Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Gamete Handling
2.3. Protein Extraction and Western Immunoblotting
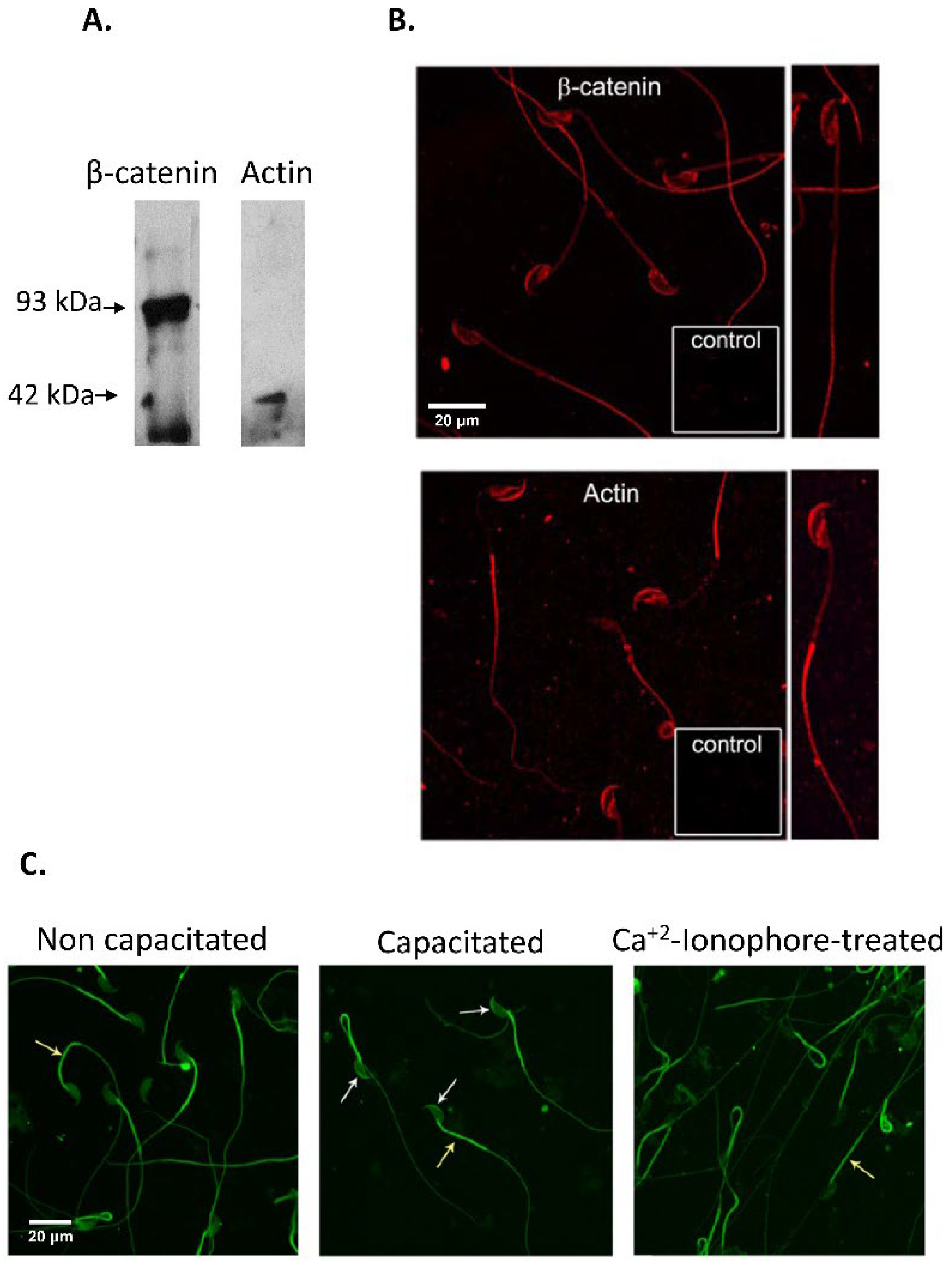
2.4. Sperm Capacitation and Acrosomal Exocytosis
2.5. Immunocytochemistry
2.6. Sperm-Oocyte Interaction Assays
2.6.1. IVF
2.6.2. Cumulus Penetration Assay
2.6.3. Sperm-Oolemma Binding and Fusion Assays
2.7. Statistical Analysis
3. Results
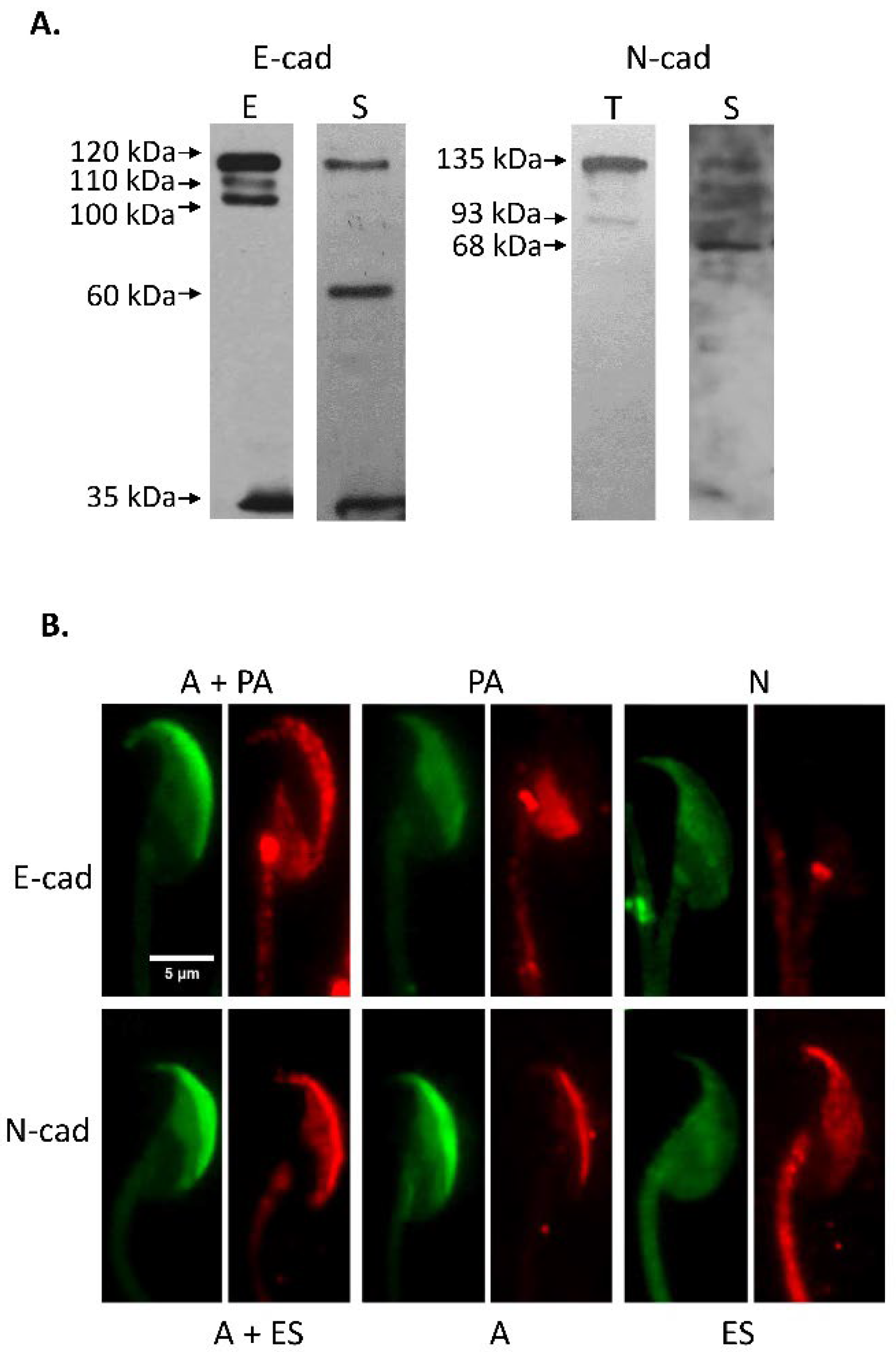
3.1. Immunodetection of E-cad and N-cad and Other Members of the Adherent Complex in Testis, Epididymis, and Cauda Epididymal Spermatozoa
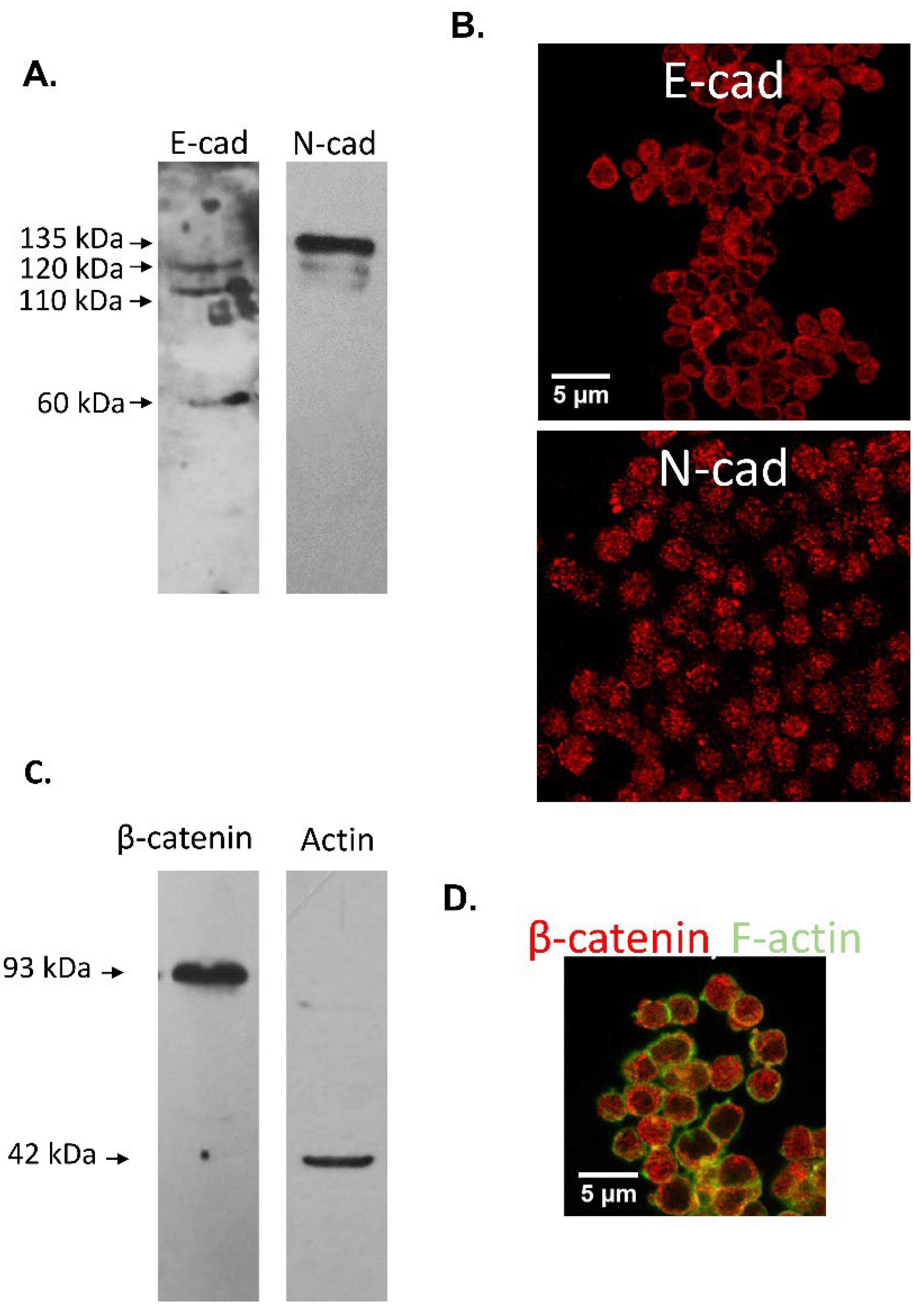
3.2. Immunodetection of E-cad and N-cad in Cumulus Oophorus Cells and Oocytes Recovered from Mature COCs
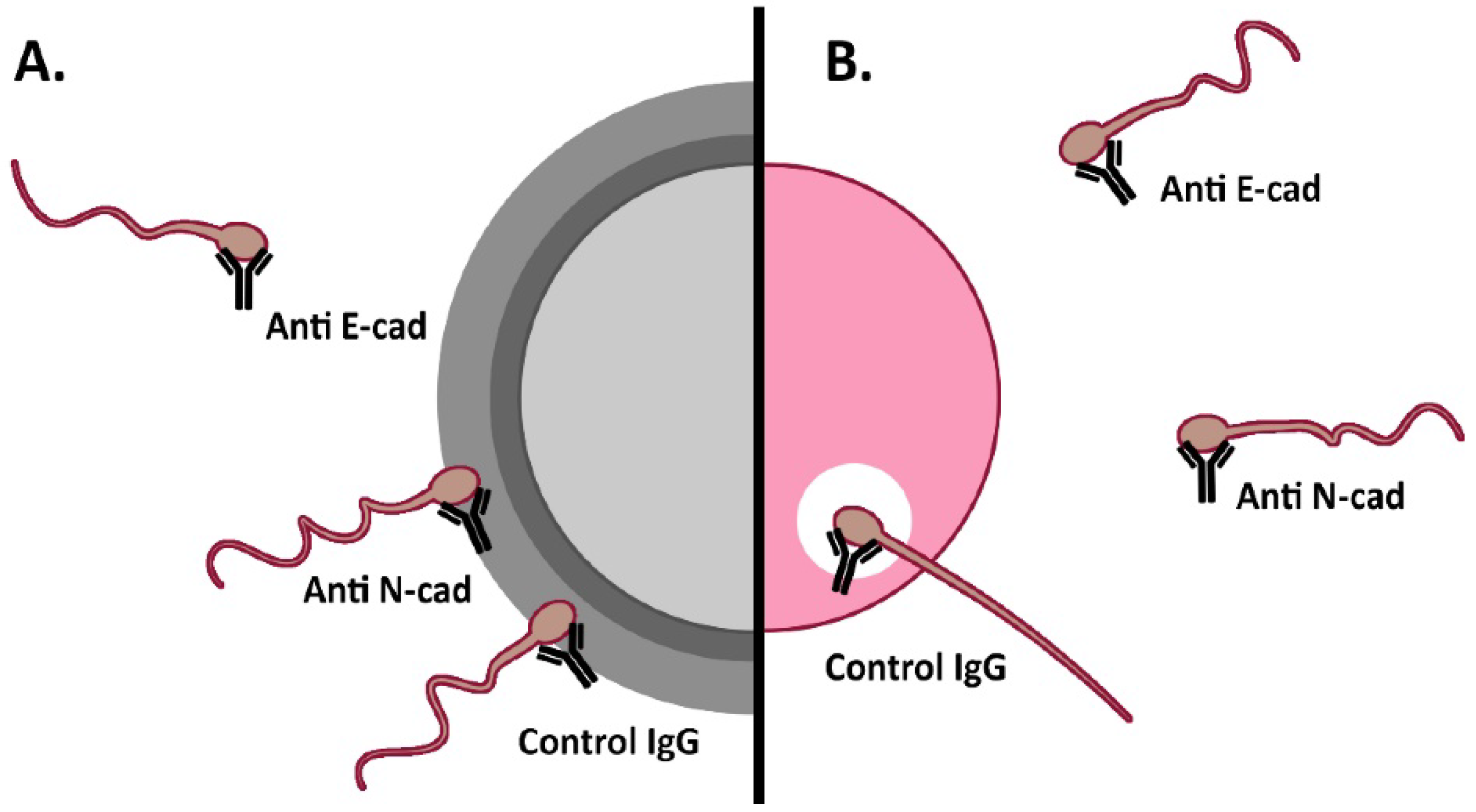
3.3. Effect of Anti-Cadherins Antibodies and Blocking Peptides upon Murine Fertilization
3.3.1. In Vitro Fertilization
3.3.2. Cumulus penetration and Sperm-Oocyte Interaction Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Nair, A.V.; Battistone, M.A. Epithelial dynamics in the epididymis: Role in the maturation, protection, and storage of spermatozoa. Andrology 2019, 7, 631–643. [Google Scholar] [CrossRef]
- Björkgren, I.; Sipilä, P. The impact of epididymal proteins on sperm function. Reproduction 2019, 158, R155–R167. [Google Scholar] [CrossRef]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- De Jonge, C. Biological basis for human capacitation-revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 27, 6–72. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Fertilization studies and assisted fertilization in mammals: Their development and future. J. Reprod. Dev. 2012, 58, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P. Sperm-egg interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef]
- Wassarman, P.M. Reproductive biology: Sperm protein finds its mate. Nature 2014, 508, 466–467. [Google Scholar] [CrossRef]
- Bianchi, E.; Wright, G.J. Sperm Meets Egg: The Genetics of Mammalian Fertilization. Annu. Rev. Genet. 2016, 50, 93–111. [Google Scholar] [CrossRef]
- Weigel Muñoz, M.; Carvajal, G.; Curci, L.; Gonzalez, S.N.; Cuasnicu, P.S. Relevance of CRISP proteins for epididymal physiology, fertilization, and fertility. Andrology 2019, 7, 610–617. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kloc, M.; Mizia, P.; Kubiak, J.Z. The Central Role of Cadherins in Gonad Development, Reproduction, and Fertility. Int. J. Mol. Sci. 2020, 21, 8264. [Google Scholar] [CrossRef] [PubMed]
- Gahlay, G.K.; Rajput, N. The enigmatic sperm proteins in mammalian fertilization: An overview. Biol. Reprod. 2020, 103, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Deneke, V.E.; Pauli, A. The Fertilization Enigma: How Sperm and Egg Fuse. Annu. Rev. Cell Dev. Biol. 2021, 37, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Wright, G.J. Find and fuse: Unsolved mysteries in sperm-egg recognition. PLoS Biol. 2020, 18, e3000953. [Google Scholar] [CrossRef]
- Angst, B.D.; Marcozzi, C.; Magee, A.I. The cadherin superfamily: Diversity in form and function. J. Cell Sci. 2001, 114, 629–641. [Google Scholar] [CrossRef]
- Takeichi, M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 1977, 75, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, B.; Sato, Y.; Kameya, T.; Okayasu, I.; Mukai, K. Differential expression of N-cadherin and E-cadherin in normal human tissues. Arch. Histol. Cytol. 2006, 69, 135–145. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef]
- Hazan, R.B.; Qiao, R.; Keren, R.; Badano, I.; Suyama, K. Cadherin switch in tumor progression. Ann. N. Y. Acad. Sci. 2004, 1014, 155–163. [Google Scholar] [CrossRef]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef]
- Takeichi, M. Self-organization of animal tissues: Cadherin-mediated processes. Dev. Cell 2011, 21, 24–26. [Google Scholar] [CrossRef]
- Arslan, F.N.; Eckert, J.; Schmidt, T.; Heisenberg, C.P. Holding it together: When cadherin meets cadherin. Biophys. J. 2021, 120, 4182–4192. [Google Scholar] [CrossRef]
- Gupta, S.; Yap, A.S. How adherens junctions move cells during collective migration. Fac. Rev. 2021, 10, 56. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef]
- Labernadie, A.; Kato, T.; Brugués, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; González-Tarragó, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ signaling in mammalian spermatozoa. Mol. Cell Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef]
- Marín-Briggiler, C.I.; Veiga, M.F.; Matos, M.L.; Echeverría, M.F.; Furlong, L.I.; Vazquez-Levin, M.H. Expression of epithelial cadherin in the human male reproductive tract and gametes and evidence of its participation in fertilization. Mol. Hum. Reprod. 2008, 14, 561–571. [Google Scholar] [CrossRef]
- Marín-Briggiler, C.I.; Lapyckyj, L.; González Echeverría, M.F.; Rawe, V.Y.; Alvarez Sedó, C.; Vazquez-Levin, M.H. Neural cadherin is expressed in human gametes and participates in sperm-oocyte interaction events. Int. J. Androl. 2010, 33, e228–e239. [Google Scholar] [CrossRef]
- Vazquez-Levin, M.H.; Marín-Briggiler, C.I.; Caballero, J.N.; Veiga, M.F. Epithelial and neural cadherin expression in the mammalian reproductive tract and gametes and their participation in fertilization-related events. Dev. Biol. 2015, 401, 2–16. [Google Scholar] [CrossRef]
- Bergin, E.; Levine, J.S.; Koh, J.S.; Lieberthal, W. Mouse proximal tubular cell-cell adhesion inhibits apoptosis by a cadherin-dependent mechanism. Am. J. Physiol. Renal. Physiol. 2000, 278, F758–F768. [Google Scholar] [CrossRef]
- Devemy, E.; Blaschuk, O.W. Identification of a novel N-cadherin antagonist. Peptides 2008, 29, 1853–1861. [Google Scholar] [CrossRef]
- Devemy, E.; Blaschuk, O.W. Identification of a novel dual E- and N-cadherin antagonist. Peptides 2009, 30, 1539–1547. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Washington, DC, USA, 2011. [Google Scholar]
- Veaute, C.; Furlong, L.I.; Cameo, M.; Harris, J.D.; Vazquez-Levin, M.H. Antiacrosin antibodies and infertility. II. Gene immunization with human proacrosin to assess the effect of immunity toward proacrosin/acrosin upon protein activities and animal fertility. Fertil. Steril. 2009, 91, 1256–1268. [Google Scholar] [CrossRef]
- Fraser, L.R.; Drury, L.M. The relationship between sperm concentration and fertilization in vitro of mouse eggs. Biol. Reprod. 1975, 13, 513–518. [Google Scholar] [CrossRef]
- Zahn, A.; Furlong, L.I.; Biancotti, J.C.; Ghiringhelli, P.D.; Marin-Briggiler, C.I.; Vazquez-Levin, M.H. Evaluation of the proacrosin/acrosin system and its mechanism of activation in human sperm extracts. J. Reprod. Immunol. 2002, 54, 43–63. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. 1979. Biotechnology 1992, 24, 145–149. [Google Scholar]
- Barraud-Lange, V.; Naud-Barriant, N.; Saffar, L.; Gattegno, L.; Ducot, B.; Drillet, A.S.; Bomsel, M.; Wolf, J.P.; Ziyyat, A. Alpha6beta1 integrin expressed by sperm is determinant in mouse fertilization. BMC Dev. Biol. 2007, 7, 102. [Google Scholar] [CrossRef]
- Battistone, M.A.; Alvau, A.; Salicioni, A.M.; Visconti, P.E.; Da Ros, V.G.; Cuasnicú, P.S. Evidence for the involvement of proline-rich tyrosine kinase 2 in tyrosine phosphorylation downstream of protein kinase A activation during human sperm capacitation. Mol. Hum. Reprod. 2014, 20, 1054–1066. [Google Scholar] [CrossRef]
- Herrero, M.B.; Mandal, A.; Digilio, L.C.; Coonrod, S.A.; Maier, B.; Herr, J.C. Mouse SLLP1, a sperm lysozyme-like protein involved in sperm-egg binding and fertilization. Dev. Biol. 2005, 284, 126–142. [Google Scholar] [CrossRef]
- Yoshida-Noro, C.; Suzuki, N.; Takeichi, M. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev. Biol. 1984, 101, 19–27. [Google Scholar] [CrossRef]
- Shirayoshi, Y.; Okada, T.S.; Takeichi, M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell 1983, 35, 631–638. [Google Scholar] [CrossRef]
- Perrais, M.; Chen, X.; Perez-Moreno, M.; Gumbiner, B.M. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol. Biol. Cell 2007, 18, 2013–2025. [Google Scholar] [CrossRef]
- Gloushankova, N.A.; Rubtsova, S.N.; Zhitnyak, I.Y. Cadherin-mediated cell-cell interactions in normal and cancer cells. Tissue Barriers 2017, 5, e1356900. [Google Scholar] [CrossRef]
- Ziv, S.; Rufas, O.; Shalgi, R. Cadherins expression during gamete maturation and fertilization in the rat. Mol. Reprod. Dev. 2002, 62, 547–556. [Google Scholar] [CrossRef]
- Tsuiki, A.; Hoshiai, H.; Takahashi, K.; Suzuki, M.; Hoshi, K. Sperm-egg interactions observed by scanning electron microscopy. Arch. Androl. 1986, 16, 35–47. [Google Scholar] [CrossRef]
- Fléchon, J.E.; Pavlok, A. Ultrastructural study of the interactions and fusion of ram spermatozoa with zona-free hamster oocytes. Reprod. Nutr. Dev. 1986, 26, 999–1008. [Google Scholar] [CrossRef]
- Hyttel, P.; Xu, K.P.; Greve, T. Scanning electron microscopy of in vitro fertilization in cattle. Anat. Embryol. 1988, 178, 41–46. [Google Scholar] [CrossRef]
- Kadam, A.L.; Fateh, M.; Naz, R.K. Fertilization antigen (FA-1) completely blocks human sperm binding to human zona pellucida: FA-1 antigen may be a sperm receptor for zona pellucida in humans. J. Reprod. Immunol. 1995, 29, 19–30. [Google Scholar] [CrossRef]
- Frolikova, M.; Manaskova-Postlerova, P.; Cerny, J.; Jankovicova, J.; Simonik, O.; Pohlova, A.; Secova, P.; Antalikova, J.; Dvorakova-Hortova, K. CD9 and CD81 Interactions and Their Structural Modelling in Sperm Prior to Fertilization. Int. J. Mol. Sci. 2018, 19, 1236. [Google Scholar] [CrossRef]
- Erikson, D.W.; Way, A.L.; Chapman, D.A.; Killian, G.J. Detection of osteopontin on Holstein bull spermatozoa, in cauda epididymal fluid and testis homogenates, and its potential role in bovine fertilization. Reproduction 2007, 133, 909–917. [Google Scholar] [CrossRef]
- Ying, X.; Liu, Y.; Guo, Q.; Qu, F.; Guo, W.; Zhu, Y.; Ding, Z. Endoplasmic reticulum protein 29 (ERp29), a protein related to sperm maturation is involved in sperm-oocyte fusion in mouse. Reprod. Biol. Endocrinol. 2010, 8, 10. [Google Scholar] [CrossRef]
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential Role of Sperm-Specific PLC-Zeta in Egg Activation and Male Factor Infertility: An Update. Front. Cell Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef]
- Gianzo, M.; Urizar-Arenaza, I.; Muñoa-Hoyos, I.; Larreategui, Z.; Garrido, N.; Irazusta, J.; Subirán, N. (Pro)renin Receptor Is Present in Human Sperm and It Adversely Affects Sperm Fertility Ability. Int. J. Mol. Sci. 2021, 22, 3215. [Google Scholar] [CrossRef]
- Miro-Moran, A.; Jardin, I.; Ortega-Ferrusola, C.; Salido, G.M.; Peña, F.J.; Tapia, J.A.; Aparicio, I.M. Identification and function of exchange proteins activated directly by cyclic AMP (Epac) in mammalian spermatozoa. PLoS ONE 2012, 7, e37713. [Google Scholar]
- Satouh, Y.; Inoue, N.; Ikawa, M.; Okabe, M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 2012, 125 Pt 21, 4985–4990. [Google Scholar] [CrossRef]
- Yamatoya, K.; Kousaka, M.; Ito, C.; Nakata, K.; Hatano, M.; Araki, Y.; Toshimori, K. Cleavage of SPACA1 regulates assembly of sperm-egg membrane fusion machinery in mature spermatozoa. Biol. Reprod. 2020, 102, 750–757. [Google Scholar] [CrossRef]
- Lamas-Toranzo, I.; Hamze, J.G.; Bianchi, E.; Fernández-Fuertes, B.; Pérez-Cerezales, S.; Laguna-Barraza, R.; Fernández-González, R.; Lonergan, P.; Gutiérrez-Adán, A.; Wright, G.J.; et al. TMEM95 is a sperm membrane protein essential for mammalian fertilization. eLife 2020, 9, e53913. [Google Scholar] [CrossRef]
- Fujihara, Y.; Lu, Y.; Noda, T.; Oji, A.; Larasati, T.; Kojima-Kita, K.; Yu, Z.; Matzuk, R.M.; Matzuk, M.M.; Ikawa, M. Spermatozoa lacking Fertilization Influencing Membrane Protein (FIMP) fail to fuse with oocytes in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 9393–9400. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Lu, Y.; Fujihara, Y.; Oura, S.; Koyano, T.; Kobayashi, S.; Matzuk, M.M.; Ikawa, M. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 11493–11502. [Google Scholar] [CrossRef]
- Larue, L.; Ohsugi, M.; Hirchenhain, J.; Kemler, R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc. Natl. Acad. Sci. USA 1994, 91, 8263–8267. [Google Scholar] [CrossRef]
- Radice, G.L.; Rayburn, H.; Matsunami, H.; Knudsen, K.A.; Takeichi, M.; Hynes, R.O. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 1997, 181, 64–78. [Google Scholar] [CrossRef]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef]
- Breitbart, H.; Finkelstein, M. Actin cytoskeleton and sperm function. Biochem. Biophys. Res. Commun. 2018, 506, 372–377. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, L.; Shi, D.S.; Jiang, J.R. Effects of latrunculin A on the relocation of sperm IZUMO1 during gamete interaction in mouse. Mol. Reprod. Dev. 2017, 84, 1183–1190. [Google Scholar] [CrossRef]
- Kumakiri, J.; Oda, S.; Kinoshita, K.; Miyazaki, S. Involvement of Rho family G protein in the cell signaling for sperm incorporation during fertilization of mouse eggs: Inhibition by Clostridium difficile toxin B. Dev. Biol. 2003, 260, 522–535. [Google Scholar] [CrossRef]
- Steinbacher, T.; Ebnet, K. The regulation of junctional actin dynamics by cell adhesion receptors. Histochem. Cell Biol. 2018, 150, 341–350. [Google Scholar] [CrossRef]
- Rufas, O.; Fisch, B.; Ziv, S.; Shalgi, R. Expression of cadherin adhesion molecules on human gametes. Mol. Hum. Reprod. 2000, 6, 163–169. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Roy, S.K. Expression of E-cadherin and N-cadherin in perinatal hamster ovary: Possible involvement in primordial follicle formation and regulation by follicle-stimulating hormone. Endocrinology 2010, 151, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Guibert, E.; Faure, M.; Ramé, C.; Foretz, M.; Viollet, B.; Dupont, J.; Froment, P. Specific deletion of AMP-activated protein kinase (α1AMPK) in murine oocytes alters junctional protein expression and mitochondrial physiology. PLoS ONE 2015, 10, e0119680. [Google Scholar] [CrossRef]
- Nose, A.; Tsuji, K.; Takeichi, M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell 1990, 61, 147–155. [Google Scholar] [CrossRef]
- McClure, M.J.; Ramey, A.N.; Rashid, M.; Boyan, B.D.; Schwartz, Z. Integrin-α7 signaling regulates connexin 43, M-cadherin, and myoblast fusion. Am. J. Physiol. Cell Physiol. 2019, 316, C876–C887. [Google Scholar] [CrossRef]
- Manibog, K.; Sankar, K.; Kim, S.A.; Zhang, Y.; Jernigan, R.L.; Sivasankar, S. Molecular determinants of cadherin ideal bond formation: Conformation-dependent unbinding on a multidimensional landscape. Proc. Natl. Acad. Sci. USA 2016, 113, E5711–E5720. [Google Scholar] [CrossRef]
- Miron, R.J.; Hedbom, E.; Ruggiero, S.; Bosshardt, D.D.; Zhang, Y.; Mauth, C.; Gemperli, A.C.; Iizuka, T.; Buser, D.; Sculean, A. Premature osteoblast clustering by enamel matrix proteins induces osteoblast differentiation through up-regulation of connexin 43 and N-cadherin. PLoS ONE 2011, 6, e23375. [Google Scholar]
- Iwahashi, N.; Ikezaki, M.; Matsuzaki, I.; Yamamoto, M.; Toujima, S.; Murata, S.I.; Ihara, Y.; Ino, K. Calreticulin Regulates Syncytialization Through Control of the Synthesis and Transportation of E-Cadherin in BeWo Cells. Endocrinology 2019, 160, 359–374. [Google Scholar] [CrossRef]
- Aghababaei, M.; Hogg, K.; Perdu, S.; Robinson, W.P.; Beristain, A.G. ADAM12-directed ectodomain shedding of E-cadherin potentiates trophoblast fusion. Cell Death Differ. 2015, 22, 1970–1984. [Google Scholar] [CrossRef] [PubMed]
- Pey, R.; Vial, C.; Schatten, G.; Hafner, M. Increase of intracellular Ca2+ and relocation of E-cadherin during experimental decompaction of mouse embryos. Proc. Natl. Acad. Sci. USA 1998, 95, 12977–12982. [Google Scholar] [CrossRef]
- Ito, K.; Okamoto, I.; Araki, N.; Kawano, Y.; Nakao, M.; Fujiyama, S.; Tomita, K.; Mimori, T.; Saya, H. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene 1999, 18, 7080–7090. [Google Scholar] [CrossRef]
- Ichinose, S.; Ogawa, T.; Hirokawa, N. Mechanism of Activity-Dependent Cargo Loading via the Phosphorylation of KIF3A by PKA and CaMKIIa. Neuron 2015, 87, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, Y.; Yoshida, K.; Miyado, K.; Sato, M.; Nakamura, A.; Kawano, N.; Sakakibara, K.; Kondo, T.; Harada, Y.; Ohnami, N.; et al. β-catenin is a molecular switch that regulates transition of cell-cell adhesion to fusion. Sci Rep 2011, 1, 68. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Savagner, P. Epithelial-mesenchymal transitions: From cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 2015, 112, 273–300. [Google Scholar] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yi, B.R.; Kim, N.H.; Choi, K.C. Role of the epithelial–mesenchymal transition and its effects on embryonic stem cells. Exp. Mol. Med. 2014, 46, e108. [Google Scholar] [CrossRef]
- Ismagulov, G.; Hamidi, S.; Sheng, G. Epithelial-Mesenchymal Transition Drives Three-Dimensional Morphogenesis in Mammalian Early Development. Front. Cell Dev. Biol. 2021, 9, 639244. [Google Scholar] [CrossRef]
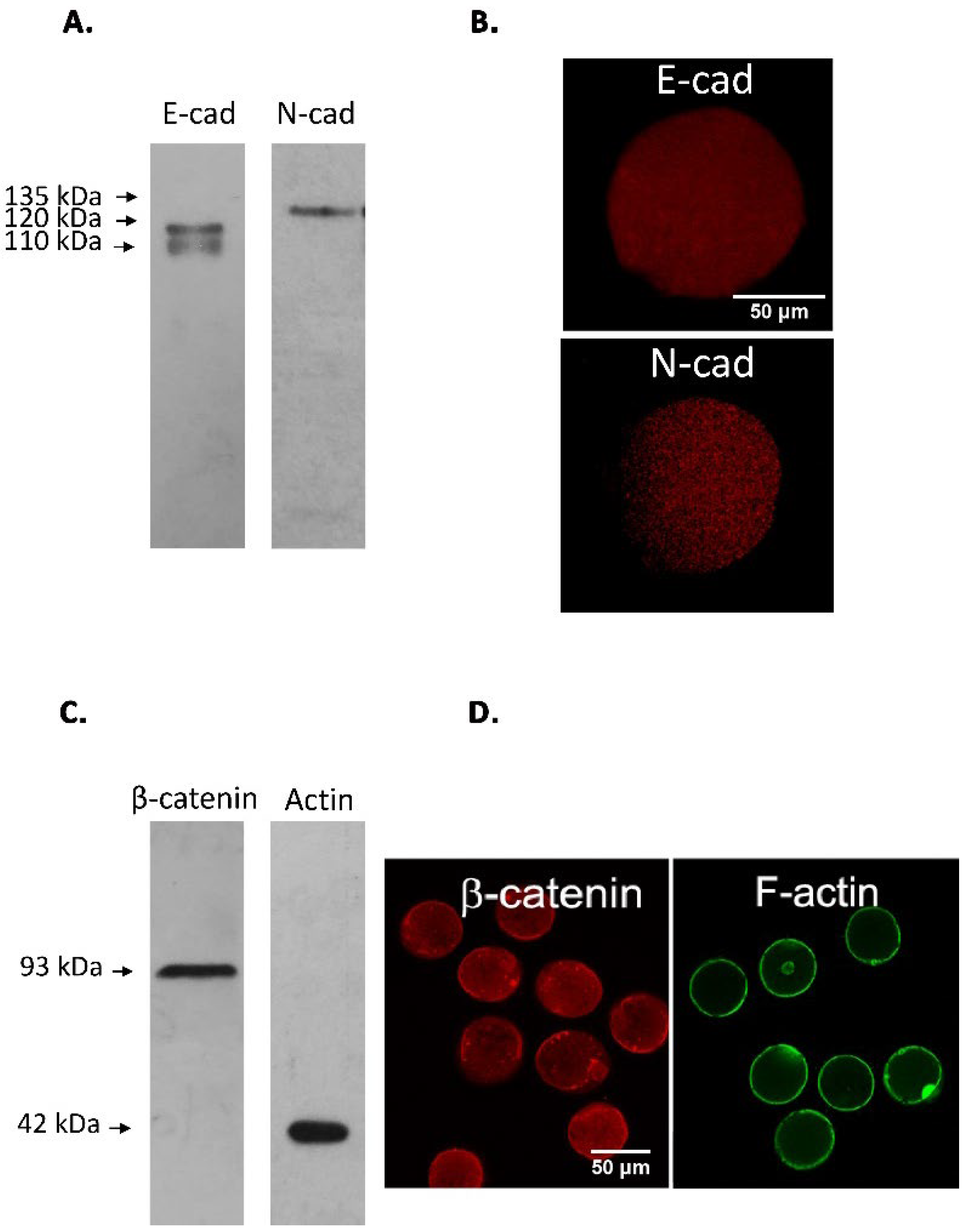
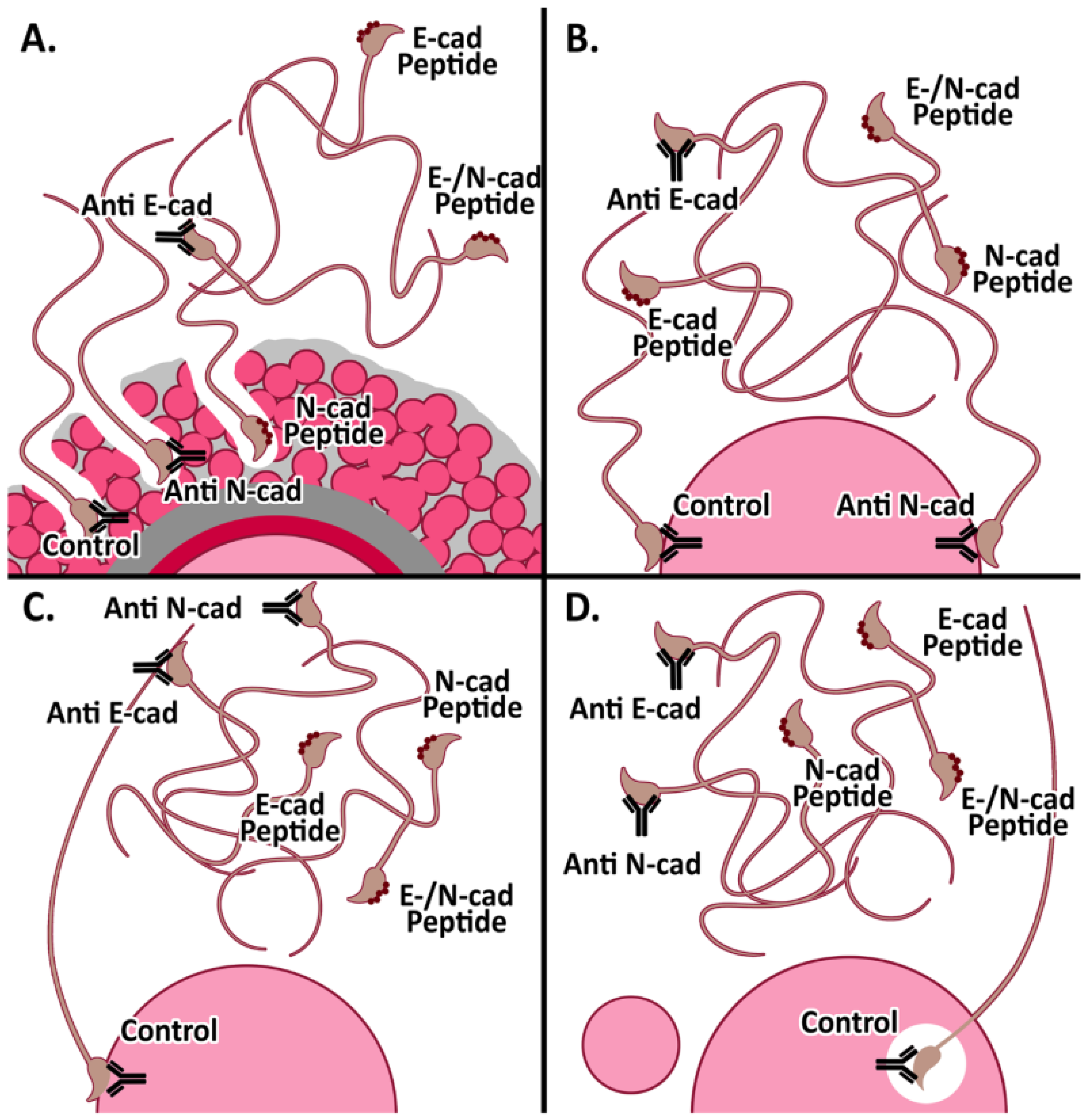
| A. | |||
| E-cad | A + PA | PA | N |
| Intact Non-Capacitated | 90.3 ± 5.7 | 8.0 ± 4.6 | 1.7 ± 1.2 |
| Intact Capacitated | 69.0 ± 6.0 | 22.7 ± 6.7 | 8.3 ± 1.5 |
| A23187 Ca2+-ionophore acrosome-reacted | 20.0 ± 2.7 | 75.3 ± 7.4 | 4.7 ± 4.7 |
| B. | |||
| N-cad | A | A + ES | ES |
| Intact Non-Capacitated | 39.8 ± 19.8 | 51.8 ± 15.6 | 8.4 ±6.0 |
| Intact Capacitated | 40.1 ± 17.9 | 40.6 ± 12.1 | 19.3 ± 6.0 |
| A23187 Ca2+-ionophore acrosome-reacted | 15.9 ± 5.2 | 17.2 ± 7.7 | 66.9 ± 10.0 |
| IVF | Control (%) | Pre-Treated (%) |
|---|---|---|
| COC E-cad Antibody | 66.6 ± 4.4 (n = 117) | 31.8 ± 7.2 * (n = 123) |
| N-cadAntibody | 43.8 ± 9.1 (n = 29) | 14.3 ± 8.3 ** (n = 40) |
| Cumulus-free Oocytes | ||
| E-cad Antibody | 57.5 ± 36.5 (n = 93) | 11.0 ± 1.6 * (n = 95) |
| N-cad Antibody | 56.4 ± 6.1 (n = 30) | 13.9 ± 2.8 ** (n = 30) |
| ZP-Free Oocytes IVF | Control (%) | Pre-Treated (%) |
|---|---|---|
| E-cad Antibody | 58.1 ± 1.0 (n = 24) | 24.5 ± 2.5 * (n = 25) |
| N-cadAntibody | 72.6 ± 6.3 (n = 29) | 27.8 ± 5.6 ** (n = 30) |
| E-cad Peptide | 50.9 ± 4.8 (n = 49) | 18.1 ± 5.9 *** (n = 55) |
| N-cadPeptide | 57.5 ± 8.5 (n = 48) | 24.3 ± 8.2 ** (n = 46) |
| E-/N-cad Dual Peptide | 69.1 ± 6.4 (n = 51) | 22.8 ± 8.6 *** (n = 45) |
| Cumulus Penetration Assay | Control (# Spermatozoa) | Pre-Treated (# Spermatozoa) |
|---|---|---|
| E-cad Antibody | 32.9 ± 2.0 (n = 16) | 16.8 ± 1.0 *** (n = 13) |
| N-cad Antibody | 32.9 ± 2.0 (n = 16) | 29.8 ± 2.3 (n = 17) |
| E-cad Peptide | 38.3 ± 2.0 (n = 32) | 27.3 ± 2.9 ** (n = 29) |
| N-cad Peptide | 32.1 ± 7.0 (n = 27) | 31.9 ± 14.5 (n = 30) |
| E-cad/N-cad Dual Peptide | 43.3 ± 2.4 (n = 28) | 33.6 ± 2.2 * (n = 25) |
| Oolemma Binding | Control (# Spermatozoa) | Pre-treated (# Spermatozoa) |
|---|---|---|
| E-cad Antibody | 25.8 ± 1.1 (n = 70) | 11.5 ± 1.0 *** (n = 60) |
| N-cad Antibody | 18.9 ± 1.6 (n = 54) | 15.8 ± 1.3 (n = 63) |
| E-cad Peptide | 9.0 ± 0.8 (n = 50) | 2.4 ± 0.6*** (n = 52) |
| N-cad Peptide | 14.2 ± 1.3 (n = 59) | 6.8 ± 1.7*** (n = 54) |
| E-cad/N-cad Dual Peptide | 14.5 ± 1.2 (n = 56) | 3.6 ± 0.6*** (n = 56) |
| Sperm-oocyte Fusion | Control (%) | Pre-Treated (%) |
|---|---|---|
| E-cad Antibody | 91.3 ± 4.2 (n = 52) | 24.3 ± 14.6 *** (n = 40) |
| N-cad Antibody | 73.7 ± 3.5 (n = 44) | 44.3 ± 9.9 * (n = 63) |
| E-cad Peptide | 76.5 ± 7.6 (n = 50) | 33.9 ± 7.5 *** (n = 52) |
| N-cad Peptide | 81.0 ± 6.6 (n = 59) | 28.3 ± 4.8 *** (n = 54) |
| E-cad/N-cad Dual Peptide | 75.9 ± 2.2 (n = 56) | 25.2 ± 10.3 *** (n = 56) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verón, G.L.; Veiga, M.F.; Cameo, M.; Marín-Briggiler, C.I.; Vazquez-Levin, M.H. Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model. Cells 2022, 11, 102. https://doi.org/10.3390/cells11010102
Verón GL, Veiga MF, Cameo M, Marín-Briggiler CI, Vazquez-Levin MH. Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model. Cells. 2022; 11(1):102. https://doi.org/10.3390/cells11010102
Chicago/Turabian StyleVerón, Gustavo Luis, María Florencia Veiga, Mónica Cameo, Clara Isabel Marín-Briggiler, and Mónica Hebe Vazquez-Levin. 2022. "Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model" Cells 11, no. 1: 102. https://doi.org/10.3390/cells11010102
APA StyleVerón, G. L., Veiga, M. F., Cameo, M., Marín-Briggiler, C. I., & Vazquez-Levin, M. H. (2022). Epithelial and Neural Cadherin in Mammalian Fertilization: Studies in the Mouse Model. Cells, 11(1), 102. https://doi.org/10.3390/cells11010102






