Cytochalasin B Modulates Nanomechanical Patterning and Fate in Human Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. hASCs: Harvesting and Culture
2.2. hASC Characterization
2.3. Cytochalasin B treatments
2.4. Resazurin-Based Assay
2.5. Cell Count
2.6. RNA Extraction, RT-PCR and Real Time-PCR
2.7. Morphological Analysis and MTT Assay
2.8. Live Cell Imaging
2.9. Immunofluorescence of Cytoskeletal Markers
2.10. Atomic Force Microscopy
2.11. Adipogenic Commitment: Immunofluorescence and Oil Red O (O.R.O) Staining
2.12. Statistical Analysis
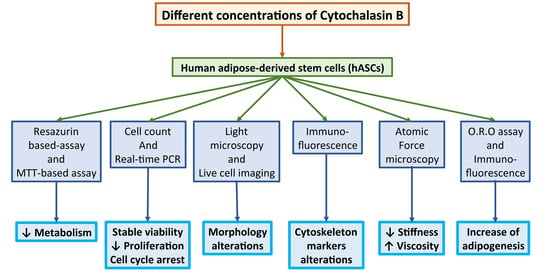
3. Results
3.1. hASC Surface Markers and Trilineage Differentiative Potential
3.2. Effects of a Prolonged Treatment with Cytochalasin B in hASCs
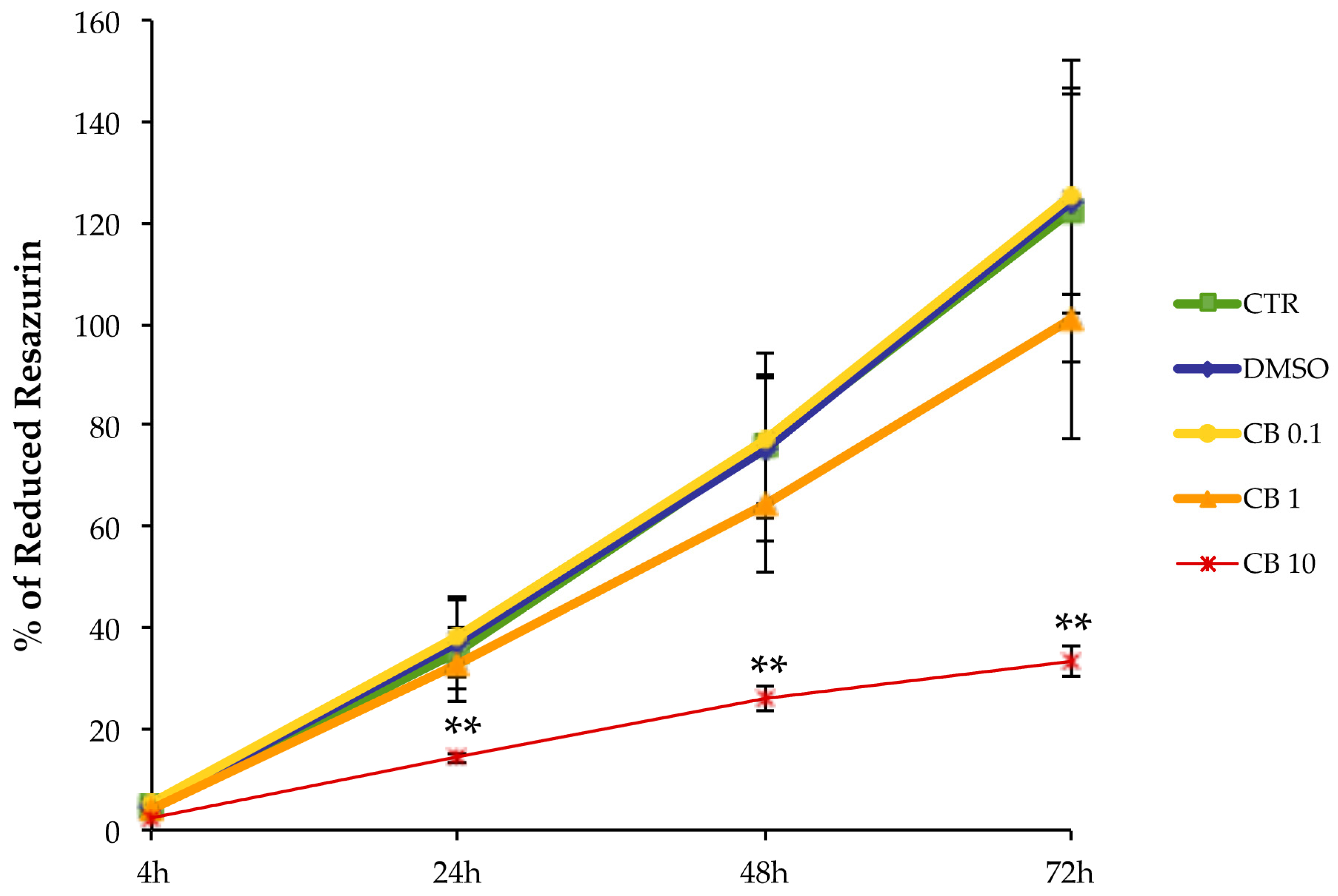
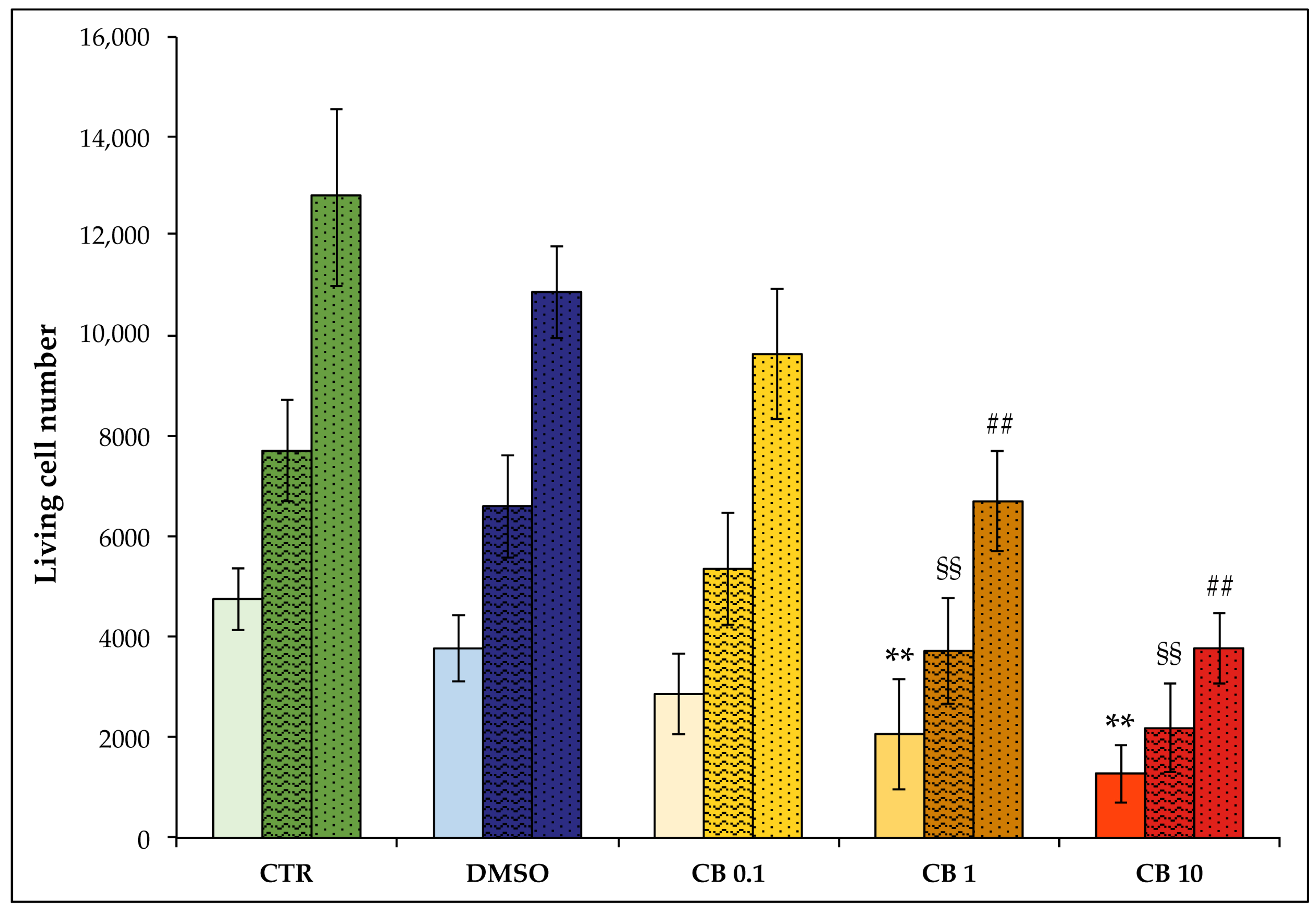
3.3. Effects of Cytochalasin B on hASC Viability, Growth Rate, and Cell Cycle Progression
3.4. Effects of Cytochalasin B on hASC Morphology and Metabolism
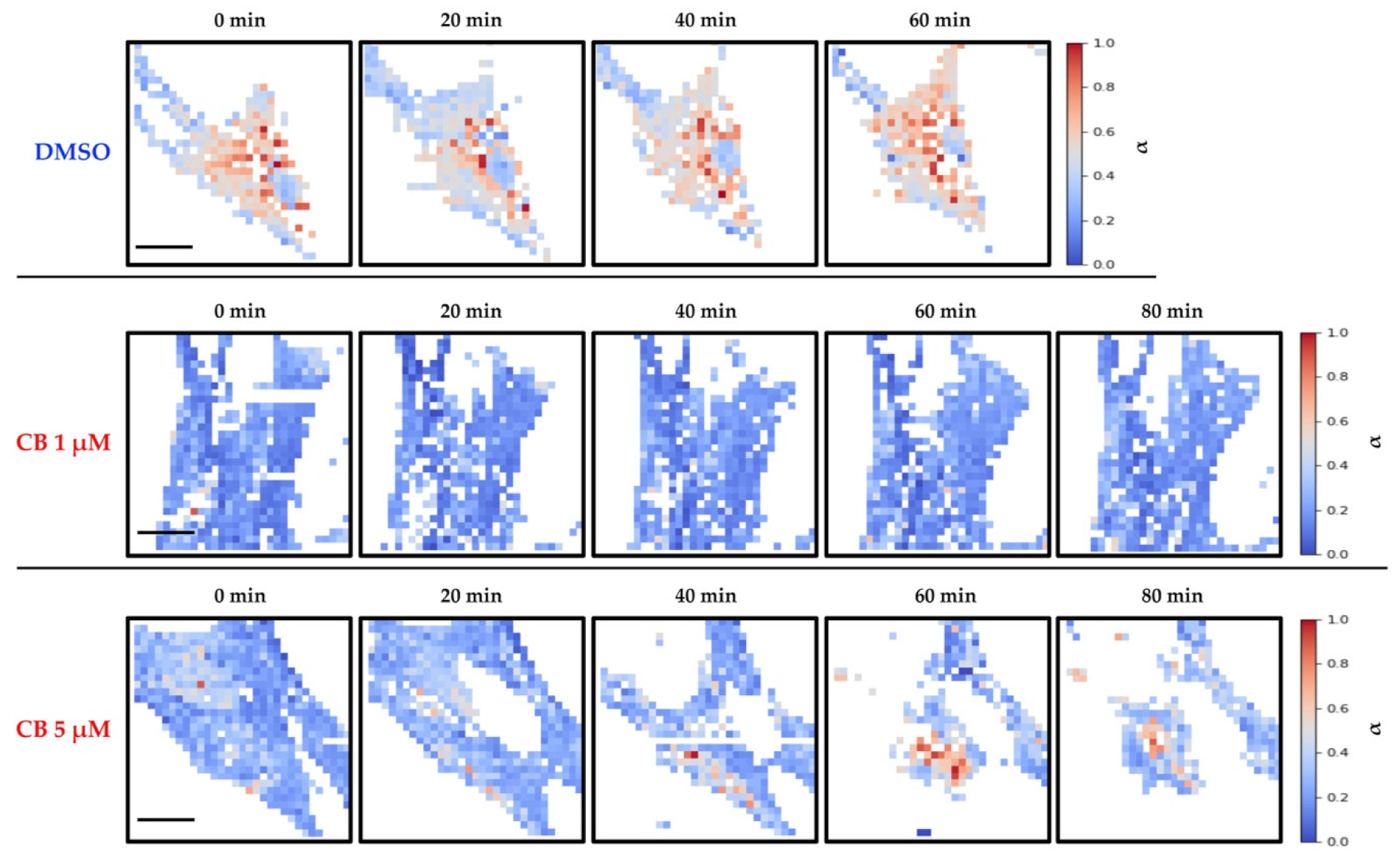
3.5. Live Cell Imaging of hASCs Treated with Cytochalasin B
3.6. Effects of Cytochalasin B on the Localization and Expression of Cytoskeletal Markers in hASCs
3.7. Effects of Cytochalasin B on the Mechanical Properties of hASCs
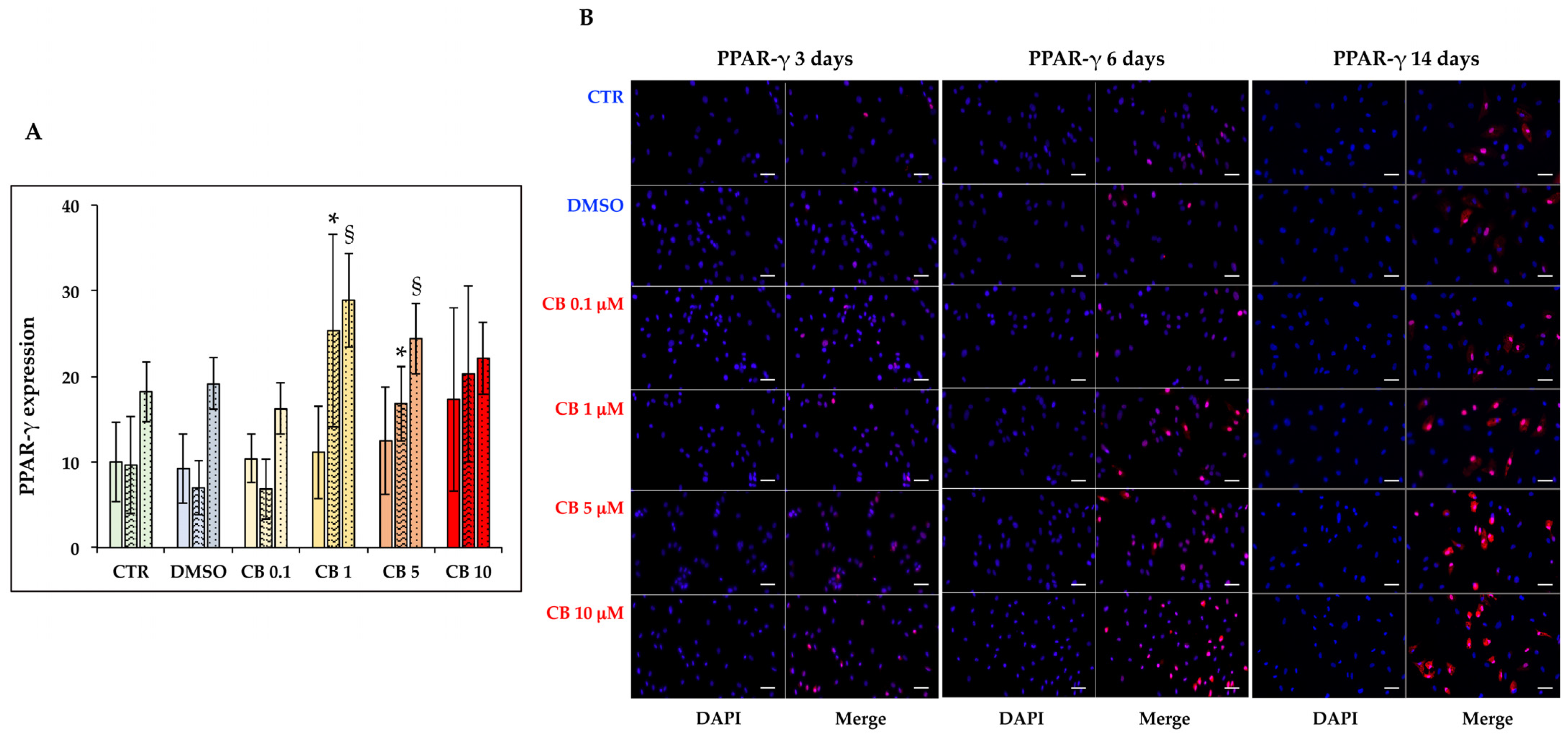
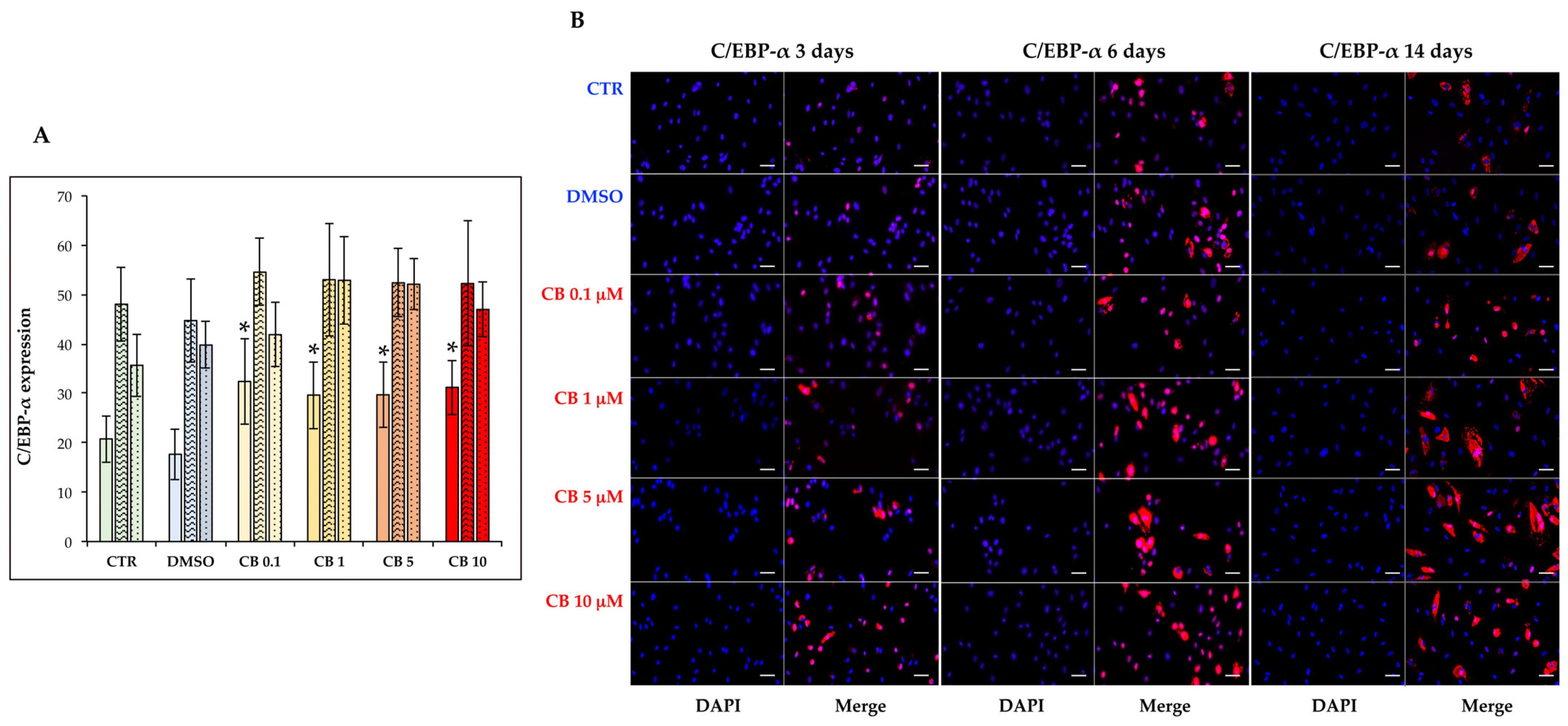
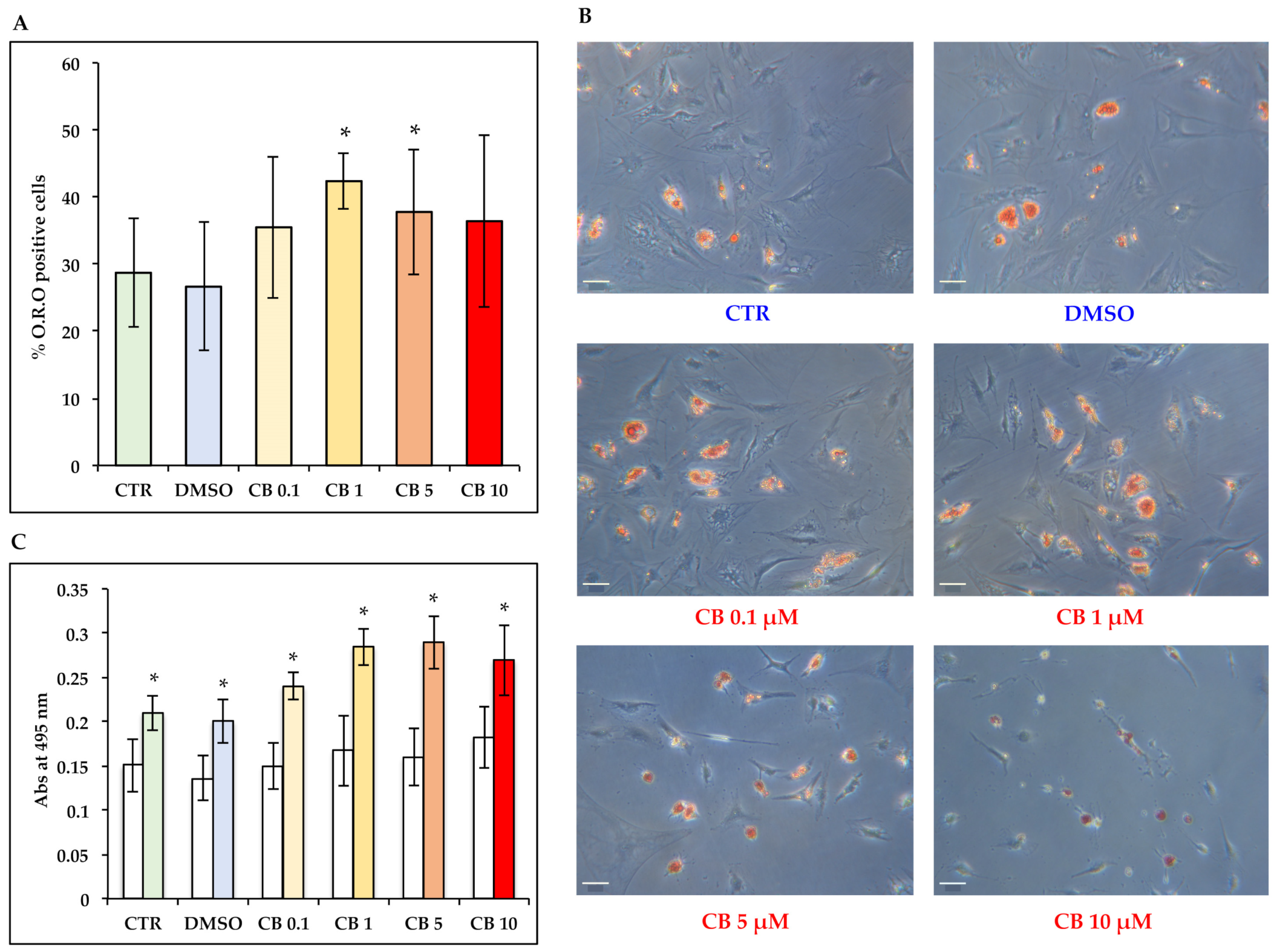
3.8. Effects of Cytochalasin B on the Adipogenic Ability of hASCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ingber, D.E. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997, 59, 575–599. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Ghosh, S.; Hirata, K.; Fujita, D.; Bandyopadhyay, A. Multi-level memory-switching properties of a single brain microtubule. Appl. Phys. Lett. 2013, 102, 123701. [Google Scholar] [CrossRef]
- Sahu, S.; Ghosh, S.; Fujita, D.; Bandyopadhyay, A. Live visualizations of single isolated tubulin protein self-assembly via tunneling current: Effect of electromagnetic pumping during spontaneous growth of microtubule. Sci. Rep. 2014, 4, 7303. [Google Scholar] [CrossRef] [PubMed]
- Facchin, F.; Canaider, S.; Tassinari, R.; Zannini, C.; Bianconi, E.; Taglioli, V.; Olivi, E.; Cavallini, C.; Tausel, M.; Ventura, C. Physical energies to the rescue of damaged tissues. World J. Stem Cells 2019, 11, 297–321. [Google Scholar] [CrossRef]
- De, R.; Zemel, A.; Safran, S.A. Theoretical concepts and models of cellular mechanosensing. Methods Cell Biol. 2010, 98, 143–175. [Google Scholar] [CrossRef]
- Zemel, A.; Rehfeldt, F.; Brown, A.E.; Discher, D.E.; Safran, S.A. Cell shape, spreading symmetry and the polarization of stress-fibers in cells. J. Phys. Condens. Matter 2010, 22, 194110. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Rodriguez, J.P.; Gonzalez, M.; Rios, S.; Cambiazo, V. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell. Biochem. 2004, 93, 721–731. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Luo, W.; Shitaye, H.; Friedman, M.; Bennett, C.N.; Miller, J.; MacDougald, O.A.; Hankenson, K.D. Disruption of cell matrix interaction by heparin enhances mesenchymal progenitor adipocyte differentiation. Exp. Cell. Res. 2008, 314, 3382–3391. [Google Scholar] [CrossRef]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef]
- Woods, A.; Wang, G.; Beier, F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 2006, 280, 11626–11634. [Google Scholar] [CrossRef]
- Docheva, D.; Padula, D.; Popov, C.; Mutschler, W.; Clausen-Schaumann, H.; Schieker, M. Researching into the cellular shape, volume and elasticity of mesenchymal stem cells, osteoblasts and osteosarcoma cells by atomic force microscopy. J. Cell. Mol. Med. 2008, 12, 537–552. [Google Scholar] [CrossRef]
- Trendowski, M.; Wong, V.; Zoino, J.N.; Christen, T.D.; Gadeberg, L.; Sansky, M.; Fondy, T.P. Preferential enlargement of leukemia cells using cytoskeletal-directed agents and cell cycle growth control parameters to induce sensitivity to low frequency ultrasound. Cancer Lett. 2015, 360, 160–170. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Armstrong, J.J.; Speake, R.N.; Turner, W.B. The cytochalasins, a new class of biologically active mould metabolites. Chem. Commun. 1967, 1, 26–27. [Google Scholar] [CrossRef]
- Rothweiler, W.; Tamm, C. Isolation and structure of Phomin. Experientia 1966, 22, 750–752. [Google Scholar] [CrossRef]
- Carter, S.B. Effects of cytochalasins on mammalian cells. Nature 1967, 213, 261–264. [Google Scholar] [CrossRef]
- Yahara, I.; Harada, F.; Sekita, S.; Yoshihira, K.; Natori, S. Correlation between effects of 24 different cytochalasins on cellular structures and cellular events and those on actin in vitro. J. Cell Biol. 1982, 92, 69–78. [Google Scholar] [CrossRef]
- Downey, G.P.; Grinstein, S.; Sue, A.Q.A.; Czaban, B.; Chan, C.K. Volume regulation in leukocytes: Requirement for an intact cytoskeleton. J. Cell. Physiol. 1995, 163, 96–104. [Google Scholar] [CrossRef]
- Cassimeris, L.; McNeill, H.; Zigmond, S.H. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J. Cell Biol. 1990, 110, 1067–1075. [Google Scholar] [CrossRef]
- Stevenson, B.R.; Begg, D.A. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J. Cell Sci. 1994, 107, 367–375. [Google Scholar] [CrossRef]
- Franki, N.; Ding, G.; Gao, Y.; Hays, R.M. Effect of cytochalasin D on the actin cytoskeleton of the toad bladder epithelial cell. Am. J. Physiol. 1992, 263, C995–C1000. [Google Scholar] [CrossRef]
- Matthews, J.B.; Smith, J.A.; Mun, E.C.; Sicklick, J.K. Osmotic regulation of intestinal epithelial Na(+)-K(+)-Cl− cotransport: Role of Cl- and Factin. Am. J. Physiol. 1998, 274, C697–C706. [Google Scholar] [CrossRef]
- Van Goietsenoven, G.; Mathieu, V.; Andolfi, A.; Cimmino, A.; Lefranc, F.; Kiss, R.; Evidente, A. In vitro growth inhibitory effects of cytochalasins and derivatives in cancer cells. Planta Med. 2011, 77, 711–717. [Google Scholar] [CrossRef]
- Trendowski, M. Using cytochalasins to improve current chemotherapeutic approaches. Anticancer Agents Med. Chem. 2015, 15, 327–335. [Google Scholar] [CrossRef]
- MacLean-Fletcher, S.; Pollard, T.D. Mechanism of action of cytochalasin B on actin. Cell 1980, 20, 329–341. [Google Scholar] [CrossRef]
- Flanagan, M.D.; Lin, S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J. Biol. Chem. 1980, 255, 835–838. [Google Scholar] [CrossRef]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett. 2010, 287, 231–239. [Google Scholar] [CrossRef]
- Trendowski, M.; Yu, G.; Wong, V.; Acquafondata, C.; Christen, T.; Fondy, T.P. The real deal: Using cytochalasin B in sonodynamic therapy to preferentially damage leukemia cells. Anticancer Res. 2014, 34, 2195–2202. [Google Scholar]
- Simpson, P.B.; Armstrong, R.C. Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia 1999, 26, 22–35. [Google Scholar] [CrossRef]
- Corradi, M.P.; Jelinek, D.F.; Ramberg, J.E.; Lipsky, P.E. Development of a cell with dendritic morphology from a precursor of B lymphocyte lineage. J. Immunol. 1987, 138, 2075–2081. [Google Scholar] [PubMed]
- Sen, B.; Uzer, G.; Samsonraj, R.M.; Xie, Z.; McGrath, C.; Styner, M.; Dudakovic, A.; van Wijnen, A.J.; Rubin, J. Intranuclear Actin Structure Modulates Mesenchymal Stem Cell Differentiation. Stem Cells 2017, 35, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Paradise, C.R.; Dudakovic, A.; Sen, B.; Nair, A.A.; Dietz, A.B.; Deyle, D.R.; Cool, S.M.; Rubin, J.; van Wijnen, A.J. Validation of Osteogenic Properties of Cytochalasin D by High-Resolution RNA-Sequencing in Mesenchymal Stem Cells Derived from Bone Marrow and Adipose Tissues. Stem Cells Dev. 2018, 27, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Qu, R.; Jiang, X.; Yang, Y.; Sun, B.; Huang, X.; Zhou, Z.; Ouyang, J.; Zhong, S.; Dai, J. Spatial organization and crosstalk of vimentin and actin stress fibers regulate the osteogenic differentiation of human adipose-derived stem cells. FASEB J. 2021, 35, e21175. [Google Scholar] [CrossRef]
- Sonowal, H.; Kumar, A.; Bhattacharyya, J.; Gogoi, P.K.; Jaganathan, B.G. Inhibition of actin polymerization decreases osteogeneic differentiation of mesenchymal stem cells through p38 MAPK pathway. J. Biomed. Sci. 2013, 20, 71. [Google Scholar] [CrossRef]
- Sun, B.; Qu, R.; Fan, T.; Yang, Y.; Jiang, X.; Khan, A.U.; Zhou, Z.; Zhang, J.; Wei, K.; Ouyang, J.; et al. Actin polymerization state regulates osteogenic differentiation in human adipose-derived stem cells. Cell. Mol. Bio. Lett. 2021, 26, 15. [Google Scholar] [CrossRef]
- Keller, V.; Deiwick, A.; Pflaum, M.; Schlie-Wolter, S. Correlation between ECM guidance and actin polymerization on osteogenic differentiation of human adipose-derived stem cells. Exp. Cell Res. 2016, 347, 339–349. [Google Scholar] [CrossRef]
- Schiller, Z.A.; Schiele, N.R.; Sims, J.K.; Lee, K.; Kuo, C.K. Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem Cell Res. Ther. 2013, 4, 79. [Google Scholar] [CrossRef]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef]
- Sawangmake, C.; Pavasant, P.; Chansiripornchai, P.; Osathanon, T. High glucose condition suppresses neurosphere formation by human periodontal ligament-derived mesenchymal stem cells. J. Cell. Biochem. 2014, 115, 928–939. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem cells applications in regenerative medicine and disease therapeutics. Int. J. Cell. Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef]
- Laurent, L.C.; Ulitsky, I.; Slavin, I.; Tran, H.; Schork, A.; Morey, R.; Lynch, C.; Harness, J.V.; Lee, S.; Barrero, M.J.; et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 2011, 8, 106–118. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods. Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [CrossRef]
- Facchin, F.; Bianconi, E.; Romano, M.; Impellizzeri, A.; Alviano, F.; Maioli, M.; Canaider, S.; Ventura, C. Comparison of oxidative stress effects on senescence patterning of human adult and perinatal tissue-derived stem cells in short and long-term cultures. Int. J. Med. Sci. 2018, 15, 1486–1501. [Google Scholar] [CrossRef]
- Facchin, F.; Vitale, L.; Bianconi, E.; Piva, F.; Frabetti, F.; Strippoli, P.; Casadei, R.; Pelleri, M.C.; Piovesan, A.; Canaider, S. Complexity of bidirectional transcription and alternative splicing at human RCAN3 locus. PLoS ONE 2011, 6, e24508. [Google Scholar] [CrossRef]
- Beraudi, A.; Bianconi, E.; Catalani, S.; Canaider, S.; De Pasquale, D.; Apostoli, P.; Bordini, B.; Stea, S.; Toni, A.; Facchin, F. In vivo response of heme-oxygenase-1 to metal ions released from metal-on-metal hip prostheses. Mol. Med. Rep. 2016, 14, 474–480. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Delitala, A.; Lotti Margotti, M.; Bagella, L.; Fontani, V.; Ventura, C. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age 2014, 36, 9–20. [Google Scholar] [CrossRef][Green Version]
- Ragazzini, G.; Mescola, A.; Corsi, L.; Alessandrini, A. Fabrication of a low-cost on-stage cell incubator with full automation. J. Biol. Educ. 2018, 53, 165–173. [Google Scholar] [CrossRef]
- Butt, H.J.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1–7. [Google Scholar] [CrossRef]
- Rico, F.; Roca-Cusachs, P.; Gavara, N.; Farré, R.; Rotger, M.; Navajas, D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 72, 21914. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Wang, W.H.; Hardy, S.D.; Geahlen, R.L.; Raman, A. Measuring nanoscale viscoelastic parameters of cells directly from AFM force-displacement curves. Sci. Rep. 2017, 7, 1541. [Google Scholar] [CrossRef]
- Marcon, B.H.; Shigunov, P.; Spangenberg, L.; Pereira, I.T.; de Aguiar, A.M.; Amorín, R.; Rebelatto, C.K.; Correa, A.; Dallagiovanna, B. Cell cycle genes are downregulated after adipogenic triggering in human adipose tissue-derived stem cells by regulation of mRNA abundance. Sci. Rep. 2019, 9, 5611. [Google Scholar] [CrossRef]
- Marquez, M.P.; Alencastro, F.; Madrigal, A.; Jimenez, J.L.; Blanco, G.; Gureghian, A.; Keagy, L.; Lee, C.; Liu, R.; Tan, L.; et al. The Role of Cellular Proliferation in Adipogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 1578–1595. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Radmacher, M. Studying the mechanics of cellular processes by atomic force microscopy. Methods Cell Biol. 2007, 83, 347–372. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Okajima, T.; Raman, A. Measuring viscoelasticity of soft biological samples using atomic force microscopy. Soft Matter 2020, 16, 64–81. [Google Scholar] [CrossRef]
- Lin, E.C.; Cantiello, H.F. A novel method to study the electrodynamic behavior of actin filaments. Evidence for cable-like properties of actin. Biophys. J. 1993, 65, 1371–1378. [Google Scholar] [CrossRef]
- Cantiello, H.F.; Patenaude, C.; Zaner, K. Osmotically induced electrical signals from actin filaments. Biophys. J. 1991, 59, 1284–1289. [Google Scholar] [CrossRef]
- Kobayasi, S.; Asai, H.; Oosawa, F. Electric birefringence of actin. Biochim. Biophys. Acta 1964, 88, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Levin, M. General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harb. Protoc. 2012, 2012, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Levin, M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012, 2012, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Levin, M. Bioelectric memory: Modeling resting potential bistability in amphibian embryos and mammalian cells. Theor. Biol. Med. Model 2015, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Willocq, V.; Pitcairn, E.J.; Lemire, J.M.; Paré, J.F.; Shi, N.Q.; McLaughlin, K.A.; Levin, M. HCN4 ion channel function is required for early events that regulate anatomical left-right patterning in a nodal and lefty asymmetric gene expression-independent manner. Biol. Open 2017, 6, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Pietak, A.; Willocq, V.; Ye, B.; Shi, N.Q.; Levin, M. HCN2 rescues brain defects by enforcing endogenous voltage pre-patterns. Nat. Commun. 2018, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Liskovykh, M.; Lee, N.C.; Larionov, V.; Kouprina, N. Moving toward a higher efficiency of microcell-mediated chromosome transfer. Mol. Ther. Methods Clin. Dev. 2016, 3, 16043. [Google Scholar] [CrossRef]
- Cho, Y.M.; Kim, J.H.; Kim, M.; Park, S.J.; Koh, S.H.; Ahn, H.S.; Kang, G.H.; Lee, J.B.; Park, K.S.; Lee, H.K. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS ONE 2012, 7, e32778. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Liou, C.W.; Chen, S.D.; Wang, P.W.; Chuang, J.H.; Tiao, M.M.; Hsu, T.Y.; Lin, H.Y.; Lin, T.K. Mitochondrial transfer from Wharton’s jelly mesenchymal stem cell to MERRF cybrid reduces oxidative stress and improves mitochondrial bioenergetics. Oxid. Med. Cell. Longev. 2017, 2017, 5691215. [Google Scholar] [CrossRef]
- Rotsch, C.; Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys. J. 2000, 78, 520–535. [Google Scholar] [CrossRef]
- Wu, H.W.; Kuhn, T.; Moy, V.T. Mechanical properties of L929 cells measured by atomic force microscopy: Effects of anticytoskeletal drugs and membrane crosslinking. Scanning 1998, 20, 389–397. [Google Scholar] [CrossRef]
- Grady, M.E.; Composto, R.J.; Eckmann, D.M. Cell elasticity with altered cytoskeletal architectures across multiple cell types. J. Mech. Behav. Biomed. Mater. 2016, 61, 197–207. [Google Scholar] [CrossRef]
- Kubiak, A.; Zieliński, T.; Pabijan, J.; Lekka, M. Nanomechanics in monitoring the effectiveness of drugs targeting the cancer cell cytoskeleton. Int. J. Mol. Sci. 2020, 21, 8786. [Google Scholar] [CrossRef]
- Garcia, P.D.; Guerrero, C.R.; Garcia, R. Time-resolved nanomechanics of a single cell under the depolymerization of the cytoskeleton. Nanoscale 2017, 9, 12051–12059. [Google Scholar] [CrossRef]
- Hecht, F.M.; Rheinlaender, J.; Schierbaum, N.; Goldmann, W.H.; Fabry, B.; Schäffer, T.E. Imaging viscoelastic properties of live cells by AFM: Power-law rheology on the nanoscale. Soft Matter 2015, 11, 4584–4591. [Google Scholar] [CrossRef]
- Yourek, G.; Hussain, M.A.; Mao, J.J. Cytoskeletal Changes of Mesenchymal Stem Cells During Differentiation. ASAIO J. 2007, 53, 219–228. [Google Scholar] [CrossRef]
- Müller, P.; Langenbach, A.; Kaminski, A.; Rychly, J. Modulating the Actin Cytoskeleton Affects Mechanically Induced Signal Transduction and Differentiation in Mesenchymal Stem Cells. PLoS ONE 2013, 8, e71283. [Google Scholar] [CrossRef]
- Xu, B.; Ju, Y.; Song, G. Role of p38, ERK1/2, focal adhesion kinase, RhoA/ROCK and cytoskeleton in the adipogenesis of human mesenchymal stem cells. J. Biosci. Bioeng. 2014, 117, 624–631. [Google Scholar] [CrossRef]
- Chen, L.; Hu, H.; Qiu, W.; Shi, K.; Kassem, M. Actin depolymerization enhances adipogenic differentiation in human stromal stem cells. Stem Cell Res. 2018, 29, 76–83. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Dudakovic, A.; Manzar, B.; Sen, B.; Dietz, A.B.; Cool, S.M.; Rubin, J.; van Wijnen, A.J. Osteogenic Stimulation of Human Adipose-Derived Mesenchymal Stem Cells Using a Fungal Metabolite That Suppresses the Polycomb Group Protein EZH2. Stem Cells Transl. Med. 2017, 7, 197–209. [Google Scholar] [CrossRef]
- Sen, B.; Xie, Z.; Uzer, G.; Thompson, W.R.; Styner, M.; Wu, X.; Rubin, J. Intranuclear Actin Regulates Osteogenesis. Stem Cells 2015, 33, 3065–3076. [Google Scholar] [CrossRef]
- Kocsis, Á.; Pasztorek, M.; Rossmanith, E.; Djinovic, Z.; Mayr, T.; Spitz, S.; Zirath, H.; Ertl, P.; Fiscjer, M.B. Dependence of mitochondrial function on the filamentous actin cytoskeleton in cultured mesenchymal stem cells treated with cytochalasin B. J. Biosci. Bioeng. 2021, 132, 310–320. [Google Scholar] [CrossRef]
- Su, X.; Zhou, H.; Bao, G.; Wang, J.; Liu, L.; Zheng, Q.; Guo, M.; Zhang, J. Nanomorphological and mechanical reconstruction of mesenchymal stem cells during early apoptosis detected by atomic force microscopy. Biol. Open 2020, 9, bio048108. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell. Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Canaider, S.; Pizzuti, V.; Pampanella, L.; Casadei, R.; Facchin, F.; Ventura, C. Herb-Derived Products: Natural Tools to Delay and Counteract Stem Cell Senescence. Stem Cells Int. 2020, 2020, 8827038. [Google Scholar] [CrossRef]
- Galderisi, U.; Giordano, A. Short Introduction to the Cell Cycle. In Cell Cycle Regulation and Differentation in Cardiovascular and Neural System; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Madsen, M.S.; Siersbæk, R.; Boergesen, M.; Nielsen, R.; Mandrup, S. Peroxisome proliferator-activated receptor γ and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell. Biol. 2014, 34, 939–954. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell. 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Mu, X.; Tseng, C.; Hambright, W.S.; Matre, P.; Lin, C.Y.; Chanda, P.; Chen, W.; Gu, J.; Ravuri, S.; Cui, Y.; et al. Cytoskeleton stiffness regulates cellular senescence and innate immune response in Hutchinson-Gilford Progeria Syndrome. Aging Cell 2020, 19, e13152. [Google Scholar] [CrossRef]
- Kiderlen, S.; Polzer, C.; Rädler, J.O.; Docheva, D.; Clausen-Schaumann, H.; Sudhop, S. Age related changes in cell stiffness of tendon stem/progenitor cells and a rejuvenating effect of ROCK-inhibition. Biochem. Biophys. Res. Commun. 2019, 509, 839–844. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell. 2017, 16, 518–528. [Google Scholar] [CrossRef]
- Shih, L.; Davis, M.J.; Winocour, S.J. The Science of Fat Grafting. Semin. Plast. Surg. 2020, 34, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Doornaert, M.; Colle, J.; De Maere, E.; Declercq, H.; Blondeel, P. Autologous fat grafting: Latest insights. Ann. Med. Surg. 2018, 37, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Grieco, M.P.; Raposio, E. The science behind autologous fat grafting. Ann. Med. Surg. 2017, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- van Goor, H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007, 9, 25–34. [Google Scholar] [CrossRef]








| Group | t of Analysis 24 h | t of Analysis 24 h + 24 h | t of Analysis 4 h + 48 h |
|---|---|---|---|
| CTR | 99.4 ± 1.5 | 97.8 ± 1.9 | 97.9 ± 2.1 |
| DMSO | 99.0 ± 2.4 | 96.1 ± 4.0 | 97.1 ± 2.7 |
| CB 0.1 µM | 100.0 ± 0.0 | 96.7 ± 2.8 | 97.0 ± 3.1 |
| CB 1 µM | 96.1 ± 4.9 | 98.4 ± 1.8 | 96.9 ± 2.8 |
| CB 10 µM | 89.1 ± 7.0 | 93.9 ± 5.8 | 94.1 ± 4.1 |
| Group | t of Analysis 6 Days |
|---|---|
| CTR | 22.4% ± 5.3 |
| DMSO | 25.9% ± 3.6 |
| CB 0.1 µM | 30.9% ± 6.7 |
| CB 1 µM | 44.9% ± 9.1 |
| CB 5 µM | 42.8% ± 4.5 |
| CB 10 µM | 49.8% ± 9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianconi, E.; Tassinari, R.; Alessandrini, A.; Ragazzini, G.; Cavallini, C.; Abruzzo, P.M.; Petrocelli, G.; Pampanella, L.; Casadei, R.; Maioli, M.; et al. Cytochalasin B Modulates Nanomechanical Patterning and Fate in Human Adipose-Derived Stem Cells. Cells 2022, 11, 1629. https://doi.org/10.3390/cells11101629
Bianconi E, Tassinari R, Alessandrini A, Ragazzini G, Cavallini C, Abruzzo PM, Petrocelli G, Pampanella L, Casadei R, Maioli M, et al. Cytochalasin B Modulates Nanomechanical Patterning and Fate in Human Adipose-Derived Stem Cells. Cells. 2022; 11(10):1629. https://doi.org/10.3390/cells11101629
Chicago/Turabian StyleBianconi, Eva, Riccardo Tassinari, Andrea Alessandrini, Gregorio Ragazzini, Claudia Cavallini, Provvidenza Maria Abruzzo, Giovannamaria Petrocelli, Luca Pampanella, Raffaella Casadei, Margherita Maioli, and et al. 2022. "Cytochalasin B Modulates Nanomechanical Patterning and Fate in Human Adipose-Derived Stem Cells" Cells 11, no. 10: 1629. https://doi.org/10.3390/cells11101629
APA StyleBianconi, E., Tassinari, R., Alessandrini, A., Ragazzini, G., Cavallini, C., Abruzzo, P. M., Petrocelli, G., Pampanella, L., Casadei, R., Maioli, M., Canaider, S., Facchin, F., & Ventura, C. (2022). Cytochalasin B Modulates Nanomechanical Patterning and Fate in Human Adipose-Derived Stem Cells. Cells, 11(10), 1629. https://doi.org/10.3390/cells11101629