribeiro, E.M.; Fiori Gradia, D.; Calin, G.A.; Carvalho de Oliveira, J. Highlighting transcribed ultraconserved regions in human diseases. Wiley Interdiscip. Rev. RNA 2019, 11, e1567. [Google Scholar] [CrossRef] [PubMed]
- Satake, Y.; Kuwano, Y.; Nishikawa, T.; Fujita, K.; Saijo, S.; Itai, M.; Tanaka, H.; Nishida, K.; Rokutan, K. Nucleolin facilitates nuclear retention of an ultraconserved region containing TRA2beta4 and accelerates colon cancer cell growth. Oncotarget 2018, 9, 26817–26833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scaruffi, P. The transcribed-ultraconserved regions: A novel class of long noncoding RNAs involved in cancer susceptibility. Sci. World J. 2011, 11, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; You, L.; Li, Y.; Zhu, L.; Zhang, F.; Xie, K.; Cao, Y.; Ji, C.; Guo, X. A transcribed ultraconserved noncoding RNA, uc.417, serves as a negative regulator of brown adipose tissue thermogenesis. FASEB J. 2016, 30, 4301–4312. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.; Sun, L.; Lu, Y.; Dou, L.; Huang, X.; Tang, W.; Yu, L.; Li, J. Ultraconserved element uc.372 drives hepatic lipid accumulation by suppressing miR-195/miR4668 maturation. Nat. Commun. 2018, 9, 612. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, W.; Xue, R.; Dong, B.; Liang, Z.; Chen, C.; Li, J.; Wang, Y.; Zhao, J.; Huang, H.; et al. Transcribed Ultraconserved Regions, Uc.323, Ameliorates Cardiac Hypertrophy by Regulating the Transcription of CPT1b (Carnitine Palmitoyl transferase 1b). Hypertension 2020, 75, 79–90. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Yu, Z.; Zhu, C.; Qian, L. Role of lncRNA uc.457 in the differentiation and maturation of cardiomyocytes. Mol. Med. Rep. 2019, 19, 4927–4934. [Google Scholar] [CrossRef]
- Ding, L.; Gong, C.; Zhao, J.; Liu, X.; Li, T.; Rao, S.; Wang, S.; Liu, Y.; Peng, S.; Xiao, W.; et al. Noncoding transcribed ultraconserved region (T-UCR) UC.48+ is a novel regulator of high-fat diet induced myocardial ischemia/reperfusion injury. J. Cell Physiol. 2019, 234, 9849–9861. [Google Scholar] [CrossRef]
- Fiorenzano, A.; Pascale, E.; Gagliardi, M.; Terreri, S.; Papa, M.; Andolfi, G.; Galasso, M.; Tagliazucchi, G.M.; Taccioli, C.; Patriarca, E.J.; et al. An Ultraconserved Element Containing lncRNA Preserves Transcriptional Dynamics and Maintains ESC Self-Renewal. Stem Cell Rep. 2018, 10, 1102–1114. [Google Scholar] [CrossRef]
- Qian, X.X.; Peng, J.C.; Xu, A.T.; Zhao, D.; Qiao, Y.Q.; Wang, T.R.; Shen, J.; Ran, Z.H. Noncoding Transcribed Ultraconserved Region (T-UCR) uc.261 Participates in Intestinal Mucosa Barrier Damage in Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2840–2852. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, P.; Fredlund, E.; Pattyn, F.; Rihani, A.; Van Maerken, T.; Vermeulen, J.; Kumps, C.; Menten, B.; De Preter, K.; Schramm, A.; et al. An integrative genomics screen uncovers ncRNA T-UCR functions in neuroblastoma tumours. Oncogene 2010, 29, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Lujambio, A.; Portela, A.; Liz, J.; Melo, S.A.; Rossi, S.; Spizzo, R.; Croce, C.M.; Calin, G.A.; Esteller, M. CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene 2010, 29, 6390–6401. [Google Scholar] [CrossRef] [PubMed]
- Colwell, M.; Drown, M.; Showel, K.; Drown, C.; Palowski, A.; Faulk, C. Evolutionary conservation of DNA methylation in CpG sites within ultraconserved noncoding elements. Epigenetics 2018, 13, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. Chronic lymphocytic leukemia: Interplay between noncoding RNAs and protein-coding genes. Blood 2009, 114, 4761–4770. [Google Scholar] [CrossRef]
- Chiang, C.W.; Derti, A.; Schwartz, D.; Chou, M.F.; Hirschhorn, J.N.; Wu, C.T. Ultraconserved elements: Analyses of dosage sensitivity, motifs and boundaries. Genetics 2008, 180, 2277–2293. [Google Scholar] [CrossRef]
- Martinez, F.; Monfort, S.; Rosello, M.; Oltra, S.; Blesa, D.; Quiroga, R.; Mayo, S.; Orellana, C. Enrichment of ultraconserved elements among genomic imbalances causing mental delay and congenital anomalies. BMC Med. Genom. 2010, 3, 54. [Google Scholar] [CrossRef]
- McCole, R.B.; Fonseka, C.Y.; Koren, A.; Wu, C.T. Abnormal dosage of ultraconserved elements is highly disfavored in healthy cells but not cancer cells. PLoS Genet. 2014, 10, e1004646. [Google Scholar] [CrossRef]
- De Grassi, A.; Segala, C.; Iannelli, F.; Volorio, S.; Bertario, L.; Radice, P.; Bernard, L.; Ciccarelli, F.D. Ultradeep sequencing of a human ultraconserved region reveals somatic and constitutional genomic instability. PLoS Biol. 2010, 8, e1000275. [Google Scholar] [CrossRef]
- Rossi, S.; Sevignani, C.; Nnadi, S.C.; Siracusa, L.D.; Calin, G.A. Cancer-associated genomic regions (CAGRs) and noncoding RNAs: Bioinformatics and therapeutic implications. Mamm. Genome 2008, 19, 526–540. [Google Scholar] [CrossRef]
- Katzman, S.; Kern, A.D.; Bejerano, G.; Fewell, G.; Fulton, L.; Wilson, R.K.; Salama, S.R.; Haussler, D. Human genome ultraconserved elements are ultraselected. Science 2007, 317, 915. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.W.; Liu, C.T.; Lettre, G.; Lange, L.A.; Jorgensen, N.W.; Keating, B.J.; Vedantam, S.; Nock, N.L.; Franceschini, N.; Reiner, A.P.; et al. Ultraconserved elements in the human genome: Association and transmission analyses of highly constrained single-nucleotide polymorphisms. Genetics 2012, 192, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Halligan, D.L.; Oliver, F.; Guthrie, J.; Stemshorn, K.C.; Harr, B.; Keightley, P.D. Positive and negative selection in murine ultraconserved noncoding elements. Mol. Biol. Evol. 2011, 28, 2651–2660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Licastro, D.; Gennarino, V.A.; Petrera, F.; Sanges, R.; Banfi, S.; Stupka, E. Promiscuity of enhancer, coding and non-coding transcription functions in ultraconserved elements. BMC Genom. 2010, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Ahituv, N.; Zhu, Y.; Visel, A.; Holt, A.; Afzal, V.; Pennacchio, L.A.; Rubin, E.M. Deletion of ultraconserved elements yields viable mice. PLoS Biol. 2007, 5, e234. [Google Scholar] [CrossRef]
- Bao, B.Y.; Lin, V.C.; Yu, C.C.; Yin, H.L.; Chang, T.Y.; Lu, T.L.; Lee, H.Z.; Pao, J.B.; Huang, C.Y.; Huang, S.P. Genetic variants in ultraconserved regions associate with prostate cancer recurrence and survival. Sci. Rep. 2016, 6, 22124. [Google Scholar] [CrossRef]
- Shen, H.; Lu, C.; Jiang, Y.; Tang, J.; Chen, W.; Zhang, H.; Zhang, Q.; Wang, J.; Liang, J.; Hu, Z.; et al. Genetic variants in ultraconserved elements and risk of breast cancer in Chinese population. Breast Cancer Res. Treat 2011, 128, 855–861. [Google Scholar] [CrossRef]
- Lin, M.; Eng, C.; Hawk, E.T.; Huang, M.; Greisinger, A.J.; Gu, J.; Ellis, L.M.; Wu, X.; Lin, J. Genetic variants within ultraconserved elements and susceptibility to right- and left-sided colorectal adenocarcinoma. Carcinogenesis 2012, 33, 841–847. [Google Scholar] [CrossRef][Green Version]
- Lin, M.; Eng, C.; Hawk, E.T.; Huang, M.; Lin, J.; Gu, J.; Ellis, L.M.; Wu, X. Identification of polymorphisms in ultraconserved elements associated with clinical outcomes in locally advanced colorectal adenocarcinoma. Cancer 2012, 118, 6188–6198. [Google Scholar] [CrossRef]
- Fassan, M.; Dall’Olmo, L.; Galasso, M.; Braconi, C.; Pizzi, M.; Realdon, S.; Volinia, S.; Valeri, N.; Gasparini, P.; Baffa, R.; et al. Transcribed ultraconserved noncoding RNAs (T-UCR) are involved in Barrett’s esophagus carcinogenesis. Oncotarget 2014, 5, 7162–7171. [Google Scholar] [CrossRef][Green Version]
- Rossi, S.; Kopetz, S.; Davuluri, R.; Hamilton, S.R.; Calin, G.A. MicroRNAs, ultraconserved genes and colorectal cancers. Int. J. Biochem. Cell Biol. 2010, 42, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Ferdin, J.; Nishida, N.; Wu, X.; Nicoloso, M.S.; Shah, M.Y.; Devlin, C.; Ling, H.; Shimizu, M.; Kumar, K.; Cortez, M.A.; et al. HINCUTs in cancer: Hypoxia-induced noncoding ultraconserved transcripts. Cell Death Differ. 2013, 20, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Lena, A.M.; Panatta, E.; Ivan, C.; Han, L.; Liang, H.; Annicchiarico-Petruzzelli, M.; Di Daniele, N.; Calin, G.A.; Candi, E.; et al. Ultraconserved long non-coding RNA uc.63 in breast cancer. Oncotarget 2017, 8, 35669–35680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sekino, Y.; Sakamoto, N.; Goto, K.; Honma, R.; Shigematsu, Y.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; Yasui, W. Transcribed ultraconserved region Uc.63+ promotes resistance to docetaxel through regulation of androgen receptor signaling in prostate cancer. Oncotarget 2017, 8, 94259–94270. [Google Scholar] [CrossRef] [PubMed]
- Sekino, Y.; Sakamoto, N.; Ishikawa, A.; Honma, R.; Shigematsu, Y.; Hayashi, T.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; et al. Transcribed ultraconserved region Uc.63+ promotes resistance to cisplatin through regulation of androgen receptor signaling in bladder cancer. Oncol. Rep. 2019, 41, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, Y.; Nishida, K.; Rokutan, K. Overexpression of the transcribed ultraconserved region Uc.138 accelerates colon cancer progression. Sci. Rep. 2021, 11, 8667. [Google Scholar] [CrossRef]
- Hudson, R.S.; Yi, M.; Volfovsky, N.; Prueitt, R.L.; Esposito, D.; Volinia, S.; Liu, C.G.; Schetter, A.J.; Van Roosbroeck, K.; Stephens, R.M.; et al. Transcription signatures encoded by ultraconserved genomic regions in human prostate cancer. Mol. Cancer 2013, 12, 13. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.; Gong, W.; Wu, Y.; Xue, H.; Jiang, Z.; Shi, M. uc.454 Inhibited Growth by Targeting Heat Shock Protein Family A Member 12B in Non-Small-Cell Lung Cancer. Mol. Ther. Nucleic Acids 2018, 12, 174–183. [Google Scholar] [CrossRef]
- Goto, K.; Ishikawa, S.; Honma, R.; Tanimoto, K.; Sakamoto, N.; Sentani, K.; Oue, N.; Teishima, J.; Matsubara, A.; Yasui, W. The transcribed-ultraconserved regions in prostate and gastric cancer: DNA hypermethylation and microRNA-associated regulation. Oncogene 2016, 35, 3598–3606. [Google Scholar] [CrossRef]
- Zhang, L.X.; Xu, L.; Zhang, C.H.; Lu, Y.H.; Ji, T.H.; Ling, L.J. uc.38 induces breast cancer cell apoptosis via PBX1. Am. J. Cancer Res. 2017, 7, 2438–2451. [Google Scholar]
- Guo, Y.; Wang, C.; Miao, X.; Chen, S.; Qian, Y.; Li, G.; Jiang, Y. Upregulation of uc.189 in patients with esophageal squamous cell carcinoma and its clinicopathologic value. Pathol. Res. Pract. 2017, 213, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.C.; Yang, T.; He, L.N.; Tao, Y.X.; Gao, Y.J. Altered T-UCRs expression profile in the spinal cord of mice with neuropathic pain. Transl. Perioper Pain Med. 2016, 1, 1–10. [Google Scholar] [PubMed]
- Sana, J.; Hankeova, S.; Svoboda, M.; Kiss, I.; Vyzula, R.; Slaby, O. Expression levels of transcribed ultraconserved regions uc.73 and uc.388 are altered in colorectal cancer. Oncology 2012, 82, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Vannini, I.; Wise, P.M.; Challagundla, K.B.; Plousiou, M.; Raffini, M.; Bandini, E.; Fanini, F.; Paliaga, G.; Crawford, M.; Ferracin, M.; et al. Transcribed ultraconserved region 339 promotes carcinogenesis by modulating tumor suppressor microRNAs. Nat. Commun. 2017, 8, 1801. [Google Scholar] [CrossRef] [PubMed]
- Kajita, K.; Kuwano, Y.; Satake, Y.; Kano, S.; Kurokawa, K.; Akaike, Y.; Masuda, K.; Nishida, K.; Rokutan, K. Ultraconserved region-containing Transformer 2beta4 controls senescence of colon cancer cells. Oncogenesis 2016, 5, e213. [Google Scholar] [CrossRef] [PubMed]
- Liz, J.; Portela, A.; Soler, M.; Gomez, A.; Ling, H.; Michlewski, G.; Calin, G.A.; Guil, S.; Esteller, M. Regulation of pri-miRNA processing by a long noncoding RNA transcribed from an ultraconserved region. Mol. Cell 2014, 55, 138–147. [Google Scholar] [CrossRef]
- Carotenuto, P.; Fassan, M.; Pandolfo, R.; Lampis, A.; Vicentini, C.; Cascione, L.; Paulus-Hock, V.; Boulter, L.; Guest, R.; Quagliata, L.; et al. Wnt signalling modulates transcribed-ultraconserved regions in hepatobiliary cancers. Gut 2017, 66, 1268–1277. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wang, C. Uc.206 regulates cell proliferation and apoptosis by targeting P53 in cervical cancer cells. Neoplasma 2016, 63, 411–418. [Google Scholar] [CrossRef]
- Vannini, I.; Ferracin, M.; Fabbri, F.; Fabbri, M. Overexpression of ultraconserved region 83- induces lung cancer tumorigenesis. PLoS ONE 2022, 17, e0261464. [Google Scholar] [CrossRef]
- Corra, F.; Crudele, F.; Baldassari, F.; Bianchi, N.; Galasso, M.; Minotti, L.; Agnoletto, C.; Di Leva, G.; Brugnoli, F.; Reali, E.; et al. UC.183, UC.110, and UC.84 Ultra-Conserved RNAs Are Mutually Exclusive with miR-221 and Are Engaged in the Cell Cycle Circuitry in Breast Cancer Cell Lines. Genes 2021, 12, 1978. [Google Scholar] [CrossRef]
- Olivieri, M.; Ferro, M.; Terreri, S.; Durso, M.; Romanelli, A.; Avitabile, C.; De Cobelli, O.; Messere, A.; Bruzzese, D.; Vannini, I.; et al. Long non-coding RNA containing ultraconserved genomic region 8 promotes bladder cancer tumorigenesis. Oncotarget 2016, 7, 20636–20654. [Google Scholar] [CrossRef] [PubMed]
- Terreri, S.; Mancinelli, S.; Ferro, M.; Vitale, M.C.; Perdona, S.; Castaldo, L.; Gigantino, V.; Mercadante, V.; De Cecio, R.; Aquino, G.; et al. Subcellular Localization of uc.8+ as a Prognostic Biomarker in Bladder Cancer Tissue. Cancers 2021, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Huang, X.; Chen, R.; Li, Y.; Xu, Y.; Zhang, W.; Zhu, Q.; Zha, X.; Wang, J. The transcribed ultraconserved element uc.51 promotes the proliferation and metastasis of breast cancer by stabilizing NONO. Clin. Exp. Metastasis 2021, 38, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Feng, Y. Up-regulation of long noncoding RNA uc.338 predicts poor survival in non-small cell lung cancer. Cancer Biomark. Sect. A Dis. Markers 2018, 22, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, X.; Li, C.; Zhang, Y.; Gao, L. Knockdown of Long Noncoding RNA uc.338 by siRNA Inhibits Cellular Migration and Invasion in Human Lung Cancer Cells. Oncol. Res. 2016, 24, 337–343. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhou, J.; Liu, S.; Wu, C.; Huang, C.; Ding, Y. TUC.338 promotes invasion and metastasis in colorectal cancer. Int. J. Cancer 2017, 140, 1457–1464. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Qian, W.; Ji, D.; Wang, Q.; Zhang, Z.; Wang, S.; Ji, B.; Fu, Z.; Sun, Y. uc.338 targets p21 and cyclin D1 via PI3K/AKT pathway activation to promote cell proliferation in colorectal cancer. Oncol. Rep. 2018, 40, 1119–1128. [Google Scholar] [CrossRef]
- Luo, H.L.; Chen, J.; Luo, T.; Wu, F.X.; Liu, J.J.; Wang, H.F.; Chen, M.; Li, L.Q.; Li, H. Downregulation of Macrophage-Derived T-UCR uc.306 Associates with Poor Prognosis in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2017, 42, 1526–1539. [Google Scholar] [CrossRef]
- Pereira Zambalde, E.; Bayraktar, R.; Schultz Jucoski, T.; Ivan, C.; Rodrigues, A.C.; Mathias, C.; Knutsen, E.; Silveira de Lima, R.; Fiori Gradia, D.; de Souza Fonseca Ribeiro, E.M.; et al. A novel lncRNA derived from an ultraconserved region: Lnc-uc.147, a potential biomarker in luminal A breast cancer. RNA Biol. 2021, 18, 416–429. [Google Scholar] [CrossRef]
- Honma, R.; Goto, K.; Sakamoto, N.; Sekino, Y.; Sentani, K.; Oue, N.; Yasui, W. Expression and function of Uc.160+, a transcribed ultraconserved region, in gastric cancer. Gastric Cancer 2017, 20, 960–969. [Google Scholar] [CrossRef]
- Pang, L.; Li, Q.; Zhang, Y.; Deng, B.; Wu, F.; Wang, J.; Wu, K.; Ding, Y.; Yu, D. Transcribed ultraconserved noncoding RNA uc.160 acts as a negative regulator in gastric cancer. Am. J. Transl. Res. 2018, 10, 2822–2833. [Google Scholar] [PubMed]
- Kottorou, A.E.; Dimitrakopoulos, F.D.; Antonacopoulou, A.G.; Diamantopoulou, G.; Tsoumas, D.; Koutras, A.; Makatsoris, T.; Stavropoulos, M.; Thomopoulos, K.C.; Hulbert, A.; et al. Differentially Methylated Ultra-Conserved Regions Uc160 and Uc283 in Adenomas and Adenocarcinomas Are Associated with Overall Survival of Colorectal Cancer Patients. Cancers 2020, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Alberca Custodio, R.W.; Mirotti, L.; Gomes, E.; Nunes, F.P.B.; Vieira, R.S.; Graca, L.; Russo, M. Dendritic Cells Expressing MyD88 Molecule Are Necessary and Sufficient for CpG-Mediated Inhibition of IgE Production In Vivo. Cells 2019, 8, 1165. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Chen, Y. Targeting long non-coding RNAs in cancers: Progress and prospects. Int. J. Biochem. Cell Biol. 2013, 45, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- Baira, E.; Greshock, J.; Coukos, G.; Zhang, L. Ultraconserved elements: Genomics, function and disease. RNA Biol. 2008, 5, 132–134. [Google Scholar] [CrossRef]
- Yang, R.; Frank, B.; Hemminki, K.; Bartram, C.R.; Wappenschmidt, B.; Sutter, C.; Kiechle, M.; Bugert, P.; Schmutzler, R.K.; Arnold, N.; et al. SNPs in ultraconserved elements and familial breast cancer risk. Carcinogenesis 2008, 29, 351–355. [Google Scholar] [CrossRef]
- Braconi, C.; Valeri, N.; Kogure, T.; Gasparini, P.; Huang, N.; Nuovo, G.J.; Terracciano, L.; Croce, C.M.; Patel, T. Expression and functional role of a transcribed noncoding RNA with an ultraconserved element in hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 786–791. [Google Scholar] [CrossRef]
- Pertea, M.; Shumate, A.; Pertea, G.; Varabyou, A.; Breitwieser, F.P.; Chang, Y.C.; Madugundu, A.K.; Pandey, A.; Salzberg, S.L. CHESS: A new human gene catalog curated from thousands of large-scale RNA sequencing experiments reveals extensive transcriptional noise. Genome Biol. 2018, 19, 208. [Google Scholar] [CrossRef]
- Zampetaki, A.; Albrecht, A.; Steinhofel, K. Long Non-coding RNA Structure and Function: Is There a Link? Front. Physiol. 2018, 9, 1201. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, J.; Yeung, P.Y.; Zhang, Q.C.; Kwok, C.K. Revealing lncRNA Structures and Interactions by Sequencing-Based Approaches. Trends Biochem. Sci. 2019, 44, 33–52. [Google Scholar] [CrossRef]
- Graf, J.; Kretz, M. From structure to function: Route to understanding lncRNA mechanism. Bioessays 2020, 42, e2000027. [Google Scholar] [CrossRef] [PubMed]
- Gross, L. Are “ultraconserved” genetic elements really indispensable? PLoS Biol. 2007, 5, e253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ward, M.; McEwan, C.; Mills, J.D.; Janitz, M. Conservation and tissue-specific transcription patterns of long noncoding RNAs. J. Hum. Transcr. 2015, 1, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, Y.; Zhao, Z.; Lu, J.; Chen, H.; Ding, N.; Wang, G.; Xu, J.; Li, X. Identifying and functionally characterizing tissue-specific and ubiquitously expressed human lncRNAs. Oncotarget 2016, 7, 7120–7133. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]


| Primary Search Term | Secondary Search Term | Number of Publications |
|---|---|---|
| TUCR, UCR, or “ultraconserved” | Cancer | 69 |
| Long non-coding RNA | Cancer | 7796 |
| Enhancer RNA | Cancer | 1207 |
| Antisense RNA | Cancer | 2310 |
| Micro RNA | Cancer | 12,141 |
| p53 | Cancer | 24,073 |
| TUCR (Citation) | Cancer | Expression | Function | Mechanism |
|---|---|---|---|---|
| uc.8 [60,61,62] | Bladder | Upregulated | Invasion, Accumulation, Proliferation, Migration, Tumor Grading and Staging, Poor Prognosis | Upregulation of MMP9 via decoy for miR-596 |
| uc.38 [50] | Breast | Downregulated | Reduced Proliferation, Induction of Apoptosis | BCL-2 family proteins through PBX-1 depletion |
| uc.51 [34,63] | Breast | Upregulated | Proliferation, Metastasis | Stabilization of NONO |
| uc.63 [43,44,45] | Breast, Prostate, Bladder, Chronic Lymphocytic Leukemia | Upregulated | Proliferation, Poor Prognosis, Docetaxel Resistance | Upregulation of MMP2 via miR-130b |
| uc.73 [53] | Colorectal | Upregulated/Downregulated | Cell Proliferation, Apoptosis | N/A |
| uc.83 [59] | Lung | Upregulated | Cell Growth | PI3K and MAPK signaling |
| uc.84 [60] | Breast | Upregulated | Cell Cycle Progression | Co-regulation w/miR-221 and CDKN1B expression |
| uc.110 [60] | Breast | Upregulated | Cell Cycle Progression | Co-regulation w/miR-221 and CDKN1B expression |
| uc.138 [46] | Colon | Upregulated | Cell Cycle Progression, Resistance to Apoptosis | N/A |
| uc.158 [57] | Hepatocellular Carcinoma | Upregulated | Cell Growth, Spheroid Formation, Migration, Apoptosis | Wnt Signaling and miR-193b |
| uc.160 [23,70,71,72] | Gastric, Colorectal, Leukemia, Bladder | Upregulated | Poor Prognosis, Proliferation, Migration, Promoter Methylation, Cell Growth | Indirect regulation of PTEN, Interaction w/miR-24-1 and -155 |
| uc.183 [60] | Breast | Upregulated | Cell Cycle Progression | Co-regulation w/miR-221 and CDKN1B expression |
| uc.189 [51,52] | Esophageal Squamous Cell Carcinoma | Upregulated | Invasion, Advanced Disease, Metastasis, Poor Prognosis | N/A |
| uc.206 [58] | Cervical | Upregulated | Cell Growth | Direct regulation of p53 |
| uc.216 [73] | Chronic Lymphocytic Leukemia | Upregulated | CpG oligodeoxynucleotide Resistance | N/A |
| uc.283 [47,56] | Prostate, Colorectal | Downregulated | Good Prognosis, Promoter Methylation | N/A |
| uc.306 [68] | Bladder, Liver | Downregulated | Good Prognosis | Wnt signaling |
| uc.338 [64,65,66,67] | Liver, Cervical, Lung | Upregulated | Proliferation, Migration, Invasion | Broad regulation of cell cycle genes |
| uc.339 [1,53,54,55] | Lung, Colorectal | Upregulated | Cell Proliferation, Clonogenic Growth, Soft Agar Growth, Adhesion | Competing RNA w/cyclin E2 for miR-339-3p, -663b-3p, and 95-5p |
| uc.346 [23,70,71,72] | Colorectal | Upregulated | Poor Prognosis, Proliferation, Migration, Promoter Methylation | N/A |
| uc.416 [49] | Renal Cell Carcinoma, Gastric | Upregulated | Cell Growth and Migration | Regulation of miR-153 and IGFBP6 |
| uc.454 [1,47,48] | Non-Small Cell Lung Cancer, Bladder, Prostate | Downregulated | Reduced Proliferation, Induction of Apoptosis | HSPA12B or Ras signaling pathway |
| uc.475 [42] | Epithelial | Upregulated | Proliferation | N/A |
| uc.147 [69] | Breast | Upregulated | Poor Prognosis | Potential regulation of miR-18 and miR-190b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibert, M.K., Jr.; Sarkar, A.; Chagari, B.; Roig-Laboy, C.; Saha, S.; Bednarek, S.; Kefas, B.; Hanif, F.; Hudson, K.; Dube, C.; et al. Transcribed Ultraconserved Regions in Cancer. Cells 2022, 11, 1684. https://doi.org/10.3390/cells11101684
Gibert MK Jr., Sarkar A, Chagari B, Roig-Laboy C, Saha S, Bednarek S, Kefas B, Hanif F, Hudson K, Dube C, et al. Transcribed Ultraconserved Regions in Cancer. Cells. 2022; 11(10):1684. https://doi.org/10.3390/cells11101684
Chicago/Turabian StyleGibert, Myron K., Jr., Aditya Sarkar, Bilhan Chagari, Christian Roig-Laboy, Shekhar Saha, Sylwia Bednarek, Benjamin Kefas, Farina Hanif, Kadie Hudson, Collin Dube, and et al. 2022. "Transcribed Ultraconserved Regions in Cancer" Cells 11, no. 10: 1684. https://doi.org/10.3390/cells11101684
APA StyleGibert, M. K., Jr., Sarkar, A., Chagari, B., Roig-Laboy, C., Saha, S., Bednarek, S., Kefas, B., Hanif, F., Hudson, K., Dube, C., Zhang, Y., & Abounader, R. (2022). Transcribed Ultraconserved Regions in Cancer. Cells, 11(10), 1684. https://doi.org/10.3390/cells11101684








