The Role of Mitochondria in Oocyte Maturation
Abstract
1. Introduction
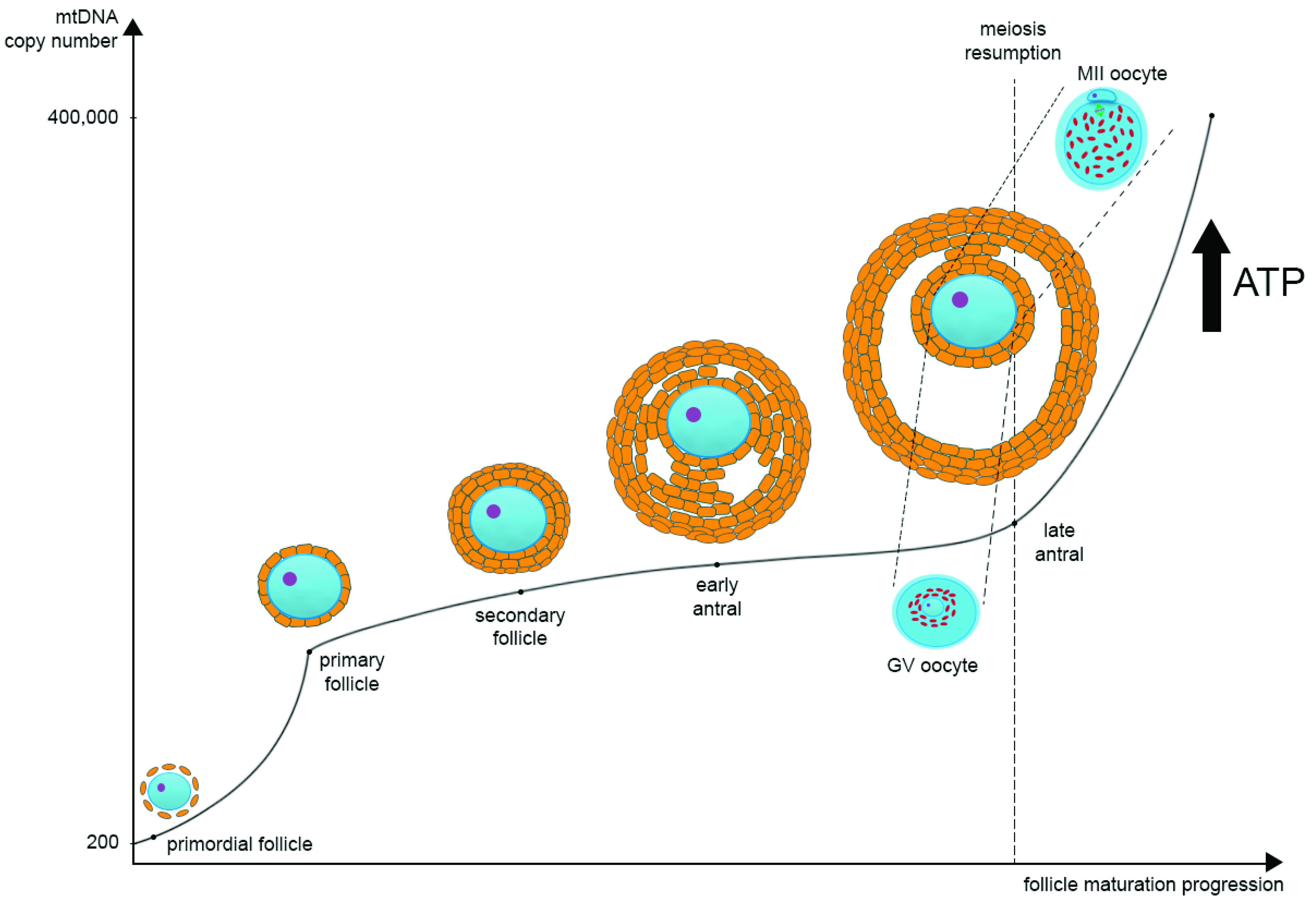
2. Mitochondrial DNA Biology in Brief
3. Mitochondrial Dynamics in Oogenesis
4. The Influence of Mitochondrial Function on Oocyte Quality
5. Mitochondria Patterns during IVM
6. Supplementation of IVM Media with Substances Enhancing the Mitochondrial Function
| Antioxidant | Model | Effect | Possible Mechanism | Reference |
|---|---|---|---|---|
| melatonin | mouse oocytes | ↑ maturation rate | ↓ excessive Ca2+ levels upregulate the SIRT1 function ↑ATP generation ↓ROS levels ↑mtDNA copy number | Nasheed Hamad Almohammed et al. 2020 [97] |
| human oocytes | ↑ implantation rates | Kim et al. 2013 [94] | ||
| rescue IVM oocytes | ↑high-quality blastocyst formation rate ↓aneuploidy rate | Zou et al. 2020 [96] | ||
| resveratrol | mouse oocytes | ↑ maturation, fertilization, and blastocyst formation rates ↓ chromosomal misalignment | ↑ATP generation content ↑mitochondrial membrane potential ↑mitochondrial number | Liu et al. 2018 [92] |
| bovine oocytes | ↑ fertilization and blastocyst formation rates ↑ blastocyst cell number | Takeo et al. 2020 [98] Sugiyama et al. 2015 [100] | ||
| porcine oocytes | ↑ blastocyst formation rate ↑ total blastocyst cell number | Saro et al. 2014 [99] | ||
| human rescue IVM oocytes | ↑ maturation rate ↓ chromosomal misalignment | Liu et al. 2018 [92] | ||
| quercetin | parthenogenetically activated porcine oocytes | ↑ blastocyst formation rate | prevents formation of abnormal mitochondrial structure ROS levels ↓ the extent of apoptosis. ↑ATP generation promotes mitophagy | Kang et al. 2013 [102] |
| goat oocytes | ↑ maturation rate | Silva et al. 2020 [103] | ||
| mouse oocytes | ↑ maturation and early embryonic development formation rates | Cao et al. 2020 [101] | ||
| human rescue IVM oocytes | ↑ maturation and blastocyst formation rates | Cao et al. 2020 [101] | ||
| anethole | bovine oocytes | ↑ cleavage and total cell number per blastocyst rates | likely ↑ the activity of primary antioxidants (superoxide dismutase, catalase, and glutathione peroxidase) | Sá et al. 2020 [104] |
| CoQ10 | ovine oocytes | ↑ blastocyst formation and hatching rates ↓ chromosomal misalignment | physiological diffused pattern of mitochondrial distribution ↑mitochondrial membrane potential ↓ intracellular ROS levels ↓glutathione levels ↑ATP generation ↑mitochondrial number ↑AMPK activity | Heydarnejad et al. 2019 [107] |
| bovine oocytes | ↓ oocyte death | Abdulhasan et al. 2017 [106]. | ||
| porcine oocytes | no effect | Maside et al. 2019 [108] | ||
| human oocytes | ↑ maturation rate ↓aneuploidy rate | Ma et al. 2020 [109] | ||
| mogroside V | porcine oocytes | ↑ maturation and blastocyst formation rates | ↑mtDNA copy number ↑mitochondrial membrane potential ↑ATP generation ↑expression of oxidative-stress-related and mitochondria-related genes ↓ intracellular ROS levels | Nie et al. 2019 [105] |
| MitoQ | murine oocytes | ↑ maturation, fertilization, and blastocyst formation rates | antioxidant against lipid peroxidation ↑mitochondrial membrane potential ↓ intracellular ROS levels | Hosseinzadeh Shirzeyli et al. 2020 [110]; Al-Zubaidi et al. 2021 [111] |
| human rescue IVM oocytes | ↑ maturation rate ↓ chromosomal misalignment | Al-Zubaidi et al. 2021 [111] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology. In Vitro Maturation: A Committee Opinion. Fertil. Steril. 2021, 115, 298–304. [Google Scholar] [CrossRef]
- Walls, M.L.; Hunter, T.; Ryan, J.P.; Keelan, J.A.; Nathan, E.; Hart, R.J. In Vitro Maturation as an Alternative to Standard in Vitro Fertilization for Patients Diagnosed with Polycystic Ovaries: A Comparative Analysis of Fresh, Frozen and Cumulative Cycle Outcomes. Hum. Reprod. 2015, 30, 88–96. [Google Scholar] [CrossRef]
- De Vos, M.; Grynberg, M.; Ho, T.M.; Yuan, Y.; Albertini, D.F.; Gilchrist, R.B. Perspectives on the Development and Future of Oocyte IVM in Clinical Practice. J. Assist. Reprod. Genet. 2021, 38, 1265–1280. [Google Scholar] [CrossRef]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, Mitochondria and Cell Metabolism: A Functional Triangle in Bioenergetics. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of Mitochondrial ATP Production: Ca2+ Signaling and Quality Control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Lill, R.; Freibert, S.-A. Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis. Annu. Rev. Biochem. 2020, 89, 471–499. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as Multifaceted Regulators of Cell Death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J. Mitochondrial Function in the Human Oocyte and Embryo and Their Role in Developmental Competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and Organization of the Human Mitochondrial Genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kukat, C.; Wurm, C.A.; Spahr, H.; Falkenberg, M.; Larsson, N.-G.; Jakobs, S. Super-Resolution Microscopy Reveals That Mammalian Mitochondrial Nucleoids Have a Uniform Size and Frequently Contain a Single Copy of MtDNA. Proc. Natl. Acad. Sci. USA 2011, 108, 13534–13539. [Google Scholar] [CrossRef]
- Bogenhagen, D.F.; Rousseau, D.; Burke, S. The Layered Structure of Human Mitochondrial DNA Nucleoids. J. Biol. Chem. 2008, 283, 3665–3675. [Google Scholar] [CrossRef]
- Song, G.J.; Lewis, V. Mitochondrial DNA Integrity and Copy Number in Sperm from Infertile Men. Fertil. Steril. 2008, 90, 2238–2244. [Google Scholar] [CrossRef]
- Wai, T.; Ao, A.; Zhang, X.; Cyr, D.; Dufort, D.; Shoubridge, E.A. The Role of Mitochondrial DNA Copy Number in Mammalian Fertility1. Biol. Reprod. 2010, 83, 52–62. [Google Scholar] [CrossRef]
- Mikhaylova, A.G.; Mikhailova, A.A.; Ushakova, K.; Tretiakov, E.O.; Shamansky, V.; Yurchenko, A.; Zazhytska, M.; Zdobnov, E.; Makeev, V.; Yurov, V.; et al. Mammalian Mitochondrial Mutational Spectrum as a Hallmark of Cellular and Organismal Aging. bioRxiv 2021. [Google Scholar] [CrossRef]
- Stewart, J.B.; Chinnery, P.F. Extreme Heterogeneity of Human Mitochondrial DNA from Organelles to Populations. Nat. Rev. Genet. 2021, 22, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, R.; Faustin, B.; Rocher, C.; Malgat, M.; Mazat, J.-P.; Letellier, T. Mitochondrial Threshold Effects. Biochem. J. 2003, 370, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, R.; Chinnegy, P.F.; Turnbull, M.; Howell, N. Mammalian mitochondrial Genetics: Heredity, Heteroplasmy and Disease. Trends Genet. 1997, 13, 450–455. [Google Scholar] [CrossRef]
- White, S.L.; Collins, V.R.; Wolfe, R.; Cleary, M.A.; Shanske, S.; DiMauro, S.; Dahl, H.-H.M.; Thorburn, D.R. Genetic Counseling and Prenatal Diagnosis for the Mitochondrial DNA Mutations at Nucleotide 8993. Am. J. Hum. Genet. 1999, 65, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Hauswirth, W.W.; Laipis, P.J. Mitochondrial DNA Polymorphism in a Maternal Lineage of Holstein Cows. Proc. Natl. Acad. Sci. USA 1982, 79, 4686–4690. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shitara, H.; Horii, T.; Nagao, Y.; Imai, H.; Abe, K.; Hara, T.; Hayashi, J.-I.; Yonekawa, H. The Mitochondrial Bottleneck Occurs without Reduction of MtDNA Content in Female Mouse Germ Cells. Nat. Genet. 2007, 39, 386–390. [Google Scholar] [CrossRef]
- Cree, L.M.; Samuels, D.C.; de Sousa Lopes, S.C.; Rajasimha, H.K.; Wonnapinij, P.; Mann, J.R.; Dahl, H.-H.M.; Chinnery, P.F. A Reduction of Mitochondrial DNA Molecules during Embryogenesis Explains the Rapid Segregation of Genotypes. Nat. Genet. 2008, 40, 249–254. [Google Scholar] [CrossRef]
- Floros, V.I.; Pyle, A.; Dietmann, S.; Wei, W.; Tang, W.C.W.; Irie, N.; Payne, B.; Capalbo, A.; Noli, L.; Coxhead, J.; et al. Segregation of Mitochondrial DNA Heteroplasmy through a Developmental Genetic Bottleneck in Human Embryos. Nat. Cell Biol. 2018, 20, 144–151. [Google Scholar] [CrossRef]
- Wai, T.; Teoli, D.; Shoubridge, E.A. The Mitochondrial DNA Genetic Bottleneck Results from Replication of a Subpopulation of Genomes. Nat. Genet. 2008, 40, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Trebichalská, Z.; Kyjovská, D.; Kloudová, S.; Otevřel, P.; Hampl, A.; Holubcová, Z. Cytoplasmic Maturation in Human Oocytes: An Ultrastructural Study. Biol. Reprod. 2021, 104, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Pikó, L.; Matsumoto, L. Number of Mitochondria and Some Properties of Mitochondrial DNA in the Mouse Egg. Dev. Biol. 1976, 49, 1–10. [Google Scholar] [CrossRef]
- Hoitzing, H.; Johnston, I.G.; Jones, N.S. What Is the Function of Mitochondrial Networks? A Theoretical Assessment of Hypotheses and Proposal for Future Research. BioEssays 2015, 37, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.P.S. Germline Passage of Mitochondria: Quantitative Considerations and Possible Embryological Sequelae. Hum. Reprod. 2000, 15, 112–128. [Google Scholar] [CrossRef]
- Santos, T.A.; El Shourbagy, S.; St. John, J.C. Mitochondrial Content Reflects Oocyte Variability and Fertilization Outcome. Fertil. Steril. 2006, 85, 584–591. [Google Scholar] [CrossRef]
- Nicholls, T.J.; Gustafsson, C.M. Separating and Segregating the Human Mitochondrial Genome. Trends Biochem. Sci. 2018, 43, 869–881. [Google Scholar] [CrossRef]
- Ban-Ishihara, R.; Ishihara, T.; Sasaki, N.; Mihara, K.; Ishihara, N. Dynamics of Nucleoid Structure Regulated by Mitochondrial Fission Contributes to Cristae Reformation and Release of Cytochrome c. Proc. Natl. Acad. Sci. USA 2013, 110, 11863–11868. [Google Scholar] [CrossRef]
- Li, H.; Ruan, Y.; Zhang, K.; Jian, F.; Hu, C.; Miao, L.; Gong, L.; Sun, L.; Zhang, X.; Chen, S.; et al. Mic60/Mitofilin Determines MICOS Assembly Essential for Mitochondrial Dynamics and MtDNA Nucleoid Organization. Cell Death Differ. 2016, 23, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Elachouri, G.; Vidoni, S.; Zanna, C.; Pattyn, A.; Boukhaddaoui, H.; Gaget, K.; Yu-Wai-Man, P.; Gasparre, G.; Sarzi, E.; Delettre, C.; et al. OPA1 Links Human Mitochondrial Genome Maintenance to MtDNA Replication and Distribution. Genome Res. 2011, 21, 12–20. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mao, C.-C.; Reyes, A.; Sembongi, H.; Di Re, M.; Granycome, C.; Clippingdale, A.B.; Fearnley, I.M.; Harbour, M.; Robinson, A.J.; et al. The AAA+ Protein ATAD3 Has Displacement Loop Binding Properties and Is Involved in Mitochondrial Nucleoid Organization. J. Cell Biol. 2007, 176, 141–146. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Mitochondrial Dynamics and Inheritance during Cell Division, Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef]
- Martínez-Diez, M.; Santamaría, G.; Ortega, Á.D.; Cuezva, J.M. Biogenesis and Dynamics of Mitochondria during the Cell Cycle: Significance of 3′UTRs. PLoS ONE 2006, 1, e107. [Google Scholar] [CrossRef]
- Cotterill, M.; Harris, S.E.; Collado Fernandez, E.; Lu, J.; Huntriss, J.D.; Campbell, B.K.; Picton, H.M. The Activity and Copy Number of Mitochondrial DNA in Ovine Oocytes throughout Oogenesis in Vivo and during Oocyte Maturation in Vitro. MHR: Basic Sci. Reprod. Med. 2013, 19, 444–450. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.Q.; Lu, S.; Guo, Y.L.; Ma, X. Deficit of Mitochondria-Derived ATP during Oxidative Stress Impairs Mouse MII Oocyte Spindles. Cell Res. 2006, 16, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Whitworth, K.M.; Spate, L.D.; Walters, E.M.; Zhao, J.; Prather, R.S. Regulation of Oocyte Mitochondrial DNA Copy Number by Follicular Fluid, EGF, and Neuregulin 1 during in Vitro Maturation Affects Embryo Development in Pigs. Theriogenology 2012, 78, 887–897. [Google Scholar] [CrossRef] [PubMed]
- St. John, J.C. Mitochondria and Female Germline Stem Cells—A Mitochondrial DNA Perspective. Cells 2019, 8, 852. [Google Scholar] [CrossRef]
- St. John, J.C. Epigenetic Regulation of the Nuclear and Mitochondrial Genomes: Involvement in Metabolism, Development, and Disease. Annu. Rev. Anim. Biosci. 2021, 9, 203–224. [Google Scholar] [CrossRef]
- Lee, W.; Johnson, J.; Gough, D.J.; Donoghue, J.; Cagnone, G.L.M.; Vaghjiani, V.; Brown, K.A.; Johns, T.G.; St. John, J.C. Mitochondrial DNA Copy Number Is Regulated by DNA Methylation and Demethylation of POLGA in Stem and Cancer Cells and Their Differentiated Progeny. Cell Death Dis. 2015, 6, e1664. [Google Scholar] [CrossRef] [PubMed]
- Dzitoyeva, S.; Chen, H.; Manev, H. Effect of Aging on 5-Hydroxymethylcytosine in Brain Mitochondria. Neurobiol. Aging 2012, 33, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Johnson, J.; St. John, J.C. Global DNA Methylation Synergistically Regulates the Nuclear and Mitochondrial Genomes in Glioblastoma Cells. Nucleic Acids Res. 2018, 46, 5977–5995. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Feng, H.L.; Yan, J.H.; Li, M.; Ma, S.Y.; Chen, Z.J. Dynamic Modulation of Cytoskeleton during in Vitro Maturation in Human Oocytes. Am. J. Obstet. Gynecol. 2010, 203, 151.e1–151.e7. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hashimoto, S.; Yamochi, T.; Goto, H.; Yamanaka, M.; Amo, A.; Matsumoto, H.; Inoue, M.; Ito, K.; Nakaoka, Y.; et al. Dynamic Changes in Mitochondrial Distribution in Human Oocytes during Meiotic Maturation. J. Assist. Reprod. Genet. 2016, 33, 929–938. [Google Scholar] [CrossRef]
- Dalton, C.M.; Carroll, J. Biased Inheritance of Mitochondria during Asymmetric Cell Division in the Mouse Oocyte. J. Cell Sci. 2013, 126, 2955–2964. [Google Scholar] [CrossRef]
- Woods, D.; Khrapko, K.; Tilly, J. Influence of Maternal Aging on Mitochondrial Heterogeneity, Inheritance, and Function in Oocytes and Preimplantation Embryos. Genes 2018, 9, 265. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondria in Human Oogenesis and Preimplantation Embryogenesis: Engines of Metabolism, Ionic Regulation and Developmental Competence. Reproduction 2004, 128, 269–280. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial Membrane Potential Probes and the Proton Gradient: A Practical Usage Guide. BioTechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Dumollard, R.; Campbell, K.; Halet, G.; Carroll, J.; Swann, K. Regulation of Cytosolic and Mitochondrial ATP Levels in Mouse Eggs and Zygotes. Dev. Biol. 2008, 316, 431–440. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chrétien, M.F.; Jacques, C.; Vasseur, C.; Malthièry, Y.; Reynier, P. Low Oocyte Mitochondrial DNA Content in Ovarian Insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, M.; Alfarawati, S.; Hurd, D.; Paolucci, M.; Shovelton, J.; Fragouli, E.; Wells, D. Simultaneous Assessment of Aneuploidy, Polymorphisms, and Mitochondrial DNA Content in Human Polar Bodies and Embryos with the Use of a Novel Microarray Platform. Fertil. Steril. 2014, 102, 1385–1392. [Google Scholar] [CrossRef]
- Desler, C.; Munch-Petersen, B.; Stevnsner, T.; Matsui, S.-I.; Kulawiec, M.; Singh, K.K.; Rasmussen, L.J. Mitochondria as Determinant of Nucleotide Pools and Chromosomal Stability. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2007, 625, 112–124. [Google Scholar] [CrossRef]
- Wilding, M.; De Placido, G.; De Matteo, L.; Marino, M.; Alviggi, C.; Dale, B. Chaotic Mosaicism in Human Preimplantation Embryos Is Correlated with a Low Mitochondrial Membrane Potential. Fertil. Steril. 2003, 79, 340–346. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y. Adenosine Triphosphate Content in Human Unfertilized Oocytes, Undivided Zygotes and Embryos Unsuitable for Transfer or Cryopreservation. J. Int. Med. Res. 2012, 40, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Miller, R.A.; Smith, I.; Bui, T.; Molgó, J.; Müller, M.; Vais, H.; Cheung, K.-H.; Yang, J.; Parker, I.; et al. Essential Regulation of Cell Bioenergetics by Constitutive InsP3 Receptor Ca2+ Transfer to Mitochondria. Cell 2010, 142, 270–283. [Google Scholar] [CrossRef]
- Baumgartner, H.K.; Gerasimenko, J.V.; Thorne, C.; Ferdek, P.; Pozzan, T.; Tepikin, A.V.; Petersen, O.H.; Sutton, R.; Watson, A.J.M.; Gerasimenko, O.V. Calcium Elevation in Mitochondria Is the Main Ca2+ Requirement for Mitochondrial Permeability Transition Pore (MPTP) Opening. J. Biol. Chem. 2009, 284, 20796–20803. [Google Scholar] [CrossRef]
- Fink, B.D.; Bai, F.; Yu, L.; Sivitz, W.I. Regulation of ATP Production: Dependence on Calcium Concentration and Respiratory State. Am. J. Physiol.—Cell Physiol. 2017, 313, C146–C153. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA Mutations in Disease and Aging. Environ. Mol. Mutagen. 2010, 51, 440–450. [Google Scholar] [CrossRef]
- Rebolledo-Jaramillo, B.; Su, M.S.-W.; Stoler, N.; McElhoe, J.A.; Dickins, B.; Blankenberg, D.; Korneliussen, T.S.; Chiaromonte, F.; Nielsen, R.; Holland, M.M.; et al. Maternal Age Effect and Severe Germ-Line Bottleneck in the Inheritance of Human Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 2014, 111, 15474–15479. [Google Scholar] [CrossRef]
- Arbeithuber, B.; Hester, J.; Cremona, M.A.; Stoler, N.; Zaidi, A.; Higgins, B.; Anthony, K.; Chiaromonte, F.; Diaz, F.J.; Makova, K.D. Age-Related Accumulation of de Novo Mitochondrial Mutations in Mammalian Oocytes and Somatic Tissues. PLoS Biol. 2020, 18, e3000745. [Google Scholar] [CrossRef]
- Colnaghi, M.; Pomiankowski, A.; Lane, N. The Need for High-Quality Oocyte Mitochondria at Extreme Ploidy Dictates Germline Development. Elife 2021, 10, e69344. [Google Scholar] [CrossRef]
- Stewart, J.B.; Freyer, C.; Elson, J.L.; Larsson, N.-G. Purifying Selection of MtDNA and Its Implications for Understanding Evolution and Mitochondrial Disease. Nat. Rev. Genet. 2008, 9, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Yang, Y.; Shi, S.; Ji, D.; Pan, J. Germline Selection by Meiosis Defends the Transmission of Defective Mitochondria with MtDNA Variants. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mao, L.; Lou, H.; Lou, Y.; Wang, N.; Jin, F. Behaviour of Cytoplasmic Organelles and Cytoskeleton during Oocyte Maturation. Reprod. BioMed. Online 2014, 28, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.; Romero, S.; De Vos, M.; Verheyen, G.; Smitz, J. Human Cumulus-Enclosed Germinal Vesicle Oocytes from Early Antral Follicles Reveal Heterogeneous Cellular and Molecular Features Associated with in Vitro Maturation Capacity. Hum. Reprod. 2015, 30, 1396–1409. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Gao, X.; Yan, J.-H.; Chen, Z.-J. Changes in the Distribution of Mitochondria before and after in Vitro Maturation of Human Oocytes and the Effect of in Vitro Maturation on Mitochondria Distribution. Fertil. Steril. 2010, 93, 1550–1555. [Google Scholar] [CrossRef]
- Shahedi, A.; Khalili, M.A.; Soleimani, M.; Morshedizad, S. Ultrastructure of in Vitro Matured Human Oocytes. Iran Red Crescent Med. J. 2013, 15, e7379. [Google Scholar] [CrossRef][Green Version]
- Coticchio, G.; Dal Canto, M.; Fadini, R.; Mignini Renzini, M.; Guglielmo, M.C.; Miglietta, S.; Palmerini, M.G.; Macchiarelli, G.; Nottola, S.A. Ultrastructure of Human Oocytes after in Vitro Maturation. Mol. Hum. Reprod. 2016, 22, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Motta, P.M.; Nottola, S.A.; Micara, G.; Familiari, G. Ultrastructure of Human Unfertilized Oocytes and Polyspermic Embryos in an IVF-ET Program. Ann. N. Y. Acad. Sci. 1988, 541, 367–383. [Google Scholar] [CrossRef]
- Motta, P.M.; Nottola, S.A.; Makabe, S.; Heyn, R. Mitochondrial Morphology in Human Fetal and Adult Female Germ Cells. Hum. Reprod. 2000, 15, 129–147. [Google Scholar] [CrossRef]
- Familiari, G.; Heyn, R.; Relucenti, M.; Nottola, S.A.; Sathananthan, A.H. Ultrastructural Dynamics of Human Reproduction, from Ovulation to Fertilization and Early Embryo Development1. Int. Rev. Cytol. 2006, 249, 53–141. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Landis, J.N.; Krisher, R.L.; Duncan, F.E.; Silva, E.; Lonczak, A.; Scott, R.T.; Zhan, Y.; Chu, T.; Scott, R.T.; et al. Mitochondrial DNA Content Is Associated with Ploidy Status, Maternal Age, and Oocyte Maturation Methods in Mouse Blastocysts. J. Assist. Reprod. Genet. 2017, 34, 1587–1594. [Google Scholar] [CrossRef]
- Ge, H.; Tollner, T.L.; Hu, Z.; Da, M.; Li, X.; Guan, H.; Shan, D.; Lu, J.; Huang, C.; Dong, Q. Impaired Mitochondrial Function in Murine Oocytes Is Associated with Controlled Ovarian Hyperstimulationand in Vitro Maturation. Reprod. Fertil. Dev. 2012, 24, 945. [Google Scholar] [CrossRef]
- Zhao, H.; Li, T.; Zhao, Y.; Tan, T.; Liu, C.; Liu, Y.; Chang, L.; Huang, N.; Li, C.; Fan, Y.; et al. Single-Cell Transcriptomics of Human Oocytes: Environment-Driven Metabolic Competition and Compensatory Mechanisms During Oocyte Maturation. Antioxid. Redox Signal. 2019, 30, 542–559. [Google Scholar] [CrossRef]
- Torner, H.; Brüssow, K.-P.; Alm, H.; Ratky, J.; Pöhland, R.; Tuchscherer, A.; Kanitz, W. Mitochondrial Aggregation Patterns and Activity in Porcine Oocytes and Apoptosis in Surrounding Cumulus Cells Depends on the Stage of Pre-Ovulatory Maturation. Theriogenology 2004, 61, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Torner, H.; Alm, H.; Kanitz, W.; Goellnitz, K.; Becker, F.; Poehland, R.; Bruessow, K.-P.; Tuchscherer, A. Effect of Initial Cumulus Morphology on Meiotic Dynamic and Status of Mitochondria in Horse Oocytes during IVM. Reprod. Domest. Anim. 2007, 42, 176–183. [Google Scholar] [CrossRef]
- Liu, S.; Feng, H.L.; Marchesi, D.; Chen, Z.-J.; Hershlag, A. Effect of Gonadotropins on Dynamic Events and Global Deoxyribonucleic Acid Methylation during in Vitro Maturation of Oocytes: An Animal Model. Fertil. Steril. 2011, 95, 1503–1506.e3. [Google Scholar] [CrossRef] [PubMed]
- Pinyopummintr, T.; Bavister, B.D. Optimum Gas Atmosphere for in Vitro Maturation and in Vitro Fertilization of Bovine Oocytes. Theriogenology 1995, 44, 471–477. [Google Scholar] [CrossRef]
- Whitty, A.; Kind, K.L.; Dunning, K.R.; Thompson, J.G. Effect of Oxygen and Glucose Availability during in Vitro Maturation of Bovine Oocytes on Development and Gene Expression. J. Assist. Reprod. Genet. 2021, 38, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N. Mechanisms of Mitochondrial ROS Production in Assisted Reproduction: The Known, the Unknown, and the Intriguing. Antioxidants 2020, 9, 933. [Google Scholar] [CrossRef]
- Sánchez, F.; Lolicato, F.; Romero, S.; De Vos, M.; Van Ranst, H.; Verheyen, G.; Anckaert, E.; Smitz, J.E.J. An Improved IVM Method for Cumulus-Oocyte Complexes from Small Follicles in Polycystic Ovary Syndrome Patients Enhances Oocyte Competence and Embryo Yield. Hum. Reprod. 2017, 32, 2056–2068. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.; Le, A.H.; Ho, V.N.A.; Romero, S.; Van Ranst, H.; De Vos, M.; Gilchrist, R.B.; Ho, T.M.; Vuong, L.N.; Smitz, J. Biphasic in Vitro Maturation (CAPA-IVM) Specifically Improves the Developmental Capacity of Oocytes from Small Antral Follicles. J. Assist. Reprod. Genet. 2019, 36, 2135–2144. [Google Scholar] [CrossRef]
- Vuong, L.N.; Le, A.H.; Ho, V.N.A.; Pham, T.D.; Sanchez, F.; Romero, S.; De Vos, M.; Ho, T.M.; Gilchrist, R.B.; Smitz, J. Live Births after Oocyte in Vitro Maturation with a Prematuration Step in Women with Polycystic Ovary Syndrome. J. Assist. Reprod. Genet. 2020, 37, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Bunyaeva, E.; Van Ranst, H.; Khabas, G.; Farmakovskaya, M.; Kamaletdinov, N.; Nazarenko, T.; Abubakirov, A.; Sukhikh, G.; Smitz, J.E.J. Improved Maturation Competence of Ovarian Tissue Oocytes Using a Biphasic in Vitro Maturation System for Patients with Gynecological Malignancy: A Study on Sibling Oocytes. J. Assist. Reprod. Genet. 2021, 38, 1331–1340. [Google Scholar] [CrossRef]
- Santiquet, N.W.; Greene, A.F.; Becker, J.; Barfield, J.P.; Schoolcraft, W.B.; Krisher, R.L. A Pre-in Vitro Maturation Medium Containing Cumulus Oocyte Complex Ligand-Receptor Signaling Molecules Maintains Meiotic Arrest, Supports the Cumulus Oocyte Complex and Improves Oocyte Developmental Competence. MHR Basic Sci. Reprod. Med. 2017, 23, 594–606. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, X.; Krysta, A.E.; Bertoldo, M.J.; Richani, D.; Gilchrist, R.B. Capacitation IVM Improves Cumulus Function and Oocyte Quality in Minimally Stimulated Mice. J. Assist. Reprod. Genet. 2020, 37, 77–88. [Google Scholar] [CrossRef]
- Rodríguez-Varela, C.; Labarta, E. Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Liu, M.-J.; Sun, A.-G.; Zhao, S.-G.; Liu, H.; Ma, S.-Y.; Li, M.; Huai, Y.-X.; Zhao, H.; Liu, H.-B. Resveratrol Improves in Vitro Maturation of Oocytes in Aged Mice and Humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Ji, D.; Liu, Z.-B.; Wang, T.-J.; Xie, F.-F.; Zhang, Z.-G.; Wei, Z.-L.; Zhou, P.; Cao, Y.-X. Melatonin Maintains Mitochondrial Membrane Potential and Decreases Excessive Intracellular Ca2+ Levels in Immature Human Oocytes. Life Sci. 2019, 235, 116810. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, E.A.; Kim, H.J.; Choi, W.Y.; Cho, J.H.; Lee, W.S.; Cha, K.Y.; Kim, Y.S.; Lee, D.R.; Yoon, T.K. Does Supplementation of In-Vitro Culture Medium with Melatonin Improve IVF Outcome in PCOS? Reprod. BioMed. Online 2013, 26, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, Z.; Han, D.; Cao, Y.; Zhou, P.; Wei, Z.; Lv, M.; Chen, D. Gene Expression Profiling of Human Blastocysts from in Vivo and ‘Rescue IVM’ with or without Melatonin Treatment. Mol. Med. Rep. 2017, 16, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, B.; Ding, D.; Gao, M.; Chen, D.; Liu, Y.; Hao, Y.; Zou, W.; Ji, D.; Zhou, P.; et al. Melatonin Promotes the Development of Immature Oocytes from the COH Cycle into Healthy Offspring by Protecting Mitochondrial Function. J. Pineal. Res. 2020, 68, e12621. [Google Scholar] [CrossRef] [PubMed]
- Nasheed Hamad Almohammed, Z.; Moghani-Ghoroghi, F.; Ragerdi kashani, I.; Fathi, R.; Tahaei, L.S.; Naji, M.; Pasbakhsh, P. The Effect of Melatonin on Mitochondrial Function and Autophagy in In Vitro Matured Oocytes of Aged Mice. Cell J. 2019, 22, 9. [Google Scholar] [CrossRef]
- Takeo, S.; Sato, D.; Kimura, K.; Monji, Y.; Kuwayama, T.; Kawahara-Miki, R.; Iwata, H. Resveratrol Improves the Mitochondrial Function and Fertilization Outcome of Bovine Oocytes. J. Reprod. Dev. 2014, 60, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Itami, N.; Tasaki, H.; Takeo, S.; Kuwayama, T.; Iwata, H. Relationship between Mitochondrial DNA Copy Number and SIRT1 Expression in Porcine Oocytes. PLoS ONE 2014, 9, e94488. [Google Scholar] [CrossRef]
- Sugiyama, M.; Kawahara-Miki, R.; Kawana, H.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol-Induced Mitochondrial Synthesis and Autophagy in Oocytes Derived from Early Antral Follicles of Aged Cows. J. Reprod. Dev. 2015, 61, 251–259. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, H.; Wang, Z.; Zhang, C.; Bian, Y.; Liu, X.; Zhang, C.; Zhang, X.; Zhao, Y. Quercetin Promotes in Vitro Maturation of Oocytes from Humans and Aged Mice. Cell Death Dis. 2020, 11, 965. [Google Scholar] [CrossRef]
- Kang, J.-T.; Kwon, D.-K.; Park, S.-J.; Kim, S.-J.; Moon, J.-H.; Koo, O.-J.; Jang, G.; Lee, B.-C. Quercetin Improves the in Vitro Development of Porcine Oocytes by Decreasing Reactive Oxygen Species Levels. J. Vet. Sci. 2013, 14, 15. [Google Scholar] [CrossRef]
- Silva, A.A.A.; Silva, M.N.P.; Figueiredo, L.B.F.; Gonçalves, J.D.; Silva, M.J.S.; Loiola, M.L.G.; Bastos, B.D.M.; Oliveira, R.A.; Ribeiro, L.G.M.; Barberino, R.S.; et al. Quercetin Influences in Vitro Maturation, Apoptosis and Metabolically Active Mitochondria of Goat Oocytes. Zygote 2018, 26, 465–470. [Google Scholar] [CrossRef]
- Sá, N.A.R.; Vieira, L.A.; Ferreira, A.C.A.; Cadenas, J.; Bruno, J.B.; Maside, C.; Sousa, F.G.C.; Cibin, F.W.S.; Alves, B.G.; Rodrigues, A.P.R.; et al. Anethole Supplementation During Oocyte Maturation Improves In Vitro Production of Bovine Embryos. Reprod. Sci. 2020, 27, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Yan, K.; Sui, L.; Zhang, H.; Zhang, H.; Yang, X.; Lu, S.; Lu, K.; Liang, X. Mogroside V Improves Porcine Oocyte in Vitro Maturation and Subsequent Embryonic Development. Theriogenology 2020, 141, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Abdulhasan, M.K.; Li, Q.; Dai, J.; Abu-Soud, H.M.; Puscheck, E.E.; Rappolee, D.A. CoQ10 Increases Mitochondrial Mass and Polarization, ATP and Oct4 Potency Levels, and Bovine Oocyte MII during IVM While Decreasing AMPK Activity and Oocyte Death. J. Assist. Reprod. Genet. 2017, 34, 1595–1607. [Google Scholar] [CrossRef]
- Heydarnejad, A.; Ostadhosseini, S.; Varnosfaderani, S.R.; Jafarpour, F.; Moghimi, A.; Nasr-Esfahani, M.H. Supplementation of Maturation Medium with CoQ10 Enhances Developmental Competence of Ovine Oocytes through Improvement of Mitochondrial Function. Mol. Reprod. Dev. 2019, 86, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Maside, C.; Martinez, C.A.; Cambra, J.M.; Lucas, X.; Martinez, E.A.; Gil, M.A.; Rodriguez-Martinez, H.; Parrilla, I.; Cuello, C. Supplementation with Exogenous Coenzyme Q10 to Media for in Vitro Maturation and Embryo Culture Fails to Promote the Developmental Competence of Porcine Embryos. Reprod. Dom. Anim. 2019, 54, 72–77. [Google Scholar] [CrossRef]
- Ma, L.; Cai, L.; Hu, M.; Wang, J.; Xie, J.; Xing, Y.; Shen, J.; Cui, Y.; Liu, X.J.; Liu, J. Coenzyme Q10 Supplementation of Human Oocyte in Vitro Maturation Reduces Postmeiotic Aneuploidies. Fertil. Steril. 2020, 114, 331–337. [Google Scholar] [CrossRef]
- Hosseinzadeh Shirzeyli, M.; Amidi, F.; Shamsara, M.; Nazarian, H.; Eini, F.; Hosseinzadeh Shirzeyli, F.; Majidi Zolbin, M.; Ghaffari Novin, M.; Daliri Joupari, M. Exposing Mouse Oocytes to MitoQ During In Vitro Maturation Improves Maturation and Developmental Competence. Iran. J. Biotechnol. 2020, 18. [Google Scholar] [CrossRef]
- Al-Zubaidi, U.; Adhikari, D.; Cinar, O.; Zhang, Q.-H.; Yuen, W.S.; Murphy, M.P.; Rombauts, L.; Robker, R.L.; Carroll, J. Mitochondria-Targeted Therapeutics, MitoQ and BGP-15, Reverse Aging-Associated Meiotic Spindle Defects in Mouse and Human Oocytes. Hum. Reprod. 2021, 36, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.J.; Murphy, M.P. Selective Targeting of a Redox-Active Ubiquinone to Mitochondria within Cells. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.J.; Murphy, M.P. Animal and Human Studies with the Mitochondria-Targeted Antioxidant MitoQ: Smith and Murphy. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bentov, Y.; Yavorska, T.; Esfandiari, N.; Jurisicova, A.; Casper, R.F. The Contribution of Mitochondrial Function to Reproductive Aging. J. Assist. Reprod. Genet. 2011, 28, 773–783. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. https://doi.org/10.3390/cells10092484
Kirillova A, Smitz JEJ, Sukhikh GT, Mazunin I. The Role of Mitochondria in Oocyte Maturation. Cells. 2021; 10(9):2484. https://doi.org/10.3390/cells10092484
Chicago/Turabian StyleKirillova, Anastasia, Johan E. J. Smitz, Gennady T. Sukhikh, and Ilya Mazunin. 2021. "The Role of Mitochondria in Oocyte Maturation" Cells 10, no. 9: 2484. https://doi.org/10.3390/cells10092484
APA StyleKirillova, A., Smitz, J. E. J., Sukhikh, G. T., & Mazunin, I. (2021). The Role of Mitochondria in Oocyte Maturation. Cells, 10(9), 2484. https://doi.org/10.3390/cells10092484






