Gluconate-Lactobionate-Dextran Perfusion Solutions Attenuate Ischemic Injury and Improve Function in a Murine Cardiac Transplant Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Heterotopic Cardiac Transplantation
2.2.1. Donor Procedure
2.2.2. Recipient Procedure
2.3. Preservation Solutions and Experimental Groups
2.4. Functional Assessment
2.5. Histopathology and Immunohistochemistry
2.6. Serum Collection and Enzyme-Linked Immunosorbent Assay
2.7. Quantitative Polymerase Chain Reaction
2.8. Statistical Analysis
3. Results
3.1. Rebeating Time and Functional Recovery
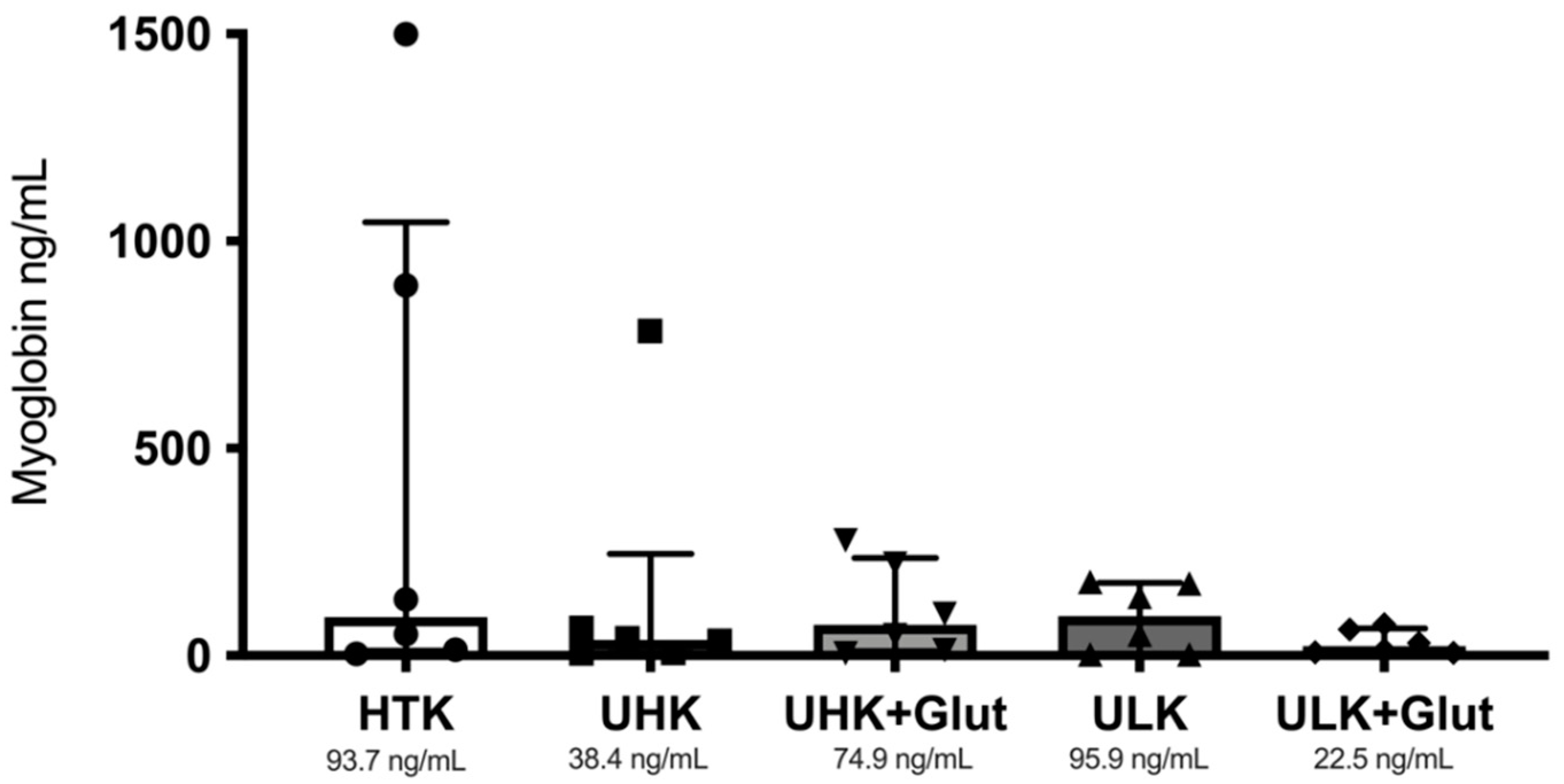
3.2. Serum Markers
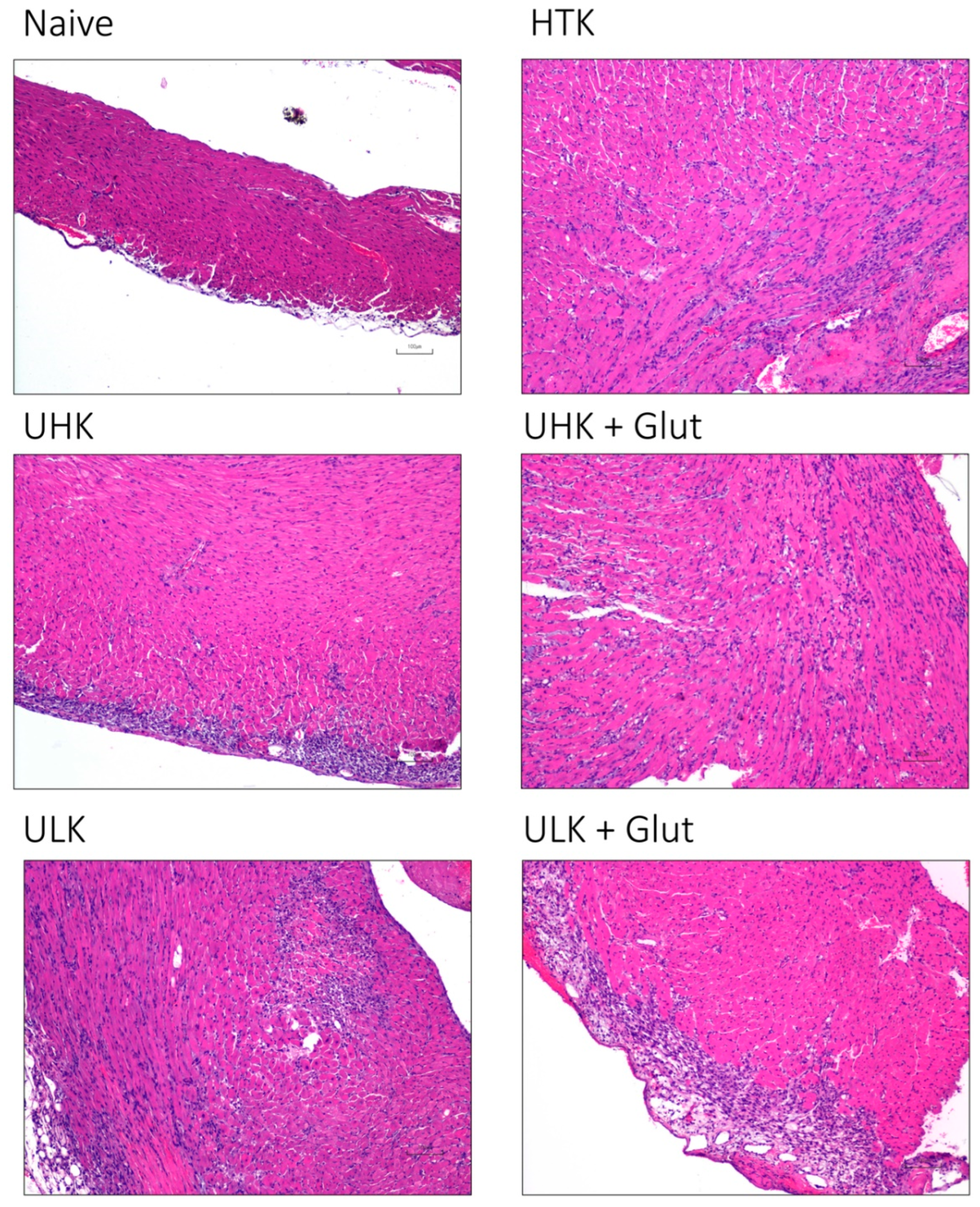
3.3. Histomorphologic Assessment
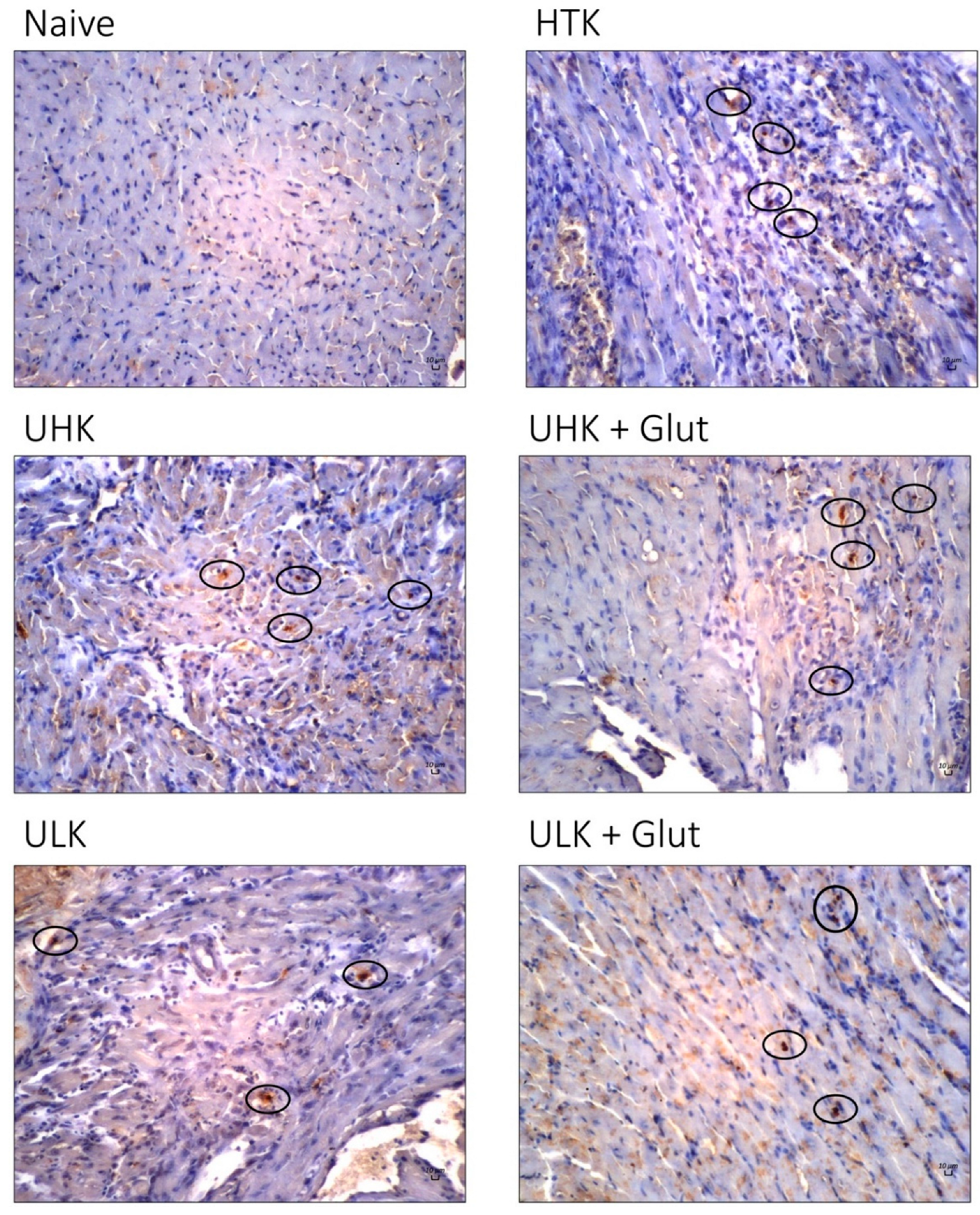
3.4. Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.; et al. The Promise of Organ and Tissue Preservation to Transform Medicine HHS Public Access Author Manuscript. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Transplant Trends–UNOS. Available online: https://unos.org/data/transplant-trends/ (accessed on 31 August 2019).
- Ward, A.; Klassen, D.K.; Franz, K.M.; Giwa, S.; Lewis, J.K. Social, Economic, and Policy Implications of Organ Preservation Advances. Curr. Opin. Organ Transplant. 2018, 23, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.K.; Bischof, J.C.; Braslavsky, I.; Brockbank, K.G.M.; Fahy, G.M.; Fuller, B.J.; Rabin, Y.; Tocchio, A.; Woods, E.J.; Wowk, B.G.; et al. The Grand Challenges of Organ Banking: Proceedings from the First Global Summit on Complex Tissue Cryopreservation. Cryobiology 2016, 72, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Messner, F.; Guo, Y.; Etra, J.W.; Brandacher, G. Emerging Technologies in Organ Preservation, Tissue Engineering and Regenerative Medicine: A Blessing or Curse for Transplantation? Transpl. Int. 2019, 32, tri.13432. [Google Scholar] [CrossRef]
- Agarwal, A.; Murdock, P.; Fridell, J.A. Comparison of Histidine-Tryptophan Ketoglutarate Solution and University of Wisconsin Solution in Prolonged Cold Preservation of Kidney Allografts. Transplantation 2006, 81, 480–482. [Google Scholar] [CrossRef]
- Parsons, R.F.; Guarrera, J.V. Preservation Solutions for Static Cold Storage of Abdominal Allografts: Which Is Best? Curr. Opin. Organ Transplant. 2014, 19, 100–107. [Google Scholar] [CrossRef]
- Fridell, J.A.; Mangus, R.S.; Powelson, J.A. Histidine-Tryptophan-Ketoglutarate for Pancreas Allograft Preservation: The Indiana University Experience. Am. J. Transplant. 2010, 10, 1284–1289. [Google Scholar] [CrossRef]
- Voigt, M.R.; Delario, G.T. Perspectives on Abdominal Organ Preservation Solutions: A Comparative Literature Review. Prog. Transpl. 2013, 23, 383–391. [Google Scholar] [CrossRef]
- Rauen, U.; de Groot, H. Inherent Toxicity of Organ Preservation Solutions to Cultured Hepatocytes. Cryobiology 2008, 56, 88–92. [Google Scholar] [CrossRef]
- Rauen, U.; de Groot, H. Mammalian Cell Injury Induced by Hypothermia—The Emerging Role for Reactive Oxygen Species. Biol. Chem. 2002, 383, 477–488. [Google Scholar] [CrossRef]
- Messner, F.; Grahammer, J.; Hautz, T.; Brandacher, G.; Schneeberger, S. Ischemia/Reperfusion Injury in Vascularized Tissue Allotransplantation: Tissue Damage and Clinical Relevance. Curr. Opin. Organ Transplant. 2016, 21, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Baust, J.M.; Snyder, K.K.; van Buskirk, R.G.; Baust, J.G. Assessment of the Impact of Post-Thaw Stress Pathway Modulation on Cell Recovery Following Cryopreservation in a Hematopoietic Progenitor Cell Model. Cells 2022, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, R.; Cardini, B.; Kofler, M.; Ritschl, P.; Oellinger, R.; Aigner, F.; Sucher, R.; Schneeberger, S.; Pratschke, J.; Brandacher, G.; et al. Murine Cervical Heart Transplantation Model Using a Modified Cuff Technique. J. Vis. Exp. 2014, 92, e50753. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Schneeberger, S.; Seiler, R.; Brandacher, G.; Mark, W.; Steurer, W.; Saks, V.; Usson, Y.; Margreiter, R.; Gnaiger, E. Mitochondrial Defects and Heterogeneous Cytochrome c Release after Cardiac Cold Ischemia and Reperfusion. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H1633–H1641. [Google Scholar] [CrossRef] [PubMed]
- Gong, W. (Ed.) Rodent Transplant Medicine; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Göser, S.; Andrassy, M.; Buss, S.J.; Leuschner, F.; Volz, C.H.; Öttl, R.; Zittrich, S.; Blaudeck, N.; Hardt, S.E.; Pfitzer, G.; et al. Cardiac Troponin I but Not Cardiac Troponin T Induces Severe Autoimmune Inflammation in the Myocardium. Circulation 2006, 114, 1693–1702. [Google Scholar] [CrossRef]
- Campbell, L.H.; Taylor, M.J.; Brockbank, K.G.M. Development of Pancreas Storage Solutions: Initial Screening of Cytoprotective Supplements for β-Cell Survival and Metabolic Status after Hypothermic Storage. Biopreserv. Biobank. 2013, 11, 12–18. [Google Scholar] [CrossRef]
- Campbell, L.H.; Taylor, M.J.; Brockbank, K.G.M. Survey of Apoptosis after Hypothermic Storage of a Pancreatic β-Cell Line. Biopreserv. Biobank. 2016, 14, 271–278. [Google Scholar] [CrossRef]
- Taylor, M.J.; Rhee, P.; Chen, Z.; Alam, H.B. Design of Preservation Solutions for Universal Tissue Preservation in Vivo: Demonstration of Efficacy in Preclinical Models of Profound Hypothermic Cardiac Arrest. Transplant. Proc. 2005, 37, 303–307. [Google Scholar] [CrossRef]
- Taylor, M.J.; Baicu, S.C. Current State of Hypothermic Machine Perfusion Preservation of Organs: The Clinical Perspective. Cryobiology 2010, 60, S20–S35. [Google Scholar] [CrossRef]
- Baicu, S.C.; Taylor, M.J.; Brockbank, K.G.M. The Role of Preservation Solution on Acid-Base Regulation during Machine Perfusion of Kidneys. Clin. Transplant. 2006, 20, 113–121. [Google Scholar] [CrossRef]
- Wu, K.; Türk, T.R.; Rauen, U.; Su, S.; Feldkamp, T.; De Groot, H.; Wiswedel, I.; Baba, H.A.; Kribben, A.; Witzke, O. Prolonged Cold Storage Using a New Histidine-Tryptophan-Ketoglutarate-Based Preservation Solution in Isogeneic Cardiac Mouse Grafts. Eur. Heart J. 2011, 32, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Hendgen-Cotta, U.B.; Kelm, M.; Rassaf, T. A Highlight of Myoglobin Diversity: The Nitrite Reductase Activity during Myocardial Ischemia-Reperfusion. Nitric Oxide-Biol. Chem. 2010, 22, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hendgen-Cotta, U.B.; Kelm, M.; Rassaf, T. Myoglobin Functions in the Heart. Free. Radic. Biol. Med. 2014, 73, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.K.; Little, T.; Zaki Masud, A.R.; Klocke, F.J. Patterns of Myoglobin Release after Reperfusion of Injured Myocardium. Circulation 1985, 72, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Botta, A.; Gottfred, S.; Nusrat, A.; Laher, I.; Ghosh, S. Glutathione Administration Reduces Mitochondrial Damage and Shifts Cell Death from Necrosis to Apoptosis in Ageing Diabetic Mice Hearts during Exercise. Br. J. Pharmacol. 2014, 171, 5345–5360. [Google Scholar] [CrossRef]
- Cheung, P.Y.; Wang, W.; Schulz, R. Glutathione Protects against Myocardial Ischemia-Reperfusion Injury by Detoxifying Peroxynitrite. J. Mol. Cell. Cardiol. 2000, 32, 1669–1678. [Google Scholar] [CrossRef]
- Kupatt, C.; Hinkel, R.; Horstkotte, J.; Deiß, M.; Von Brühl, M.L.; Bilzer, M.; Boekstegers, P. Selective Retroinfusion of GSH and Cariporide Attenuates Myocardial Ischemia-Reperfusion Injury in a Preclinical Pig Model. Cardiovasc. Res. 2004, 61, 530–537. [Google Scholar] [CrossRef][Green Version]
- Schneeberger, S.; Kuznetsov, A.V.; Seiler, R.; Renz, O.; Meusburger, H.; Mark, W.; Brandacher, G.; Margreiter, R.; Gnaiger, E. Mitochondrial Ischemia-Reperfusion Injury of the Transplanted Rat Heart: Improved Protection by Preservation versus Cardioplegic Solutions. Shock 2008, 30, 365–371. [Google Scholar] [CrossRef]
- Plenter, R.J.; Grazia, T.J. Murine Heterotopic Heart Transplant Technique. J. Vis. Exp. 2014, 89, e51511. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Bolli, R.; Canty, J.M.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for Experimental Models of Myocardial Ischemia and Infarction. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef]
- Silvis, M.J.M.; Kaffka Genaamd Dengler, S.E.; Odille, C.A.; Mishra, M.; van der Kaaij, N.P.; Doevendans, P.A.; Sluijter, J.P.G.; de Kleijn, D.P.V.; de Jager, S.C.A.; Bosch, L.; et al. Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success. Front. Immunol. 2020, 11, 599511. [Google Scholar] [CrossRef] [PubMed]



| Base Components | Mechanism of Action | Supplier | Cat # | UHK mmol/L | ULK mmol/L | Base Components | HTK mmol/L |
|---|---|---|---|---|---|---|---|
| K-Gluconate | Large ions that balance intracellular ions when ion pumps are off in cold. Prevents cell swelling. | Sigma | G-4500 | 50 | 25 | Mannitol | 30 |
| Na-Gluconate | Sigma | G-9005 | 20 | 45 | |||
| Lactobionic acid | Sigma | L-2398 | 30 | 30 | |||
| NaOH (1N soln) | Ions and pH (final pH adjusted using HCl and NaOH). | Fisher | SS266 | 40 | 50 | NaCl | 15 |
| KOH (1N soln) | Fisher | SP208 | 15 | 0 | KCl | 9 | |
| NaH2PO4 | Sigma | S-0751 | 2.5 | 0 | |||
| KH2PO4 | Sigma | P-5655 | 0 | 2.5 | |||
| NaHCO3 | Fisher | BP328-1 | 0 | 5 | |||
| KHCO3 | Sigma | P-4913 | 5 | 0 | |||
| MgCl2-6H2O | Sigma | M-9272 | 15 | 15 | MgCl2-6H2O | 4 | |
| CaCl2-2H2O | Sigma | C-3881 | 0.05 | 0.05 | CaCl2-2H2O | 0.015 | |
| HEPES | Zwitterion pH buffer effective in the cold, prevents acidosis. | Sigma | H-3375 | 35 | 35 | Ketoglutarate | 1 |
| Glucose | Substrates for regeneration of high energy compounds in the cold and stimulation of recovery upon rewarming and reperfusion. | Sigma | G-5767 | 5 | 5 | Tryptophan | 2 |
| Sucrose | Sigma | S-5016 | 15 | 15 | Histidine | 198 | |
| Mannitol | Sigma | M-1902 | 25 | 25 | |||
| Adenosine | Sigma | A-4036 | 2 | 2 | |||
| Glutathione | In supplemented groups. | Sigma | G-4251 | 3 | 3 | ||
| Dextran 40,000 or 70,000 | Oncotic pressure, prevents cell swelling. | MP Biomedical | 6% | 6% | |||
| Group No. | Strain Combination | Ischemic Time (h) | Perfusate | Number |
|---|---|---|---|---|
| 1 | Balb/c-Balb/c | 18 h | HTK | 7 |
| 2 | Balb/c-Balb/c | 18 h | UHK | 7 |
| 3 | Balb/c-Balb/c | 18 h | UHK + Glut | 7 |
| 4 | Balb/c-Balb/c | 18 h | ULK | 9 |
| 5 | Balb/c-Balb/c | 18 h | ULK + Glut | 9 |
| score 0 | no beating |
| score 1 | fibrillations, no real contractions |
| score 2 | weak or partial contractions |
| score 3 | homogenous contractions of both ventricles at reduced frequency and intensity |
| score 4 | normal contraction intensity and frequency |
| HTK | UHK | UHK + Glut | ULK | ULK + Glut | |
|---|---|---|---|---|---|
| Median | 119.5 | 56 | 44 | 45 | 47 |
| IQR | 94.5–173.5 | 40–81 | 37.5–58 | 43.5–57 | 35–57 |
| HTK | UHK | UHK + Glut | ULK | ULK + Glut | |
|---|---|---|---|---|---|
| 2 min | 1 [0–1] | 1 [0.25–2] | 1 [1–1] | 2 [1.25–2] | 2 [1–2] |
| p-value | Ref. | 0.310 | 0.440 | <0.001 | 0.014 |
| 5 min | 1 [1–2] | 2 [2–2] | 2 [1.25–2] | 2 [1.25–3] | 2 [1–3] |
| p-value | Ref. | 0.054 | 0.360 | 0.015 | 0.072 |
| 7 min | 2 [1.25–2] | 2.5 [2–3] | 2 [1–3] | 2.5 [2–3] | 1 [1–2.75] |
| p-value | Ref. | 0.590 | 0.990 | 0.450 | 0.990 |
| POD 3 | 1.5 [0–3] | 4 [3–4] | 3.5 [3–4] | 3 [3–4] | 3 [2–4] |
| p-value | Ref. | <0.001 | 0.002 | 0.009 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Messner, F.; Beck, S.E.; Iglesias Lozano, M.; Schwelberger, H.; Zhang, Y.; Kammers, K.; Oh, B.C.; Greene, E.D.; Brandacher, G.; et al. Gluconate-Lactobionate-Dextran Perfusion Solutions Attenuate Ischemic Injury and Improve Function in a Murine Cardiac Transplant Model. Cells 2022, 11, 1653. https://doi.org/10.3390/cells11101653
Guo Y, Messner F, Beck SE, Iglesias Lozano M, Schwelberger H, Zhang Y, Kammers K, Oh BC, Greene ED, Brandacher G, et al. Gluconate-Lactobionate-Dextran Perfusion Solutions Attenuate Ischemic Injury and Improve Function in a Murine Cardiac Transplant Model. Cells. 2022; 11(10):1653. https://doi.org/10.3390/cells11101653
Chicago/Turabian StyleGuo, Yinan, Franka Messner, Sarah E. Beck, Marcos Iglesias Lozano, Hubert Schwelberger, Yichuan Zhang, Kai Kammers, Byoung Chol Oh, Elizabeth D. Greene, Gerald Brandacher, and et al. 2022. "Gluconate-Lactobionate-Dextran Perfusion Solutions Attenuate Ischemic Injury and Improve Function in a Murine Cardiac Transplant Model" Cells 11, no. 10: 1653. https://doi.org/10.3390/cells11101653
APA StyleGuo, Y., Messner, F., Beck, S. E., Iglesias Lozano, M., Schwelberger, H., Zhang, Y., Kammers, K., Oh, B. C., Greene, E. D., Brandacher, G., & Brockbank, K. G. M. (2022). Gluconate-Lactobionate-Dextran Perfusion Solutions Attenuate Ischemic Injury and Improve Function in a Murine Cardiac Transplant Model. Cells, 11(10), 1653. https://doi.org/10.3390/cells11101653






