Beneficial Effects of Resveratrol in Mouse Gastrocnemius: A Hint to Muscle Phenotype and Proteolysis
Abstract
1. Introduction
2. Methods
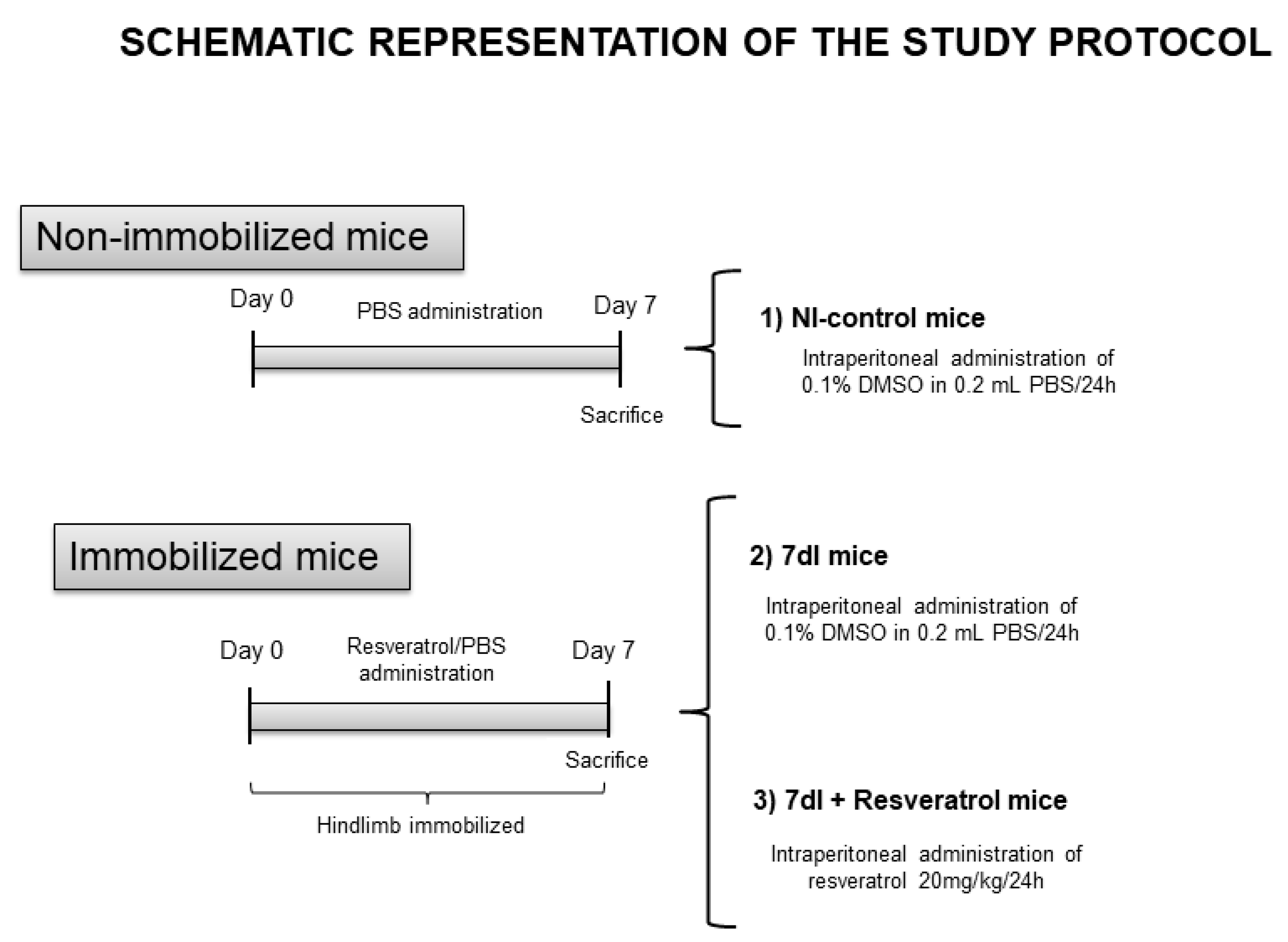
2.1. Animal Experiments
2.2. Ethics
2.3. In Vivo Measurements in the Mice
2.4. Sacrifice and Sample Collection
2.5. Biological Analyses
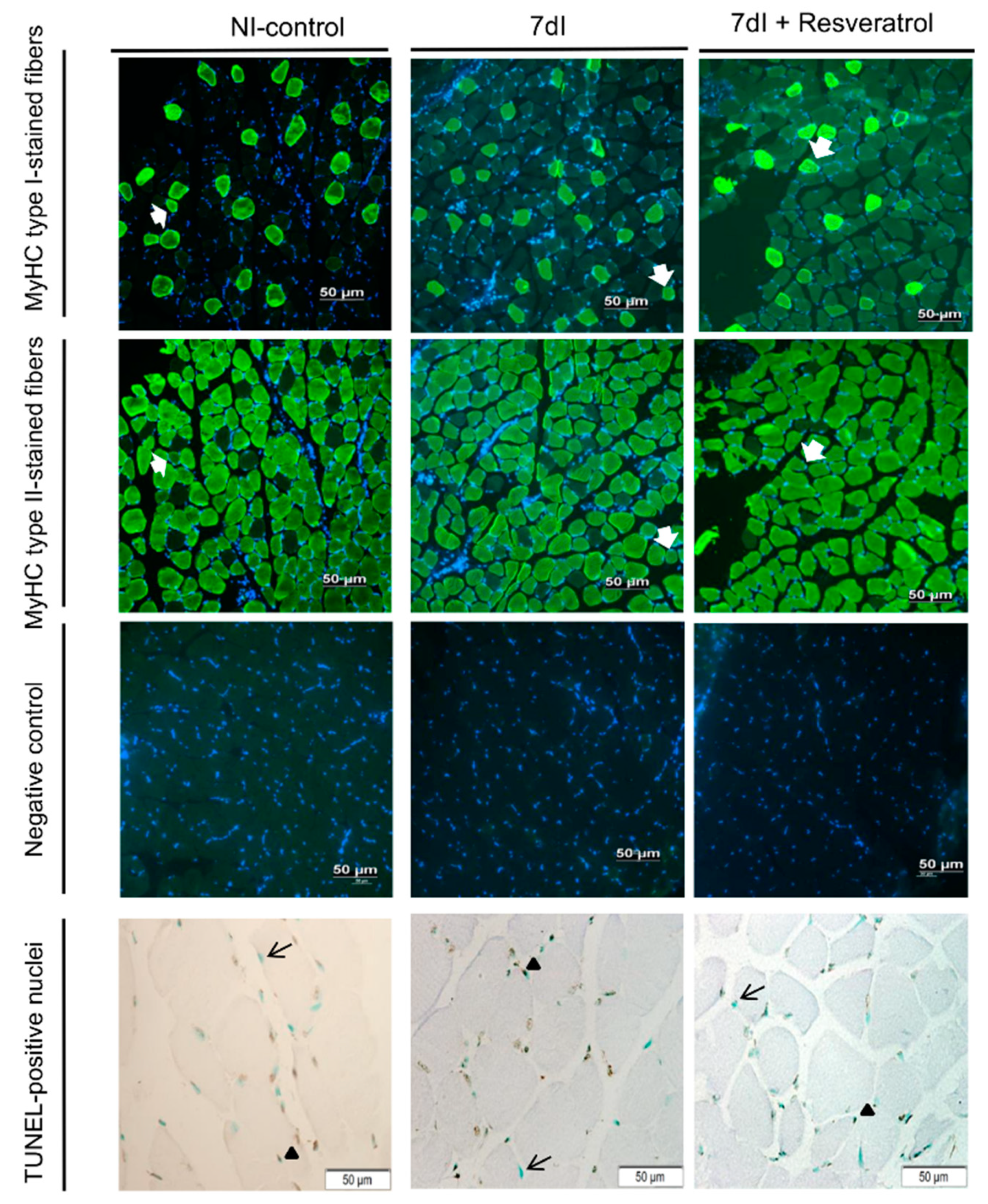
2.5.1. Muscle Fiber Type and Morphometry
2.5.2. Terminal Deoxynucleotidyl Transferase-Mediated Uridine 5′-Triphosphate (UTP) Nick-End Labeling (TUNEL) Assay
2.5.3. Ribonucleic Acid (RNA) Extraction
2.5.4. Procedures of Messenger (mRNA) Reverse Transcription (RT)
2.5.5. Quantitative Real Time-Polymerase Chain Reaction Amplification (qRT-PCR)
2.5.6. Immunoblotting of 1D Electrophoresis
2.5.7. Protein Catabolism
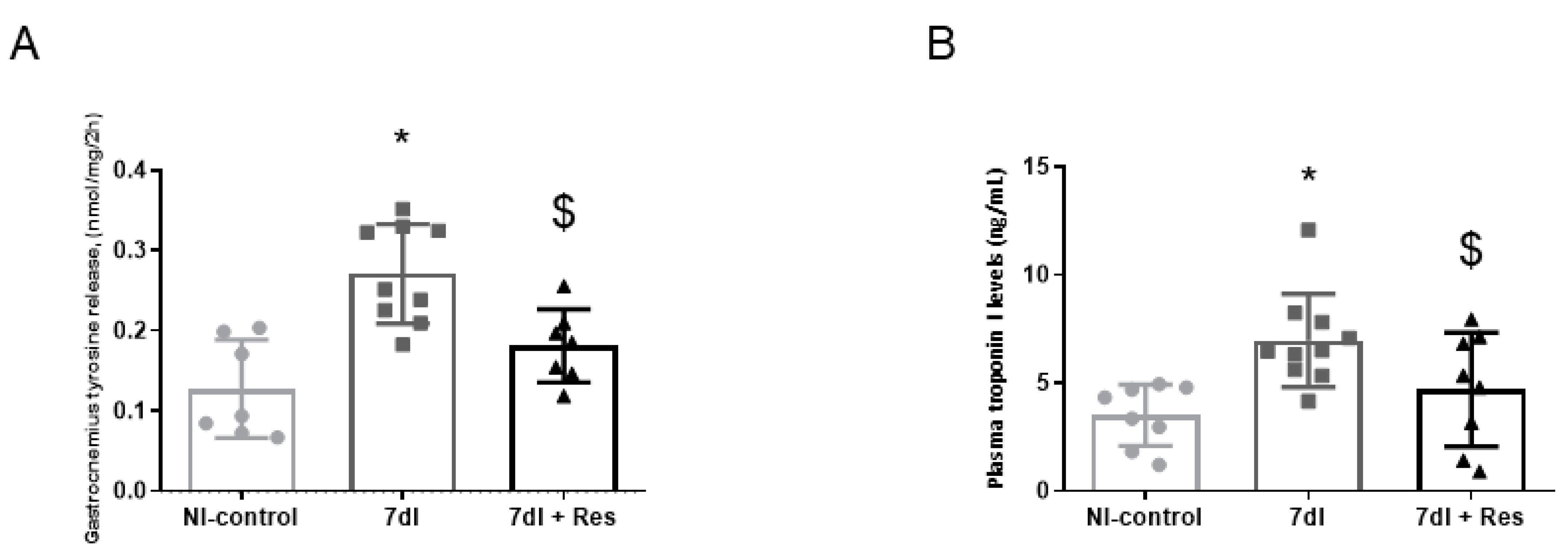
2.5.8. Enzyme-Linked Immunosorbent Assay (ELISA) Plasma Skeletal Muscle Troponin-I Levels
2.6. Statistical Analysis
3. Results
3.1. Physiological Characteristics of the Study Animals
3.1.1. Non-Immobilized versus Unloading Conditions
3.1.2. Unloading with Resveratrol versus Unloading
3.2. Structural Phenotypic Characteristics
3.2.1. Non-Immobilized versus Unloading Conditions
3.2.2. Unloading with Resveratrol versus Unloading
3.3. Muscle Proteolysis
3.3.1. Non-Immobilized versus Unloading Conditions
3.3.2. Unloading with Resveratrol versus Unloading
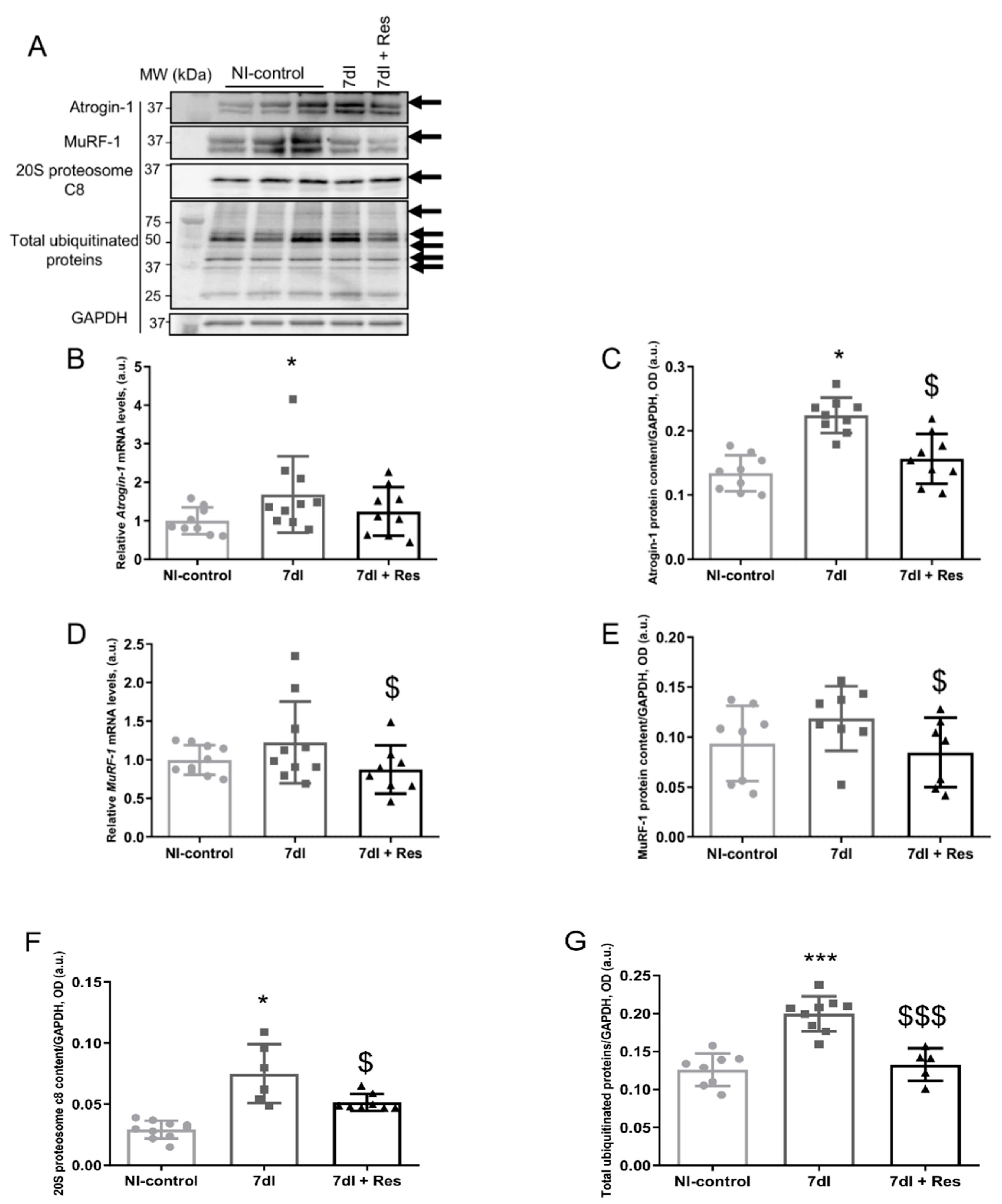
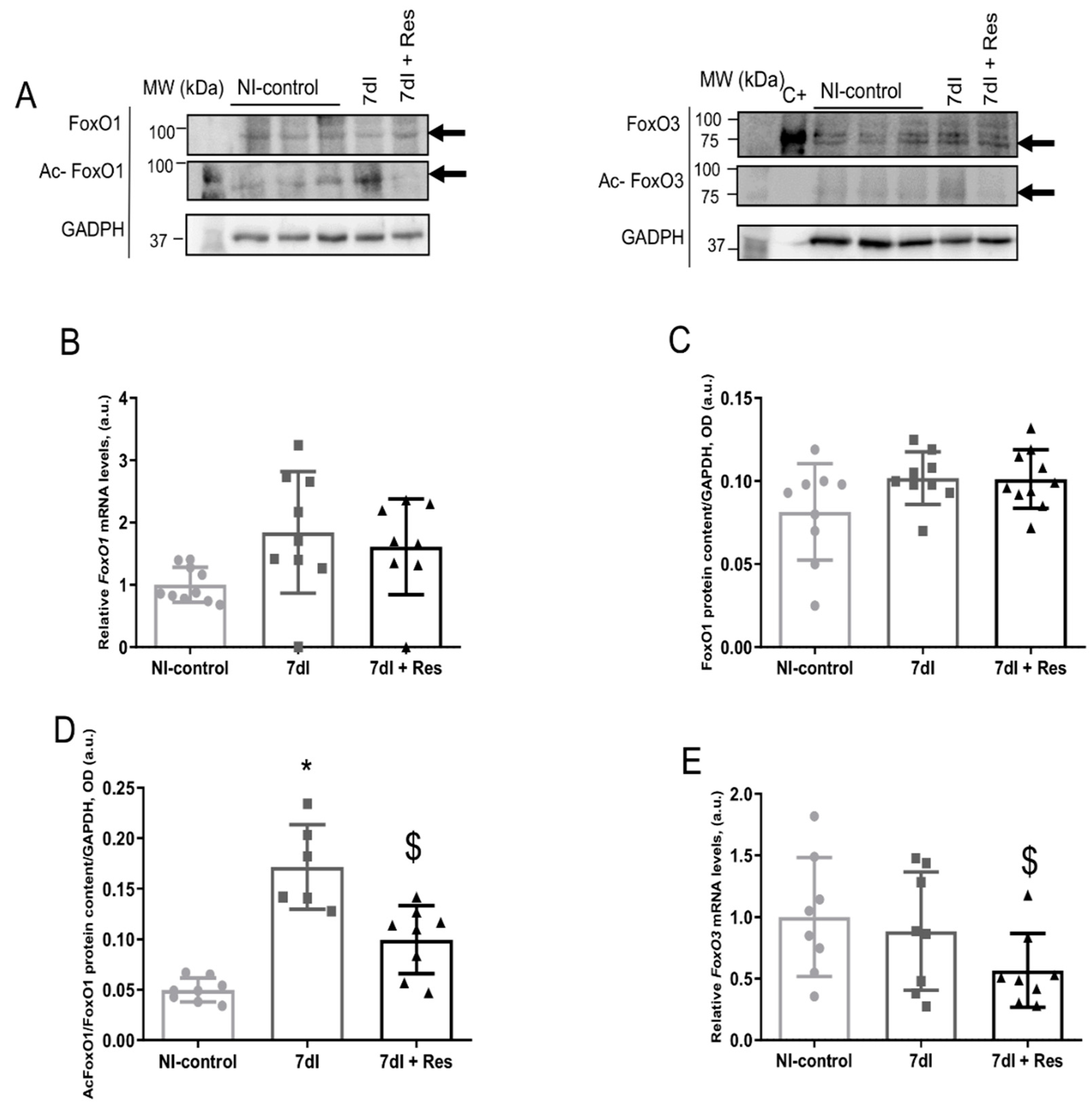
3.4. Markers of Proteolysis
3.4.1. Non-Immobilized versus Unloading Conditions
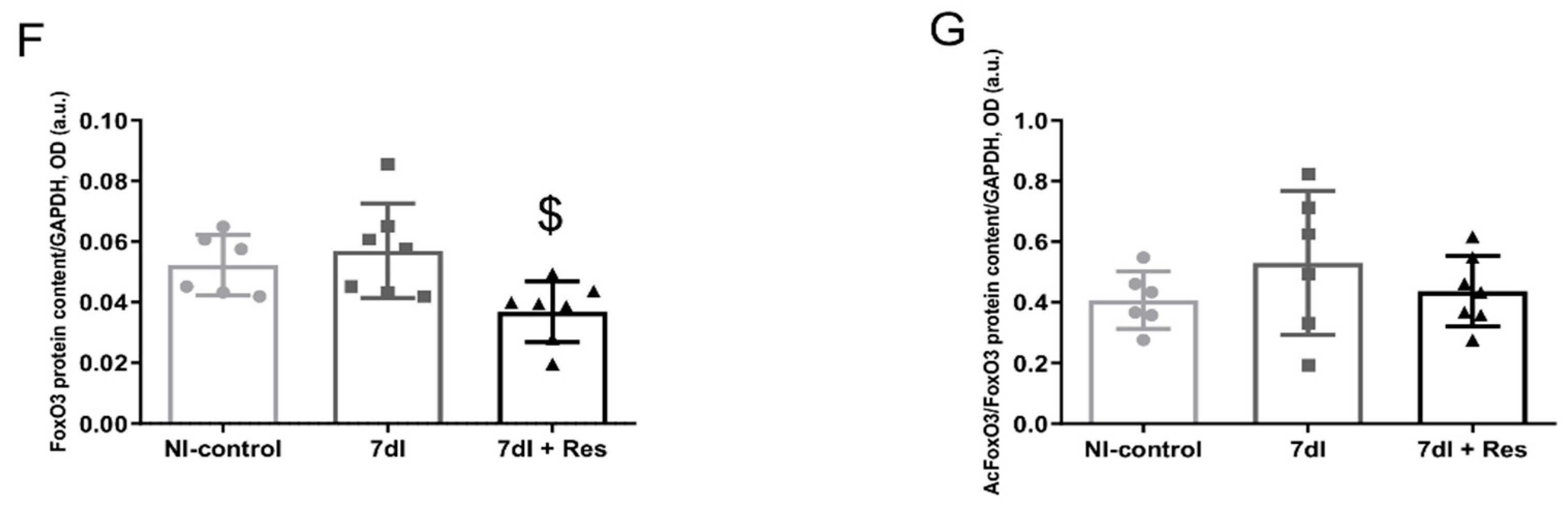
3.4.2. Unloading with Resveratrol versus Unloading
3.5. Muscle Atrophy Signaling Markers
3.5.1. Non-Immobilized versus Unloading Conditions
3.5.2. Unloading with Resveratrol versus Unloading
4. Discussion
4.1. Study Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreiro, E. Impact of Physical Activity and Exercise on Chronic Obstructive Pulmonary Disease Phenotypes: The Relevance of Muscle Adaptation. Arch. Bronconeumol. 2019, 55, 613–614. [Google Scholar] [CrossRef]
- Barreiro, E. Skeletal Muscle Dysfunction in COPD: Novelties in The Last Decade. Arch. Bronconeumol. 2017, 53, 43–44. [Google Scholar] [CrossRef][Green Version]
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. The BIOMEPOC Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2019, 55, 93–99. [Google Scholar] [CrossRef]
- Fearon, K.C.H.; Argiles, J.M.; Baracos, V.E.; Bernabei, R.; Coats, A.J.S.; Crawford, J.; Deutz, N.E.; Doehner, W.; Evans, W.J.; Ferrucci, L.; et al. Request for regulatory guidance for cancer cachexia intervention trials. J. Cachexia Sarcopenia Muscle 2015, 6, 272. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaŕe, R.; Dekhuijzen, P.N.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef]
- Marquis, K.; Debigaré, R.; Lacasse, Y.; LeBlanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Swallow, E.B.; Barreiro, E.; Gosker, H.; Sathyapala, S.A.; Sanchez, F.; Hopkinson, N.S.; Moxham, J.; Schols, A.; Gea, J.; Polkey, M.I.; et al. Quadriceps muscle strength in scoliosis. Eur. Respir. J. 2009, 34, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Shrikrishna, D.; Patel, M.; Tanner, R.J.; Seymour, J.M.; Connolly, B.A.; Puthucheary, Z.A.; Walsh, S.L.; Bloch, S.A.; Sidhu, P.; Hart, N.; et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur. Respir. J. 2012, 40, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Busquets, S.; Garcia-Martínez, C.; Olivan, M.; Barreiro, E.; López-Soriano, F.J.; Argilés, J.M. Overexpression of UCP3 in both murine and human myotubes is linked with the activation of proteolytic systems: A role in muscle wasting? Biochim. Biophys Acta Gen. Subj. 2006, 1760, 253–258. [Google Scholar] [CrossRef]
- Toledo, M.; Penna, F.; Oliva, F.; Luque, M.; Betancourt, A.; Marmonti, E.; López-Soriano, F.J.; Argiles, J.M.; Busquets, S. A multifactorial anti-cachectic approach for cancer cachexia in a rat model undergoing chemotherapy. J. Cachexia Sarcopenia Muscle 2016, 7, 48–59. [Google Scholar] [CrossRef]
- Salazar-Degracia, A.; Blanco, D.; Vilà-Ubach, M.; Biurrun, G.; Solórzano, C.O.; Montuenga, L.M.; Barreiro, E. Phenotypic and metabolic features of mouse diaphragm and gastrocnemius muscles in chronic lung carcinogenesis: Influence of underlying emphysema. J. Transl. Med. 2016, 14, s12967–s13016. [Google Scholar] [CrossRef]
- Salazar-Degracia, A.; Busquets, S.; Argilés, J.M.; Bargalló-Gispert, N.; López-Soriano, F.J.; Barreiro, E. Effects of the beta2 agonist formoterol on atrophy signaling, autophagy, and muscle phenotype in respiratory and limb muscles of rats with cancer-induced cachexia. Biochimie 2018, 149, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Degracia, A.; Busquets, S.; Argilés, J.M.; López-Soriano, F.J.; Barreiro, E. Formoterol attenuates increased oxidative stress and myosin protein loss in respiratory and limb muscles of cancer cachectic rats. PeerJ 2017, 5, e4109. [Google Scholar] [CrossRef]
- Salazar-Degracia, A.; Granado-Martínez, P.; Millán-Sánchez, A.; Tang, J.; Pons-Carreto, A.; Barreiro, E. Reduced lung cancer burden by selective immunomodulators elicits improvements in muscle proteolysis and strength in cachectic mice. J. Cell. Physiol. 2019, 234, 18041–18052. [Google Scholar] [CrossRef]
- Chacon-Cabrera, A.; Gea, J.; Barreiro, E. Short- and Long-Term Hindlimb Immobilization and Reloading: Profile of Epigenetic Events in Gastrocnemius. J. Cell. Physiol. 2017, 232, 1415–1427. [Google Scholar] [CrossRef]
- Guitart, M.; Lloreta, J.; Mañas-Garcia, L.; Barreiro, E. Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. J. Cell. Physiol. 2018, 233, 4360–4372. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Lund-Palau, H.; Gea, J.; Barreiro, E. Time-Course of muscle mass loss, damage, and proteolysis in gastrocnemius following unloading and reloading: Implications in chronic diseases. PLoS ONE 2016, 11, e0164951. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.M.; Kazi, A.A.; Hong-Brown, L.; Lang, C.H. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS ONE 2012, 7, e38910. [Google Scholar]
- Jackson, J.R.; Ryan, M.J.; Hao, Y.; Alway, S.E. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1572–R1581. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- Villar Álvarez, F.; Muguruza Trueba, I.; Belda Sanchis, J.; Molins López-Rodó, L.; Rodríguez Suárez, P.M.; Sánchez de Cos Escuín, J.; Barreiro, E.; Pintado, M.H.B.; Vicente, C.D.; Aldeyturriaga, J.F.; et al. Executive summary of the SEPAR recommendations for the diagnosis and treatment of non-small cell lung cancer. Arch. Bronconeumol. 2016, 52, 378–388. [Google Scholar] [CrossRef][Green Version]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, S.; Li, Z.; Zhao, X.; Li, W.; Sun, Y.; Zhang, Z.; Ling, W.; Feng, X. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr. Metab. 2014, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Cheng, X.; Cui, Y.; Xia, Q.; Yan, X.; Zhang, M.; Lan, G.; Liu, J.; Shan, T.; Huang, Y. Resveratrol regulates skeletal muscle fibers switching through the AdipoR1-AMPK-PGC-1α pathway. Food Funct. 2019, 10, 3334–3343. [Google Scholar] [CrossRef]
- Feng, Y.; He, Z.; Mao, C.; Shui, X.; Cai, L. Therapeutic Effects of Resveratrol Liposome on Muscle Injury in Rats. Med. Sci. Monit. 2019, 25, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.T.; Mohamed, J.S.; Alway, S.E. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS ONE 2013, 8, e83518. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Ho, C.S.; Lee, M.C.; Ho, C.S.; Huang, C.C.; Kan, N.W. Protective effects of resveratrol supplementation on contusion induced muscle injury. Int. J. Med. Sci. 2020, 17, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Mañas-García, L.; Guitart, M.; Duran, X.; Barreiro, E. Satellite cells and markers of muscle regeneration during unloading and reloading: Effects of treatment with resveratrol and curcumin. Nutrients 2020, 12, 1870. [Google Scholar] [CrossRef]
- Momken, I.; Stevens, L.; Bergouignan, A.; Desplanches, D.; Rudwill, F.; Chery, I.; Zahariev, A.; Zahn, S.; Stein, T.P.; Sebedio, J.L.; et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011, 25, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Mortreux, M.; Riveros, D.; Bouxsein, M.; Rutkove, S. A Moderate Daily Dose of Resveratrol Mitigates Muscle Deconditioning in a Martian Gravity Analog. Front. Physiol. 2019, 10, 899. [Google Scholar] [CrossRef]
- Mañas-García, L.; Penedo-Vázquez, A.; López-Postigo, A.; Deschrevel, J.; Durán, X.; Barreiro, E. Prolonged immobilization exacerbates the loss of muscle mass and function induced by cancer-associated cachexia through enhanced proteolysis in mice. Int. J. Mol. Sci. 2020, 21, 8167. [Google Scholar] [CrossRef] [PubMed]
- Mañas-García, L.; Bargalló, N.; Gea, J.; Barreiro, E. Muscle phenotype, proteolysis, and atrophy signaling during reloading in mice: Effects of curcumin on the gastrocnemius. Nutrients 2020, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Yu, W.; Fu, Y.-C.; Wang, X.; Li, J.-L.; Wang, W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem. Biophys. Res. Commun. 2009, 378, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef]
- Penedo-Vázquez, A.; Duran, X.; Mateu, J.; López-Postigo, A.; Barreiro, E. Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia. Molecules 2021, 26, 4904. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Tung, H.-C.; Chang, C.-Y.; Tsai, Y.-L.; Huang, J.-P.; Yen, T.-H.; Hung, L.-M. Resveratrol exhibits differential protective effects on fast- and slow-twitch muscles in streptozotocin-induced diabetic rats. J. Diabetes 2014, 6, 60–67. [Google Scholar] [CrossRef]
- Liu, H.W.; Su, Y.K.; Bamodu, O.A.; Hueng, D.Y.; Lee, W.H.; Huang, C.C.; Deng, L.; Hsiao, M.; Chien, M.-H.; Yeh, C.-T.; et al. The disruption of the β-catenin/TCF-1/STAT3 signaling axis by 4-acetylantroquinonol b inhibits the tumorigenesis and cancer stem-cell-like properties of glioblastoma cells, in vitro and in vivo. Cancers 2018, 10, 491. [Google Scholar] [CrossRef]
- Turner, P.V.; Pekow, C.; Vasbinder, M.A.; Brabb, T. Administration of substances to laboratory animals: Equipment considerations, vehicle selection, and solute preparation. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 614–627. [Google Scholar]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Resveratrol and exercise (review). Biomed. Rep. 2016, 5, 525–530. [Google Scholar] [CrossRef]
- Barreiro, E.; Ferrer, D.; Sanchez, F.; Minguella, J.; Marin-Corral, J.; Martinez-Llorens, J.; Lloreta, J.; Gea, J. Inflammatory cells and apoptosis in respiratory and limb muscles of patients with COPD. J. Appl. Physiol. 2011, 111, 808–817. [Google Scholar] [CrossRef]
- Barreiro, E.; Puig-Vilanova, E.; Marin-Corral, J.; Chacón-Cabrera, A.; Salazar-Degracia, A.; Mateu, X.; Puente-Maestu, L.; García-Arumí, E.; Andreu, A.L.; Molina, L. Therapeutic Approaches in Mitochondrial Dysfunction, Proteolysis, and Structural Alterations of Diaphragm and Gastrocnemius in Rats with Chronic Heart Failure. J. Cell. Physiol. 2016, 231, 1495–1513. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Fermoselle, C.; Urtreger, A.J.; Mateu-Jimenez, M.; Diament, M.J.; de Kier Joffé, E.D.B.; Sandri, M.; Barreiro, E. Pharmacological Strategies in Lung Cancer-Induced Cachexia: Effects on Muscle Proteolysis, Autophagy, Structure, and Weakness. J. Cell. Physiol. 2014, 229, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Marín-Corral, J.; Sanchez, F.; Mielgo, V.; Alvarez, F.J.; Gáldiz, J.B.; Gea, J. Reference values of respiratory and peripheral muscle function in rats. J. Anim. Physiol. Anim. Nutr. 2010, 94, e393–e401. [Google Scholar] [CrossRef]
- Chacon-Cabrera, A.; Fermoselle, C.; Salmela, I.; Yelamos, J.; Barreiro, E. MicroRNA expression and protein acetylation pattern in respiratory and limb muscles of Parp-1−/− and Parp-2−/− mice with lung cancer cachexia. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2530–2543. [Google Scholar] [CrossRef]
- Kuang, J.; Yan, X.; Genders, A.J.; Granata, C.; Bishop, D.J. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLoS ONE 2018, 13, e0196438. [Google Scholar] [CrossRef] [PubMed]
- Touchberry, C.D.; Wacker, M.J.; Richmond, S.R.; Whitman, S.A.; Godard, M.P. Age-related changes in relative expression of real-time PCR housekeeping genes in human skeletal muscle. J. Biomol. Tech. 2006, 17, 157–162. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tischler, M.E.; Desautels, M.; Goldberg, A.L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J. Biol. Chem. 1982, 257, 1613–1621. [Google Scholar] [CrossRef]
- Furuno, K.; Goodman, M.N.; Goldberg, A.L. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J. Biol. Chem. 1990, 265, 8550–8557. [Google Scholar] [CrossRef]
- Fermoselle, C.; Sanchez, F.; Barreiro, E. Reduction of Muscle Mass Mediated by Myostatin in an Experimental Model of Pulmonary Emphysema. Arch. Bronconeumol. 2011, 47, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, J.D.; Janovitz, E.B.; Wescott, D.M.; Chadwick, C.; Lowe-Krentz, L.J.; Lehman-McKeeman, L.D. Biomarkers of drug-induced skeletal muscle injury in the rat: Troponin I and myoglobin. Toxicol. Sci. 2009, 111, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.A.; Labugger, R.; Collier, C.; Brison, R.J.; Iscoe, S.; Van Eyk, J.E. Fast and slow skeletal troponin I in serum from patients with various skeletal muscle disorders: A pilot study. Clin. Chem. 2005, 51, 966–972. [Google Scholar] [CrossRef]
- Foster, G.E.; Nakano, J.; Sheel, A.W.; Simpson, J.A.; Road, J.D.; Reid, W.D. Serum skeletal troponin I following inspiratory threshold loading in healthy young and middle-aged men. Eur. J. Appl. Physiol. 2012, 112, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.W.; Simpson, J.A.; Iscoe, S.; Robins, T.; Nosaka, K. Changes in serum fast and slow skeletal troponin I concentration following maximal eccentric contractions. J. Sci. Med. Sport 2013, 16, 82–85. [Google Scholar] [CrossRef]
- Jarolim, S.; Millen, J.; Heeren, G.; Laun, P.; Goldfarb, D.S.; Breitenbach, M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004, 5, 169–177. [Google Scholar] [CrossRef]
- Pellmé, F.; Smith, U.; Funahashi, T.; Matsuzawa, Y.; Brekke, H.; Wiklund, O.; Taskinen, M.-R.; Jansson, P.-A. Circulating adiponectin levels are reduced in nonobese but insulin-resistant first-degree relatives of type 2 diabetic patients. Diabetes 2003, 52, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Krakoff, J.; Funahashi, T.; Stehouwer, C.D.A.; Schalkwijk, C.G.; Tanaka, S.; Matsuzawa, Y.; Kobes, S.; Tataranni, P.A.; Hanson, R.; Knowler, W.C.; et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care 2003, 26, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Liu, Y.; Vu, V.; Chan, L.; Xu, A.; Riddell, M.C.; Sweeney, G.; Hawke, T.J. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am. J. Physiol. Cell. Physiol. 2008, 295. [Google Scholar] [CrossRef]
- Kan, N.-W.; Lee, M.-C.; Tung, Y.-T.; Chiu, C.-C.; Huang, C.-C.; Huang, W.-C. The Synergistic Effects of Resveratrol combined with Resistant Training on Exercise Performance and Physiological Adaption. Nutrients 2018, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Higashi, A. Combined intake of astaxanthin, β-carotene, and resveratrol elevates protein synthesis during muscle hypertrophy in mice. Nutrition 2020, 69, 110561. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.; Conboy, J.J. Sirt1-Independent Rescue of Muscle Regeneration by Resveratrol in Type I Diabetes. J. Diabetes. Metab. 2013, 4, 1–6. [Google Scholar]
- Liu, J.; Peng, Y.; Wang, X.; Fan, Y.; Qin, C.; Shi, L.; Tang, Y.; Cao, K.; Li, H.; Long, J.; et al. Mitochondrial Dysfunction Launches Dexamethasone-Induced Skeletal Muscle Atrophy via AMPK/FOXO3 Signaling. Mol. Pharm. 2016, 13, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards resolving the enigma of the dichotomy of resveratrol: Cis- and trans-resveratrol have opposite effects on TyrRS-regulated PARP1 activation. GeroScience 2021, 43, 1171–1200. [Google Scholar] [CrossRef]
- Perris, A.; Bhattacharya, S.; Jawed, J.; Hoda, M. Oncotherapeutic application of resveratrol-based inorganic nanoparticles. Pharm. Nanotechnol. 2021, 9. [Google Scholar] [CrossRef]
- de Ligt, M.; Hesselink, M.; Jorgensen, J.; Hoebers, N.; Blaak, E.; Goossens, G. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411. [Google Scholar] [CrossRef] [PubMed]
| NI-Control | 7DI | 7DI + Resveratrol | |
|---|---|---|---|
| Food intake (g/24 h) | 3.20 (0.10) | 3.24 (0.18) | 3.25 (0.078) |
| Total body weight gain (%) | +6.25 (1.02) | −0.78 (3.46) ** | +1.03 (1.87) |
| Gastrocnemius weight (g) | 0.113 (0.009) | 0.097 (0.011) * | 0.099 (0.011) |
| Limb strength gain (%) | +13.44 (6.10) | −14.22 (8.97) * | −9.04 (15.21) |
| NI-Control | 7DI | 7DI + Resveratrol | |
|---|---|---|---|
| Muscle fiber type, % | |||
| Type I fibers | 15.40 (1.99) | 16.88 (2.70) | 14.60 (2.35) |
| Type II fibers | 84.60 (5.74) | 83.12 (2.70) | 85.40 (2.35) |
| Cross-sectional area, µm2 | |||
| Type I fibers | 1240.46 (122.93) | 882.28 (199.96) *** | 1032.89 (160.48) $ |
| Type II fibers | 1252.52 (95.32) | 879.85 (183.05) ** | 1065.02 (133.06) $ |
| Muscle hybrid fiber, % | 0.59 (0.10) | 3.55 (1.61) ** | 2.37 (2.45) |
| Cross-sectional area hybrid fibers, µm2 | 1012.90 (57.85) | 881.97 (270.88) | 855.71 (238.68) |
| Number of apoptotic nuclei (TUNEL), % | 52.53 (9.16) | 69.80 (5.58) * | 56.98 (7.14) $ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mañas-García, L.; Denhard, C.; Mateu, J.; Duran, X.; Gea, J.; Barreiro, E. Beneficial Effects of Resveratrol in Mouse Gastrocnemius: A Hint to Muscle Phenotype and Proteolysis. Cells 2021, 10, 2436. https://doi.org/10.3390/cells10092436
Mañas-García L, Denhard C, Mateu J, Duran X, Gea J, Barreiro E. Beneficial Effects of Resveratrol in Mouse Gastrocnemius: A Hint to Muscle Phenotype and Proteolysis. Cells. 2021; 10(9):2436. https://doi.org/10.3390/cells10092436
Chicago/Turabian StyleMañas-García, Laura, Charlotte Denhard, Javier Mateu, Xavier Duran, Joaquim Gea, and Esther Barreiro. 2021. "Beneficial Effects of Resveratrol in Mouse Gastrocnemius: A Hint to Muscle Phenotype and Proteolysis" Cells 10, no. 9: 2436. https://doi.org/10.3390/cells10092436
APA StyleMañas-García, L., Denhard, C., Mateu, J., Duran, X., Gea, J., & Barreiro, E. (2021). Beneficial Effects of Resveratrol in Mouse Gastrocnemius: A Hint to Muscle Phenotype and Proteolysis. Cells, 10(9), 2436. https://doi.org/10.3390/cells10092436








