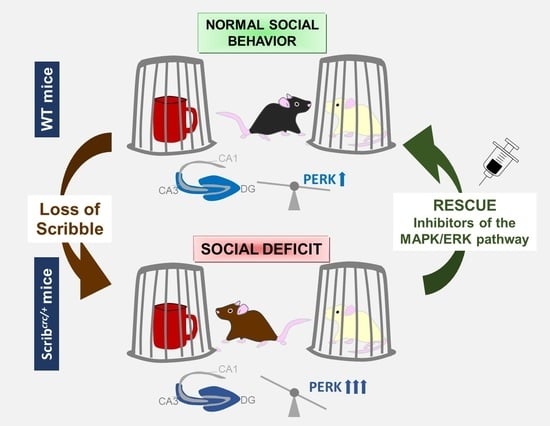
Scribble Controls Social Motivation Behavior through the Regulation of the ERK/Mnk1 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavioral Analyses
2.3. Assessment of Social Behaviors
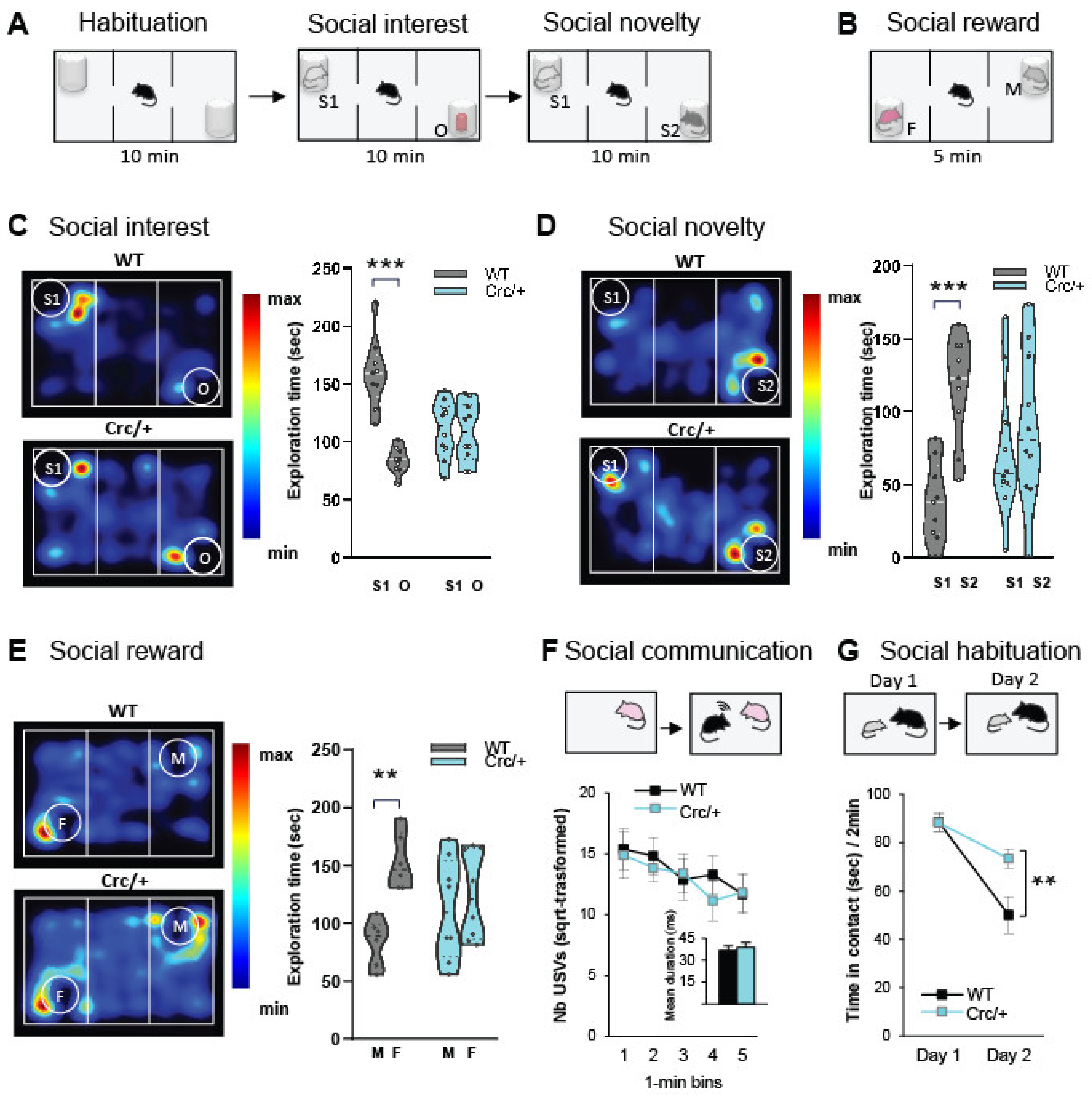
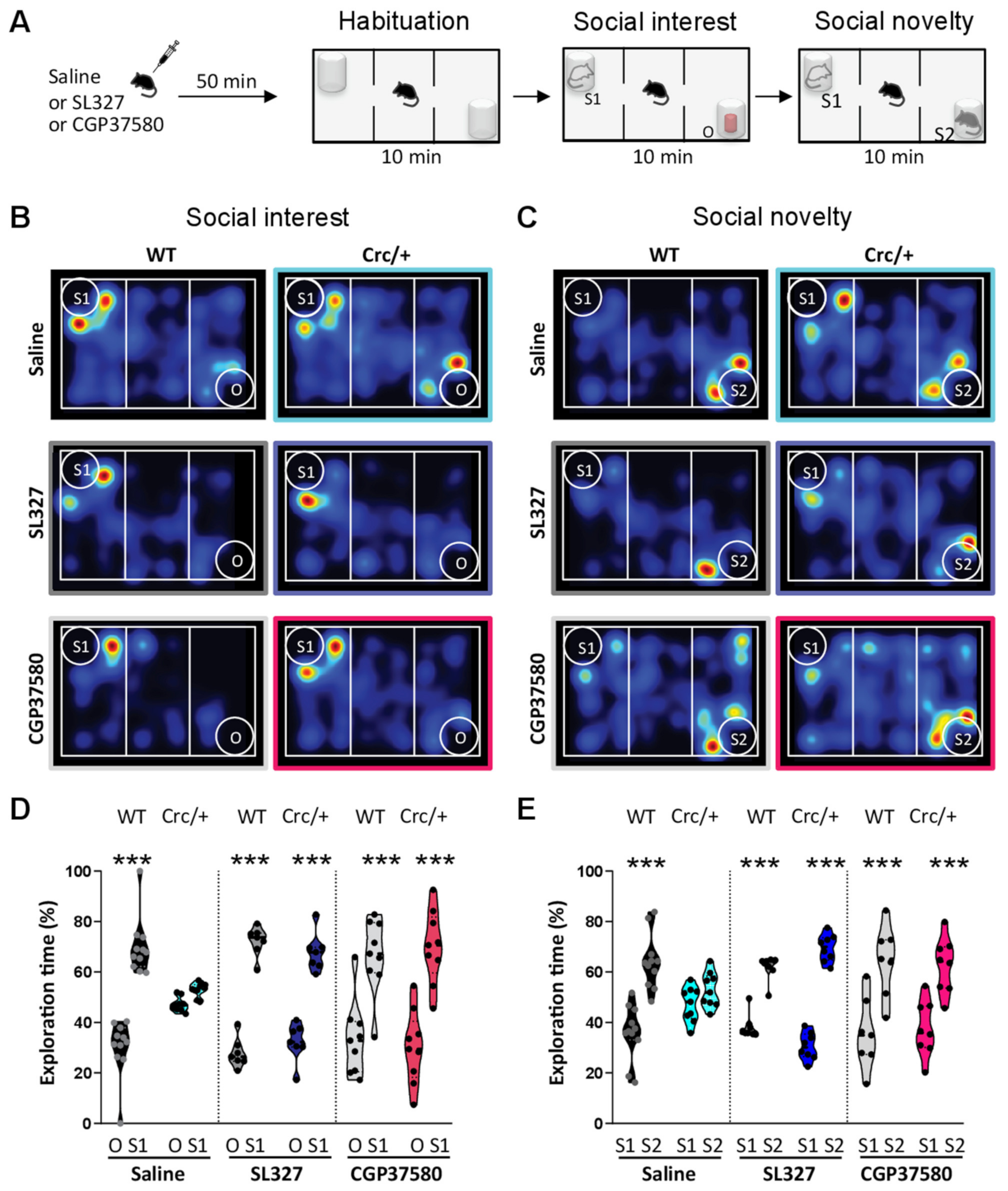
2.4. Social Motivation Task in the Three-Compartment Test
2.5. Preference for a Social Reward in the Three-Compartment Test
2.6. Direct Social Interaction and Communication
2.7. Social Habituation
2.8. Open Field Activity and Nonsocial Neophobia
2.9. Elevated Plus Maze
2.10. Dark-Light Emergence Test
2.11. Self-Grooming Test
2.12. Marble-Burying Test
2.13. Buried Food Test
2.14. Olfactory Habituation and Dishabituation
2.15. Prepulse Inhibition of the Acoustic Startle Reflex
2.16. Recognition of Nonsocial Novelty
2.17. Spontaneous Alteration in the Y-Maze
2.18. Treatments
2.19. Analysis Following the Social Interest Test
2.20. c-Fos and Zif-268 Immunoreactivity
2.21. Laser Capture Microdissection (LCM) Analysis
2.22. Cell Culture, Transfection, and the SRE-Luciferase Reporter Assay
2.23. Magnetic Resonance Imaging (MRI)
2.24. D Volume Reconstitution and Surface Rendering
2.25. Statistical Analysis
3. Results
3.1. Scribcrc/+ Mice Had Deficits in Social Motivation Task (or the Three-Compartment Test for Social Preference)
3.2. Scribcrc/+ Mice Displayed No Deficits in Direct Social Interaction and Communication
3.3. c-Fos Levels Were Increased in the DG and CA3 Regions of the Hippocampus in Scribcrc/+ Mice after Social Exposure
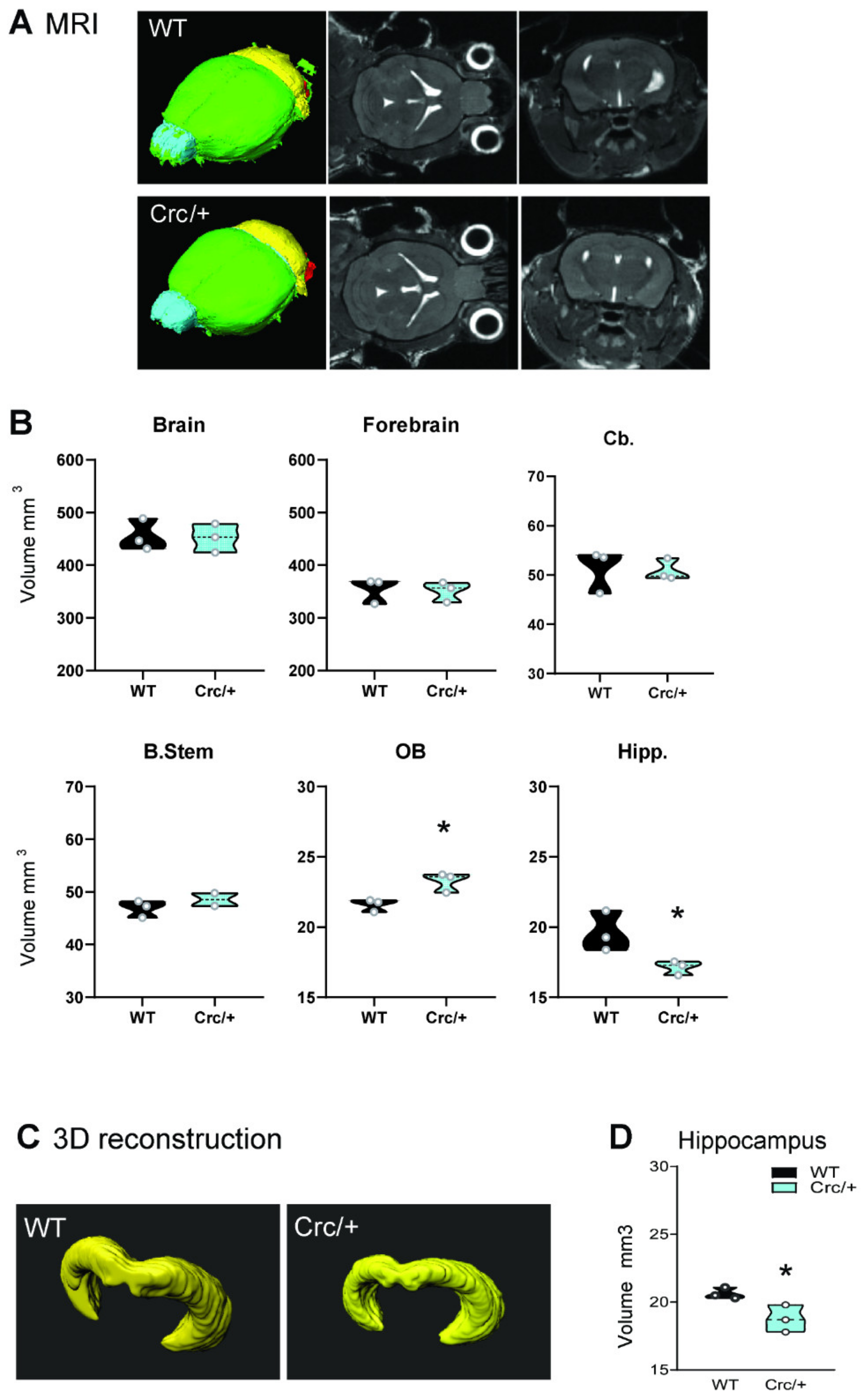
3.4. Reduced Structural Integrity of the Hippocampus in Scribcrc/+ Mice
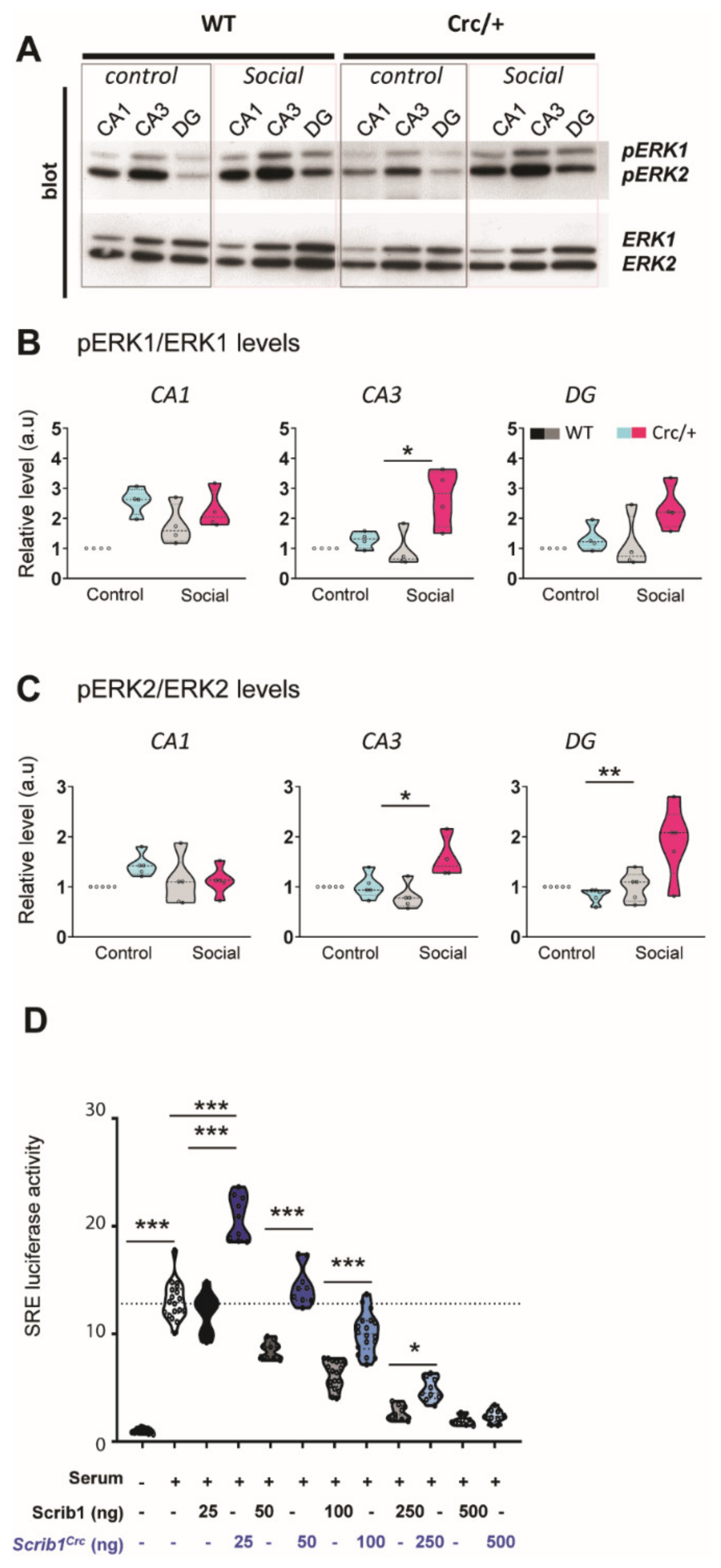
3.5. Reduction of Scrib Levels Leading to a Significant Increase in ERK Phosphorylation in the Hippocampi of Scribcrc/+ Mice after Social Exposure
3.6. The Scribcrc Form Was Crucial for ERK Pathway Activation
3.7. Specific Inhibition of ERK Rescued the Social Motivation Deficits in the Scribcrc/+ Mice
3.8. Downstream of ERK Signaling, the Specific Inhibition of Mnk1 in Scribcrc/+ Mice Also Rescued Social Motivation Deficits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adolphs, R. The Social Brain: Neural Basis of Social Knowledge. Annu. Rev. Psychol. 2009, 60, 693–716. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pramparo, T.; Gazestani, V.H.; Lombardo, M.V.; Pierce, K.; Lewis, N.E. The ASD Living Biology: From Cell Proliferation to Clinical Phenotype. Mol. Psychiatry 2019, 24, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Bonello, T.T.; Peifer, M. Scribble: A Master Scaffold in Polarity, Adhesion, Synaptogenesis, and Proliferation. J. Cell Biol. 2019, 218, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.N.; Henderson, D.J.; Doudney, K.; Gaston-Massuet, C.; Phillips, H.M.; Paternotte, C.; Arkell, R.; Stanier, P.; Copp, A.J. Disruption of Scribble (Scrb1) Causes Severe Neural Tube Defects in the Circletail Mouse. Hum. Mol. Genet. 2003, 12, 87–98. [Google Scholar] [CrossRef]
- Moreau, M.M.; Piguel, N.; Papouin, T.; Koehl, M.; Durand, C.M.; Rubio, M.E.; Loll, F.; Richard, E.M.; Mazzocco, C.; Racca, C.; et al. The Planar Polarity Protein Scribble1 Is Essential for Neuronal Plasticity and Brain Function. J. Neurosci. 2010, 30, 9738–9752. [Google Scholar] [CrossRef]
- Montcouquiol, M.; Rachel, R.A.; Lanford, P.J.; Copeland, N.G.; Jenkins, N.A.; Kelley, M.W. Identification of Vangl2 and Scrb1 as Planar Polarity Genes in Mammals. Nature 2003, 423, 173–177. [Google Scholar] [CrossRef]
- Montcouquiol, M.; Sans, N.; Huss, D.; Kach, J.; Dickman, J.D.; Forge, A.; Rachel, R.A.; Copeland, N.G.; Jenkins, N.A.; Bogani, D.; et al. Asymmetric Localization of Vangl2 and Fz3 Indicate Novel Mechanisms for Planar Cell Polarity in Mammals. J. Neurosci. 2006, 26, 5265–5275. [Google Scholar] [CrossRef]
- Lei, Y.; Zhu, H.; Duhon, C.; Yang, W.; Ross, M.E.; Shaw, G.M.; Finnell, R.H. Mutations in Planar Cell Polarity Gene SCRIB Are Associated with Spina Bifida. PLoS ONE 2013, 8, e69262. [Google Scholar] [CrossRef]
- Robinson, A.; Escuin, S.; Doudney, K.; Vekemans, M.; Stevenson, R.E.; Greene, N.D.E.; Copp, A.J.; Stanier, P. Mutations in the Planar Cell Polarity Genes CELSR1 and SCRIB Are Associated with the Severe Neural Tube Defect Craniorachischisis. Hum. Mutat. 2012, 33, 440–447. [Google Scholar] [CrossRef]
- Wang, M.; de Marco, P.; Capra, V.; Kibar, Z. Update on the Role of the Non-Canonical Wnt/Planar Cell Polarity Pathway in Neural Tube Defects. Cells 2019, 8, 1198. [Google Scholar] [CrossRef]
- Lauritsen, M.B.; Mors, O.; Mortensen, P.B.; Ewald, H. Medical Disorders among Inpatients with Autism in Denmark According to ICD-8: A Nationwide Register-Based Study. J. Autism Dev. Disord. 2002, 32, 115–119. [Google Scholar] [CrossRef]
- Dawson, S.; Glasson, E.J.; Dixon, G.; Bower, C. Birth Defects in Children With Autism Spectrum Disorders: A Population-Based, Nested Case-Control Study. Am. J. Epidemiol. 2009, 169, 1296–1303. [Google Scholar] [CrossRef]
- Timonen-Soivio, L.; Sourander, A.; Malm, H.; Hinkka-Yli-Salomäki, S.; Gissler, M.; Brown, A.; Vanhala, R. The Association Between Autism Spectrum Disorders and Congenital Anomalies by Organ Systems in a Finnish National Birth Cohort. J. Autism Dev. Disord. 2015, 45, 3195–3203. [Google Scholar] [CrossRef]
- Sans, N.; Ezan, J.; Moreau, M.M.; Montcouquiol, M. Chapter 13—Planar Cell Polarity Gene Mutations in Autism Spectrum Disorder, Intellectual Disabilities, and Related Deletion/Duplication Syndromes. In Neuronal and Synaptic Dysfunction in Autism Spectrum Disorder and Intellectual Disability; Sala, C., Verpelli, C., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 189–219. [Google Scholar] [CrossRef]
- Dauber, A.; Golzio, C.; Guenot, C.; Jodelka, F.M.; Kibaek, M.; Kjaergaard, S.; Leheup, B.; Martinet, D.; Nowaczyk, M.J.M.; Rosenfeld, J.A.; et al. SCRIB and PUF60 Are Primary Drivers of the Multisystemic Phenotypes of the 8q24.3 Copy-Number Variant. Am. J. Hum. Genet. 2013, 93, 798–811. [Google Scholar] [CrossRef]
- Neale, B.M.; Kou, Y.; Liu, L.; Ma’ayan, A.; Samocha, K.E.; Sabo, A.; Lin, C.-F.; Stevens, C.; Wang, L.-S.; Makarov, V.; et al. Patterns and Rates of Exonic de Novo Mutations in Autism Spectrum Disorders. Nature 2012, 485, 242–245. [Google Scholar] [CrossRef]
- Iossifov, I.; Ronemus, M.; Levy, D.; Wang, Z.; Hakker, I.; Rosenbaum, J.; Yamrom, B.; Lee, Y.-H.; Narzisi, G.; Leotta, A.; et al. De Novo Gene Disruptions in Children on the Autistic Spectrum. Neuron 2012, 74, 285–299. [Google Scholar] [CrossRef]
- Hu, J.; Sathanoori, M.; Kochmar, S.; Azage, M.; Mann, S.; Madan-Khetarpal, S.; Goldstein, A.; Surti, U. A Novel Maternally Inherited 8q24.3 and a Rare Paternally Inherited 14q23.3 CNVs in a Family with Neurodevelopmental Disorders. Am. J. Med. Genet. A 2015, 167A, 1921–1926. [Google Scholar] [CrossRef]
- Nagasaka, K.; Massimi, P.; Pim, D.; Subbaiah, V.K.; Kranjec, C.; Nakagawa, S.; Yano, T.; Taketani, Y.; Banks, L. The Mechanism and Implications of HScrib Regulation of ERK. Small GTPases 2010, 1, 108–112. [Google Scholar] [CrossRef][Green Version]
- Campbell, D.B.; Li, C.; Sutcliffe, J.S.; Persico, A.M.; Levitt, P. Genetic Evidence Implicating Multiple Genes in the MET Receptor Tyrosine Kinase Pathway in Autism Spectrum Disorder. Autism Res. 2008, 1, 159–168. [Google Scholar] [CrossRef]
- Fernandez, B.A.; Roberts, W.; Chung, B.; Weksberg, R.; Meyn, S.; Szatmari, P.; Joseph-George, A.M.; Mackay, S.; Whitten, K.; Noble, B.; et al. Phenotypic Spectrum Associated with de Novo and Inherited Deletions and Duplications at 16p11.2 in Individuals Ascertained for Diagnosis of Autism Spectrum Disorder. J. Med. Genet. 2010, 47, 195–203. [Google Scholar] [CrossRef]
- Saitta, S.C.; Harris, S.E.; McDonald-McGinn, D.M.; Emanuel, B.S.; Tonnesen, M.K.; Zackai, E.H.; Seitz, S.C.; Driscoll, D.A. Independent de Novo 22q11.2 Deletions in First Cousins with DiGeorge/Velocardiofacial Syndrome. Am. J. Med. Genet. A 2004, 124A, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Bagni, C.; Zukin, R.S. A Synaptic Perspective of Fragile X Syndrome and Autism Spectrum Disorders. Neuron 2019, 101, 1070–1088. [Google Scholar] [CrossRef] [PubMed]
- Faridar, A.; Jones-Davis, D.; Rider, E.; Li, J.; Gobius, I.; Morcom, L.; Richards, L.J.; Sen, S.; Sherr, E.H. Mapk/Erk Activation in an Animal Model of Social Deficits Shows a Possible Link to Autism. Mol. Autism 2014, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, J.; Pucilowska, J.; Landreth, G.E. ERK/MAPK Signaling and Autism Spectrum Disorders. Prog. Brain Res. 2018, 241, 63–112. [Google Scholar] [CrossRef]
- Hilal, M.L.; Moreau, M.M.; Racca, C.; Pinheiro, V.L.; Piguel, N.H.; Santoni, M.-J.; Dos Santos Carvalho, S.; Blanc, J.-M.; Abada, Y.-S.K.; Peyroutou, R.; et al. Activity-Dependent Neuroplasticity Induced by an Enriched Environment Reverses Cognitive Deficits in Scribble Deficient Mouse. Cereb. Cortex 2017, 27, 5635–5651. [Google Scholar] [CrossRef]
- Cheng, N.; Alshammari, F.; Hughes, E.; Khanbabaei, M.; Rho, J.M. Dendritic Overgrowth and Elevated ERK Signaling during Neonatal Development in a Mouse Model of Autism. PLoS ONE 2017, 12, e0179409. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Frei, J.A.; Kilander, M.B.C.; Shen, W.; Blatt, G.J. A Subset of Autism-Associated Genes Regulate the Structural Stability of Neurons. Front. Cell. Neurosci. 2016, 10, 263. [Google Scholar] [CrossRef]
- Diaz-Beltran, L.; Esteban, F.J.; Wall, D.P. A Common Molecular Signature in ASD Gene Expression: Following Root 66 to Autism. Transl. Psychiatry 2016, 6, e705. [Google Scholar] [CrossRef]
- Lim, S.; Saw, T.Y.; Zhang, M.; Janes, M.R.; Nacro, K.; Hill, J.; Lim, A.Q.; Chang, C.-T.; Fruman, D.A.; Rizzieri, D.A.; et al. Targeting of the MNK-EIF4E Axis in Blast Crisis Chronic Myeloid Leukemia Inhibits Leukemia Stem Cell Function. Proc. Natl. Acad. Sci. USA 2013, 110, E2298–E2307. [Google Scholar] [CrossRef]
- Rosina, E.; Battan, B.; Siracusano, M.; Di Criscio, L.; Hollis, F.; Pacini, L.; Curatolo, P.; Bagni, C. Disruption of MTOR and MAPK Pathways Correlates with Severity in Idiopathic Autism. Transl. Psychiatry 2019, 9, 50. [Google Scholar] [CrossRef]
- Assémat, E.; Bazellières, E.; Pallesi-Pocachard, E.; Le Bivic, A.; Massey-Harroche, D. Polarity Complex Proteins. Biochim. Biophys. Acta 2008, 1778, 614–630. [Google Scholar] [CrossRef]
- Murdoch, J.N.; Rachel, R.A.; Shah, S.; Beermann, F.; Stanier, P.; Mason, C.A.; Copp, A.J. Circletail, a New Mouse Mutant with Severe Neural Tube Defects: Chromosomal Localization and Interaction with the Loop-Tail Mutation. Genomics 2001, 78, 55–63. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-Background Modulation of Core and Variable Autistic-like Symptoms in Fmr1 Knock-out Mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef]
- Portfors, C.V. Types and Functions of Ultrasonic Vocalizations in Laboratory Rats and Mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2007, 46, 28–34. [Google Scholar]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural Phenotyping Assays for Mouse Models of Autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Grandy, D.K.; Low, M.J.; Paulus, M.P.; Geyer, M.A. Dopamine D4 Receptor-Knock-out Mice Exhibit Reduced Exploration of Novel Stimuli. J. Neurosci. 1999, 19, 9550–9556. [Google Scholar] [CrossRef]
- Yang, M.; Crawley, J.N. Simple Behavioral Assessment of Mouse Olfaction. Curr. Protoc. Neurosci. 2009, 48, 8–24. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Crusio, W.E. Strain-Dependent Changes in Acoustic Startle Response and Its Plasticity across Adolescence in Mice. Behav. Genet. 2009, 39, 623–631. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Feldon, J.; Yee, B.K. Nonphysical Contact between Cagemates Alleviates the Social Isolation Syndrome in C57BL/6 Male Mice. Behav. Neurosci. 2008, 122, 505–515. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Singer, P.; Feldon, J.; Yee, B.K. The Postweaning Social Isolation in C57BL/6 Mice: Preferential Vulnerability in the Male Sex. Psychopharmacology 2008, 197, 613–628. [Google Scholar] [CrossRef]
- Yee, B.K.; Chang, T.; Pietropaolo, S.; Feldon, J. The Expression of Prepulse Inhibition of the Acoustic Startle Reflex as a Function of Three Pulse Stimulus Intensities, Three Prepulse Stimulus Intensities, and Three Levels of Startle Responsiveness in C57BL6/J Mice. Behav. Brain Res. 2005, 163, 265–276. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object Recognition Test in Mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Hughes, R.N. The Value of Spontaneous Alternation Behavior (SAB) as a Test of Retention in Pharmacological Investigations of Memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. [Google Scholar] [CrossRef]
- King, D.L.; Arendash, G.W. Behavioral Characterization of the Tg2576 Transgenic Model of Alzheimer’s Disease through 19 Months. Physiol. Behav. 2002, 75, 627–642. [Google Scholar] [CrossRef]
- Satoh, Y.; Endo, S.; Nakata, T.; Kobayashi, Y.; Yamada, K.; Ikeda, T.; Takeuchi, A.; Hiramoto, T.; Watanabe, Y.; Kazama, T. ERK2 Contributes to the Control of Social Behaviors in Mice. J. Neurosci. 2011, 31, 11953–11967. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Maitre, M.; Roullot-Lacarrière, V.; Piazza, P.V.; Revest, J.-M. Western Blot Detection of Brain Phosphoproteins after Performing Laser Microdissection and Pressure Catapulting (LMPC). J. Neurosci. Methods 2011, 198, 204–212. [Google Scholar] [CrossRef]
- Sans, N.; Wang, P.Y.; Du, Q.; Petralia, R.S.; Wang, Y.-X.; Nakka, S.; Blumer, J.B.; Macara, I.G.; Wenthold, R.J. MPins Modulates PSD-95 and SAP102 Trafficking and Influences NMDA Receptor Surface Expression. Nat. Cell Biol. 2005, 7, 1179–1190. [Google Scholar] [CrossRef]
- Ossipova, O.; Ezan, J.; Sokol, S.Y. PAR-1 Phosphorylates Mind Bomb to Promote Vertebrate Neurogenesis. Dev. Cell 2009, 17, 222–233. [Google Scholar] [CrossRef]
- Miraux, S.; Massot, P.; Ribot, E.J.; Franconi, J.-M.; Thiaudiere, E. 3D TrueFISP Imaging of Mouse Brain at 4.7T and 9.4T. J. Magn. Reson. Imaging JMRI 2008, 28, 497–503. [Google Scholar] [CrossRef]
- Ribot, E.J.; Wecker, D.; Trotier, A.J.; Dallaudière, B.; Lefrançois, W.; Thiaudière, E.; Franconi, J.-M.; Miraux, S. Water Selective Imaging and BSSFP Banding Artifact Correction in Humans and Small Animals at 3T and 7T, Respectively. PLoS ONE 2015, 10, e0139249. [Google Scholar] [CrossRef]
- Kogan, J.H.; Frankland, P.W.; Silva, A.J. Long-Term Memory Underlying Hippocampus-Dependent Social Recognition in Mice. Hippocampus 2000, 10, 47–56. [Google Scholar] [CrossRef]
- Jarjour, A.A.; Boyd, A.; Dow, L.E.; Holloway, R.K.; Goebbels, S.; Humbert, P.O.; Williams, A.; ffrench-Constant, C. The Polarity Protein Scribble Regulates Myelination and Remyelination in the Central Nervous System. PLoS Biol. 2015, 13, e1002107. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.M.; Lewis, M.C.; Pechenino, A.S.; Harburger, L.L.; Orr, P.T.; Gresack, J.E.; Schafe, G.E.; Frick, K.M. Estradiol-Induced Enhancement of Object Memory Consolidation Involves Hippocampal Extracellular Signal-Regulated Kinase Activation and Membrane-Bound Estrogen Receptors. J. Neurosci. 2008, 28, 8660–8667. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; Martire, A.; Cartocci, G.; Ferrante, A.; Ricceri, L. Reduced Social Interaction, Behavioural Flexibility and BDNF Signalling in the BTBR T+tf/J Strain, a Mouse Model of Autism. Behav. Brain Res. 2013, 251, 35–40. [Google Scholar] [CrossRef]
- Stanfield, A.C.; McIntosh, A.M.; Spencer, M.D.; Philip, R.; Gaur, S.; Lawrie, S.M. Towards a Neuroanatomy of Autism: A Systematic Review and Meta-Analysis of Structural Magnetic Resonance Imaging Studies. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2008, 23, 289–299. [Google Scholar] [CrossRef]
- Via, E.; Radua, J.; Cardoner, N.; Happé, F.; Mataix-Cols, D. Meta-Analysis of Gray Matter Abnormalities in Autism Spectrum Disorder: Should Asperger Disorder Be Subsumed under a Broader Umbrella of Autistic Spectrum Disorder? Arch. Gen. Psychiatry 2011, 68, 409–418. [Google Scholar] [CrossRef]
- Treble-Barna, A.; Juranek, J.; Stuebing, K.K.; Cirino, P.T.; Dennis, M.; Fletcher, J.M. Prospective and Episodic Memory in Relation to Hippocampal Volume in Adults with Spina Bifida Myelomeningocele. Neuropsychology 2015, 29, 92–101. [Google Scholar] [CrossRef]
- Tanimizu, T.; Kenney, J.W.; Okano, E.; Kadoma, K.; Frankland, P.W.; Kida, S. Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory. J. Neurosci. 2017, 37, 4103–4116. [Google Scholar] [CrossRef]
- Okuyama, T. Social Memory Engram in the Hippocampus. Neurosci. Res. 2018, 129, 17–23. [Google Scholar] [CrossRef]
- Raam, T.; McAvoy, K.M.; Besnard, A.; Veenema, A.H.; Sahay, A. Hippocampal Oxytocin Receptors Are Necessary for Discrimination of Social Stimuli. Nat. Commun. 2017, 8, 2001. [Google Scholar] [CrossRef]
- Tzakis, N.; Holahan, M.R. Social Memory and the Role of the Hippocampal CA2 Region. Front. Behav. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef]
- Maaswinkel, H.; Baars, A.-M.; Gispen, W.-H.; Spruijt, B.M. Roles of the Basolateral Amygdala and Hippocampus in Social Recognition in Rats. Physiol. Behav. 1996, 60, 55–63. [Google Scholar] [CrossRef]
- Etherton, M.; Földy, C.; Sharma, M.; Tabuchi, K.; Liu, X.; Shamloo, M.; Malenka, R.C.; Südhof, T.C. Autism-Linked Neuroligin-3 R451C Mutation Differentially Alters Hippocampal and Cortical Synaptic Function. Proc. Natl. Acad. Sci. USA 2011, 108, 13764–13769. [Google Scholar] [CrossRef]
- Bramham, C.R.; Jensen, K.B.; Proud, C.G. Tuning Specific Translation in Cancer Metastasis and Synaptic Memory: Control at the MNK-EIF4E Axis. Trends Biochem. Sci. 2016, 41, 847–858. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreau, M.M.; Pietropaolo, S.; Ezan, J.; Robert, B.J.A.; Miraux, S.; Maître, M.; Cho, Y.; Crusio, W.E.; Montcouquiol, M.; Sans, N. Scribble Controls Social Motivation Behavior through the Regulation of the ERK/Mnk1 Pathway. Cells 2022, 11, 1601. https://doi.org/10.3390/cells11101601
Moreau MM, Pietropaolo S, Ezan J, Robert BJA, Miraux S, Maître M, Cho Y, Crusio WE, Montcouquiol M, Sans N. Scribble Controls Social Motivation Behavior through the Regulation of the ERK/Mnk1 Pathway. Cells. 2022; 11(10):1601. https://doi.org/10.3390/cells11101601
Chicago/Turabian StyleMoreau, Maïté M., Susanna Pietropaolo, Jérôme Ezan, Benjamin J. A. Robert, Sylvain Miraux, Marlène Maître, Yoon Cho, Wim E. Crusio, Mireille Montcouquiol, and Nathalie Sans. 2022. "Scribble Controls Social Motivation Behavior through the Regulation of the ERK/Mnk1 Pathway" Cells 11, no. 10: 1601. https://doi.org/10.3390/cells11101601
APA StyleMoreau, M. M., Pietropaolo, S., Ezan, J., Robert, B. J. A., Miraux, S., Maître, M., Cho, Y., Crusio, W. E., Montcouquiol, M., & Sans, N. (2022). Scribble Controls Social Motivation Behavior through the Regulation of the ERK/Mnk1 Pathway. Cells, 11(10), 1601. https://doi.org/10.3390/cells11101601