Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fig and Olive Leave Extracts
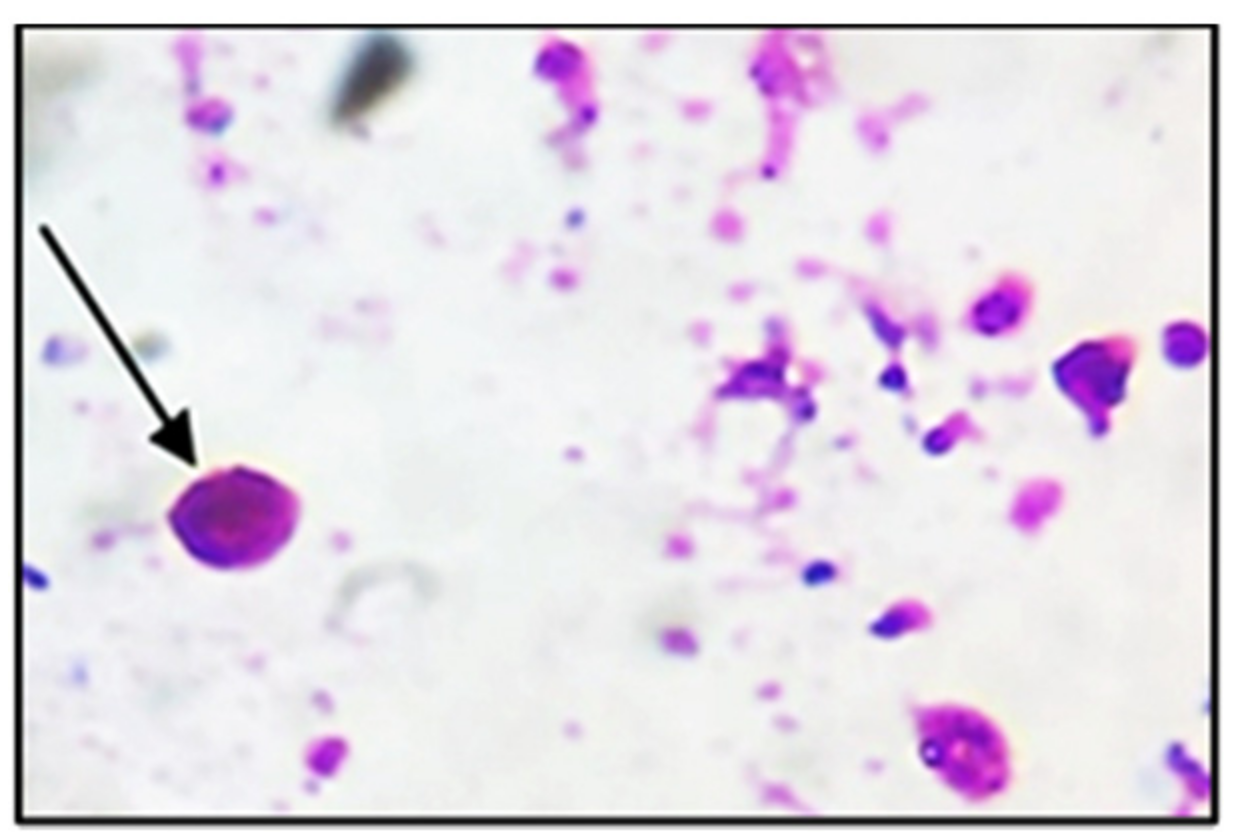
2.2. Oocysts Preparation and Isolation
2.3. The Animals
2.4. Immunosuppression
2.5. The Infection
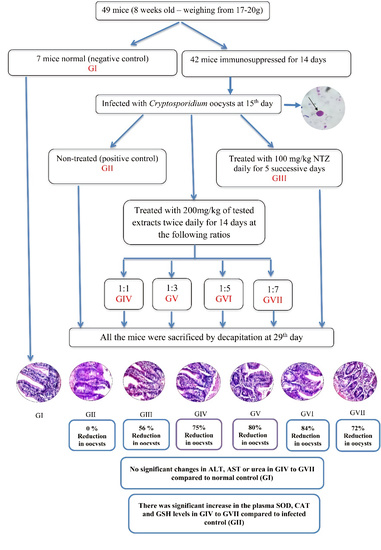
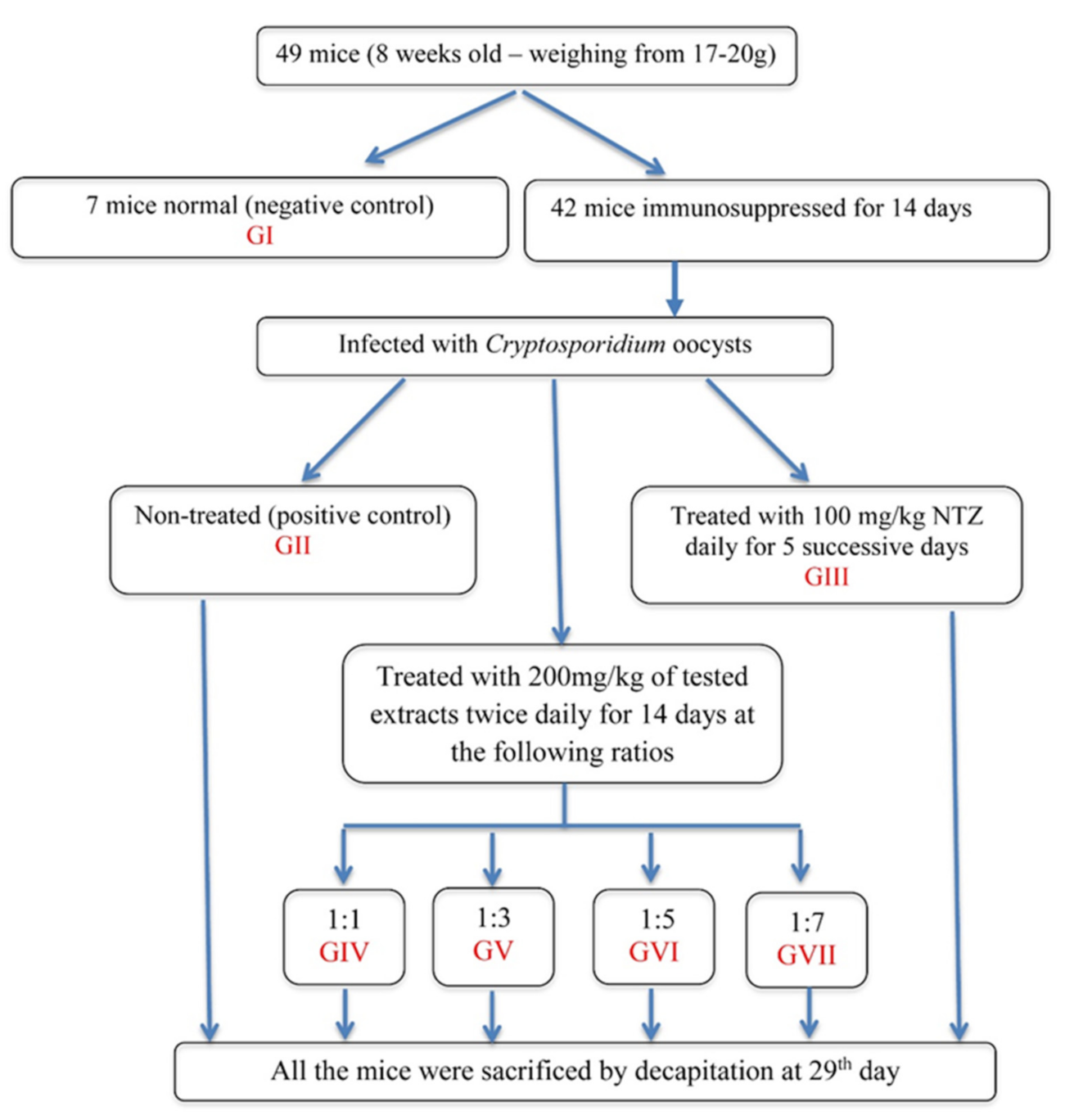
2.6. Experimental Design
2.7. Fecal Samples
2.8. Measurement of Serum Biochemical Parameters
2.9. Evaluation of Oxidative Stress and Antioxidant Markers
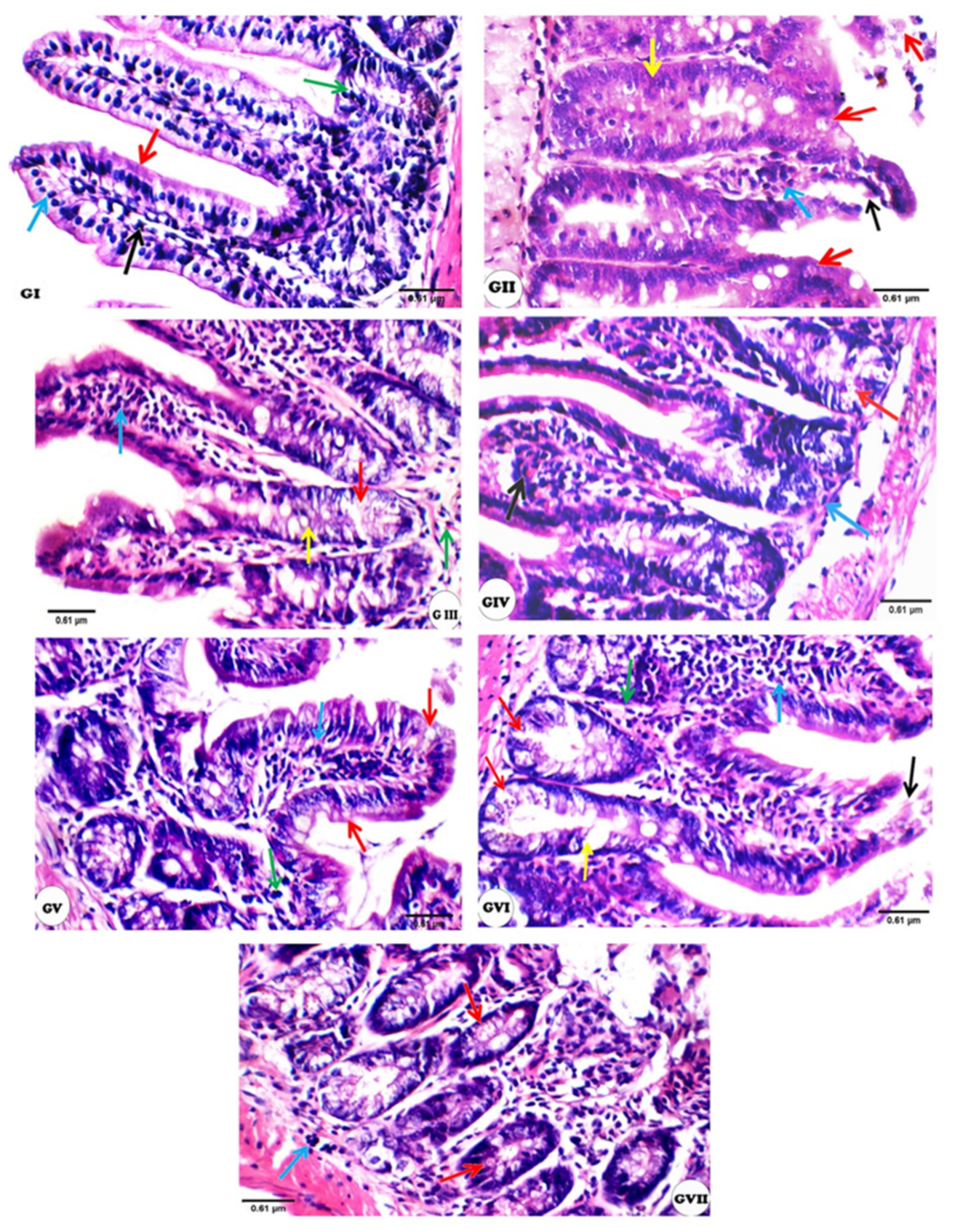
2.10. Histopathological Examinations
2.11. Statistical Analysis
3. Results
3.1. Parasitological Results
3.2. Biochemical Results
3.3. Histopathological Changes
3.4. Oxidative Stress Parameters in the Plasma of Mice Infected with Cryptosporidium parvum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hafez, E.N.; El Hamed, W.F. The Efficacy of Citrus maxima Peels Aqueous Extract Against Cryptosporidiosis in Immunecompromised Mice. Acta Parasitol. 2021, 66, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, A.; Gullicksrud, J.A.; Engiles, J.B.; McLeod, B.I.; Kugler, E.M.; Henao-Mejia, J.; Zhou, T.; Ring, A.M.; Brodsky, I.E.; Hunter, C.A.; et al. The intestinal parasite Cryptosporidium is controlled by an enterocyte intrinsic inflammasome that depends on NLRP6. Proc. Natl. Acad. Sci. USA 2021, 118, e2007807118. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, M.; Sood, S.; Yadav, A.; Verma, P.; Manzoor, N.; Chakraborty, D.; Katoch, R.; Sangha, N. Alterations in oxidative stress parameters and its associated correlation with clinical disease on experimental Cryptosporidium parvum infection in Swiss albino mice. J. Parasit Dis. 2017, 41, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Kwok, L.Y.; Schlüter, D.; Clayton, C.; Soldati, D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol. Microbiol. 2004, 51, 47–61. [Google Scholar] [PubMed]
- Kim, Y.J.; Kim, E.H.; Hahm, K.B. Oxidative stress in inflammation based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef]
- Celi, P. Oxidative Stress in Ruminants. Part of the Oxidative Stress in Applied Basic Research and Clinical Practice Book Series (OXISTRESS). Studies on Veterinary Medicine; Humana Press: Totowa, NJ, USA, 2011; pp. 191–231. [Google Scholar] [CrossRef]
- Sood, S.; Yadav, A.; Katoch, R.; Bhagat, M.; Sharma, A.; Rahman, S.; Verma, P.; Rajat, A.; Ganai, K.A.; Sharma, S. Oxidative stress and clinico-pathological alterations induced by Cryptosporidium parvum infection in a rat model. Indian J. Anim Res. 2019, 53, 1431–1435. [Google Scholar] [CrossRef]
- Sarıkaya, E.; Doğan, S. Glutathione Peroxidase in Health and Diseases. Chapter 3. In Glutathione System and Oxidative Stress in Health and Disease; Bagatini, M.D., Ed.; IntechOpen Limited: London, UK, 2020; pp. 49–64. [Google Scholar]
- MacRae, J.I.; Sheiner, L.; Nahid, A.; Tonkin, C.; Striepen, B.; McConville, M.J. Mitochondrial metabolism of glucose and glutamine is required for intracellular growth of Toxoplasma gondii. Cell Host Microbe 2012, 12, 682–692. [Google Scholar] [CrossRef]
- Almeer, R.S.; Alarifi, S.; Alkahtani, S.; Ibrahim, S.R.; Ali, D.; Moneim, A. The potential hepatoprotective effect of royal jelly against cadmium chloride- induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed. Pharmacother. 2018, 106, 1490–1498. [Google Scholar] [CrossRef]
- Wang, X.N.; Zhang, C.J.; Diao, H.L.; Zhang, Y. Protective effects of curcumin against sodium arsenite-induced ovarian oxidative injury in a mouse model. Chin. Med. J. 2017, 130, 1026–1032. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; AlKahtani, M.D.F.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The pivotal role of plant growth promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Al-Ateeq, T.; Ali, M.A.M.; Hasanuzzaman, M.; Abdelaal, K.A.A. Bacillus thuringiensis and Silicon Modulate Antioxidant Metabolism and Improve the Physiological Traits to Confer Salt Tolerance in Lettuce. Plants 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Omara, R.I.; El-Kot, G.; Fadel, F.M.; Abdelaal, K.A.A.; Saleh, E. Efficacy of certain bioagents on patho-physiological characters of wheat plants under wheat leaf rust stress. Physiol. Mol. Plant. Pathol. 2019, 106, 102–108. [Google Scholar] [CrossRef]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.M.; Abdelaal, K.A.A. Evaluation of Silicon and Proline Application on the Oxidative Machinery in Drought-Stressed Sugar Beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Mazrou, Y.S.A.; Hafez, Y.M. Silicon Foliar Application Mitigates Salt Stress in Sweet Pepper Plants by Enhancing Water Status, Photosynthesis, Antioxidant Enzyme Activity and Fruit Yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Obiad, H.M.; Al-Alousi, T.I.; Al-Jboori, A.H. The in vivo effect of some medicinal plant extracts on Cryptosporidium parasite. JUAPS 2012, 6, 15–26. [Google Scholar]
- Elmahallawy, E.K.; Elshopakey, G.E.; Saleh, A.A.; Agil, A.; El-Morsey, A.; Dina, M.M.; El-Shewehy, D.M.M.; Ahmed, S.; Sad, A.S.; Yanai, T.; et al. S-Methylcysteine (SMC) ameliorates intestinal hepatic and splenic damage induced by Cryptosporidium parvum infection via targeting inflammatory modulators and oxidative stress in Swiss albino mice. Biomedicines 2020, 8, 423. [Google Scholar] [CrossRef]
- Dumaine, J.E.; Tandel, J.; Striepen, B. Cryptosporidium parvum. Trends Parasitol 2020, 36, 485–486. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Clinically tested medicinal plant: A review (Part 1). SMU Med. J. 2016, 3, 99–128. [Google Scholar]
- Orhan, I.E. Biotechnological Production of Plant Secondary Metabolites; Bentham ebook: Dubai, United Arab Emirates, 2012; p. 107. [Google Scholar] [CrossRef]
- Smith, H.V.; Corcoran, G.D. New drugs and treatment for cryptosporidiosis. Curr. Opin. Infect. Dis. 2004, 17, 557–564. [Google Scholar] [CrossRef]
- Yousef, N.S.; El-Ghandour, A.A.; El-Shershaby, S.S.A. Antimicrobial activity of fig and olive leaves extracts. J. Food Dairy Sci. Mansoura Univ. 2019, 10, 503–508. [Google Scholar] [CrossRef]
- Haloui, E.; Marzouk, Z.; Marzouk, B.; Bouftira, I.; Bouraoui, A.; Fenina, N. Pharmacological activities and chemical composition of the Olea europaea L. leaf essential oils from Tunisia. J. Food Agric. Environ. 2010, 8, 204–208. [Google Scholar]
- Ayoub, L.; Hassan, F.; Hamid, S.; Abdelhamid, Z.; Souad, A. Phytochemical screening, antioxidant activity and inhibitory potential of Ficus carica and Olea europaea leaves. Bioinformation 2019, 15, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ademe, A.; Ayalew, A.; Woldetsadik, K. In Vitro and In Vivo activity of selected plant extracts against Papaya (Carica papaya L.) Anthracnose (Colletotrichum gloeosporioides). J. Hortic. 2014, 1, 1–4. [Google Scholar] [CrossRef]
- Current, W.L.; Reese, N.C. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J. Protozool. 1986, 33, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, S.; Pohlenz, J. Staining of cryptosporida by a modified Ziehl-Neelsentechnique. Acta Vet. Scand. 1981, 22, 594–596. [Google Scholar] [CrossRef]
- Abdou, A.G.; Harba, N.M.; Afifi, A.F.; Elnaidany, N.F. Assessment of Cryptosporidium parvum infection in immunocompetent and immunocompromised mice and its role in triggering intestinal dysplasia. Int. J. Infect. Dis. 2013, 17, 593–600. [Google Scholar] [CrossRef]
- Ridley, D.S.; Hawgood, B.C. The value of formol–ether concentration of faecal cysts and ova. Am. J. Clin. Pathol. 1956, 9, 74–76. [Google Scholar] [CrossRef]
- Sadek, G.; El-Aswad, B. Role of COX-2 in pathogenesis of intestinal cryptosporidiosis and effect of some drugs on treatment of infection. J. Parasitol. Res. 2014, 9, 21–40. [Google Scholar] [CrossRef]
- Heodos, C.M.; Griffiths, J.K.; D’Onfro, J.; Fairfield, A.; Tzipori, S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 1998, 42, 1959–1965. [Google Scholar] [CrossRef]
- Garcia, L.S.; Bruckner, D.A. Diagnostic Medical Parasitology, 3rd ed.; ASM Press: Washington, DC, USA, 1993; pp. 131–135. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxaloacetate and glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Patton, C.J.; Crauch, S.R. Spectrophotometer and kinetic investigation of Berthelot reaction for determination of ammonia. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Nishikimi, M.; Roa, N.A.; Yogi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem. Biophys. Res. Common 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Bancroft, J.D.; Stevens, A.; Turner, D.R. Theory and Practice of Histological Techniques, 4th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 1996; pp. 86–88. [Google Scholar]
- Pickerd, N.; Tuthill, D. Resolution of cryptosporidiosis with probiotic treatment. Postgrad. Med. J. 2004, 80, 112–113. [Google Scholar] [CrossRef]
- WHO. World Health Organization Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS); WHO Press: Geneva, Switzerland, 2010; Volume 450. [Google Scholar]
- Khater, M.M.; El-Sayed, S.H.; Yousof, H.S.; Mahmoud, S.S.; El-Diba, N.; El-Badrya, A.A. Anti-Cryptosporidium efficacy of Olea europaea and Actinidia deliciosa in a neonatal mouse model. Kasr Al Ainy Med. J. 2017, 23, 32–37. [Google Scholar] [CrossRef]
- Al-abodi HRJ (2018). Comparison between the effect of metronidazole and some plant extracts on parasites Cryptosporidium parvum in vivo. Int. J. Res. Pharm. Sci. 2018, 10, 388–398. [Google Scholar]
- Soufy, H.; El-Beih, N.M.; Nasr, S.M.; Abd El-Aziz, T.H.; Khalil, F.A.M.; Ahmed, Y.F.; AbouZeina, H.A.A. Effect of Egyptian propolis on cryptosporidiosis in immunosuppressed rats with special emphasis on oocysts shedding, leukogram, protein profile and ileum histopathology. Asian Pac. J. Trop Med. 2017, 10, 253–262. [Google Scholar] [CrossRef]
- Aboelsoued, D.; Abo-Aziza, F.; Mahmoud, M.; Megeed, K.A.; El Ezz, N.A.; Abu-Salem, F. Anticryptosporidial effect of pomegranate peels water extract in experimentally infected mice with special reference to some biochemical parameters and antioxidant activity. J. Parasit Dis. 2019, 43, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Abou Gabal, A.; Aboul-Ela, H.M.; Ali, E.; Khaled, A.E.M.; Shalaby, O.K. Hepatoprotective, DNA damage prevention and antioxidant potential of spirulina platensis on CCl4-induced hepatotoxicity in mice. Am. J. Biomed. Res. 2015, 3, 29–34. [Google Scholar]
- Abu El Ezz, N.M.T.; Khalil, A.M.; Shaapan, R.M. Therapeutic effect of onion (Allium cepa) and cinnamon (Cinnamomum zeylanicum) oils on Cryptosporidiosis in experimentally infected mice. Glob. Vet. 2011, 7, 179–183. [Google Scholar]
- Abdelrahman, K.A.; Abdel Megeed, K.N.; Hammam, A.M.; Morsy, G.H.; Seliem, M.M.E.; Aboelsoued, D. Molecular characterization of bubaline isolate of Cryptosporidium species from Egypt. Res. J. Parasitol. 2015, 10, 127–141. [Google Scholar] [CrossRef][Green Version]
- Cui, Z.; Song, D.; Qi, M.; Zhang, S.; Wang, R.; Jian, F.; Ning, C.; Zhang, L. Revisiting the infectivity and pathogenicity of Cryptosporidium parvum provides new information on parasitic sites within the host. Parasite Vector 2018, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Harp, J.A.; Jardon, P.; Atwill, E.R.; Zylstra, M.; Checel, S.; Goff, J.P.; De Simone, C. Field-testing of prophylactic measures against Cryptosporidium parvum infection in calves in a California dairy herd. Am. J. Vet. Res. 1996, 57, 1568–1588. [Google Scholar]
- Tabrez, S.; Ahmad, M. Effect of wastewater intake on antioxidant and marker enzymes of tissue damage in rat tissues: Implications for the use of biochemical markers. Food Chem. Toxicol. 2009, 47, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Aziz, E.; Beih, E.; Soufy, H.; Nasr, S.M.; Khalil, A.M.; Sharaf, M. Effect of Egyptian propolis on lipid profile and oxidative status in comparison with nitazoxanide in immunosuppressed rats infected with Cryptosporidium spp. Glob. Vet. 2014, 13, 17–27. [Google Scholar]
- Kyriazis, I.D.; Koutsoni, O.S.; Aligiannis, N.; Karampetsou, K.; Skaltsounis, A.L.; Dotsika, E. The leishmanicidal activity of oleuropein is selectively regulated through inflammation-and oxidative stress-related genes. Parasites Vectors 2016, 9, 1–16. [Google Scholar] [CrossRef]
- Didwania, N.; Shadab, M.; Sabur, A.; Ali, N. Alternative to Chemotherapy—The Unmet Demand against Leishmaniasis. Front. Immunol. 2017, 8, 1779. [Google Scholar] [CrossRef]
- Yeganeh, F.; Hoseini, M.H. Current Approaches to Develop a Live Vaccine against Leishmania major. Nov. Biomed. 2017, 5, 133–137. [Google Scholar]
- Almayouf, M.A.; El-khadragy, M.; Awad, M.A.; Alolayan, E.M. The effects of silver nanoparticles biosynthesized using fig and olive extracts on cutaneous leishmaniasis-induced inflammation in female balb/c mice. Biosci. Rep. 2020, 40, BSR20202672. [Google Scholar] [CrossRef]
- Abouzaid, O.A.; El-Mageid, A.; Afaf, D.; Alsadek, E.A. Protective and treatment effect of garlic extract on biochemical changes induced by pesticides in rats. Can. J. Physiol Pharmacol. 2016, 94, 571–578. [Google Scholar] [CrossRef]
- Zaidi, S.K.; Ansari, S.A.; Tabrez, S.; Naseer, M.I.; Shahwan, M.J.; Banu, N.; Al-Qahtani, M.H. Antioxidant potential of Solanum nigrum aqueous leaves extract in modulating restraint stress-induced changes in rat’s liver. J. Pharm Bioallied. Sci. 2019, 11, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Chammem, N.; Reyes-Batlle, M.; Mejri, M.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Activity of olive leaf extracts against the promastigote stage of Leishmania species and their correlation with the antioxidant activity. Exp. Parasitol. 2014, 141, 106–111. [Google Scholar] [CrossRef] [PubMed]
| Experimental Groups | Oocysts Number | Resistance to Infection (%) |
|---|---|---|
| II | ± 203.7 +++ | − |
| III | 5362 ± 23.8 *** | 56.4 |
| IV | 3000 ± 10.8 ***,+++ | 75.6 |
| V | 2476 ± 30.4 ***,+++ | 79.9 |
| VI | 1995 ± 6.5 ***,+++ | 83.8 |
| VII | 3434 ± 22.9 ***,+++ | 72.0 |
| Experimental Groups | ALT | AST | Urea |
|---|---|---|---|
| I | 86 ± 3.5 +++,### | 176.6 ± 16.5 +++ | 37.3 ± 0.9 +++,### |
| II | 124 ± 5 ***,### | 226.9 ± 2.8 ***,# | 52.7 ± 3.6 *** |
| III | 198.6 ± 11.4 ***,+++ | 198.6 ± 11.8 + | 57.2 ± 2.8 *** |
| IV | 92.5 ± 6.6 ++,### | 183 ± 2.4 ++ | 39 ± 1 +++,### |
| V | 89 ± 6.2 +++,### | 172.5 ± 5.4 +++,# | 37 ± 2.4 +++,### |
| VI | 88 ± 2.4 +++,### | 174.5 ± 1.2 +++,# | 38 ± 1.4 +++,### |
| VII | 88.7 ± 2.4 +++,### | 178.4 ± 3.6 +++ | 38.7 ± 2.4 +++,### |
| Experimental Groups | Mucosal Thickness | Villi | Crypts | Inflammatory Infiltrate | Oocysts | Submucosa | Musculosa |
|---|---|---|---|---|---|---|---|
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| II | ++ | ++ | ++ | ++ | ++ | ++ | 0 |
| III | 0 | + | + | + | + | + | 0 |
| IV | 0 | + | + | + | + | 0 | 0 |
| V | + | ++ | + | ++ | + | ++ | 0 |
| VI | 0 | + | + | ++ | ++ | 0 | 0 |
| VII | + | ++ | + | ++ | + | + | 0 |
| Experimental Groups | Oxidative Stress Parameters | ||
|---|---|---|---|
| GSH, U/L | SOD, U/mL | CAT, U/mL | |
| I | 7.6 ± 0.08 +++,### | 273.4 ± 3.3 +++,### | 88.8 ± 3.2 +++,### |
| II | 1.01 ± 0.07 ***,### | 72 ± 2.5 ***,### | 52.6 ± 0.9 ***,### |
| III | 2.7 ± 0.03 ***,+++ | 218.6 ± 1.03 ***,+++ | 113.8 ± 1.8 ***,+++ |
| IV | 3.5 ± 0.04 ***,+++,### | 214.2 ± 3.2 ***,+++ | 78.2 ± 2.03 ***,+++,### |
| V | 3.4 ± 0.03 ***,+++,### | 227.2 ± 1.9 ***,+++,# | 88.4 ± 1.5 +++,### |
| VI | 3.6 ± 0.04 ***,+++,### | 250.6 ± 3.1 ***,+++,### | 75.2 ± 1.7 ***,+++,### |
| VII | 3.8 ± 0.07 ***,+++,### | 287.4 ± 4.5 ***,+++,### | 78.2 ± 3.5 ***,+++,### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Hamed, W.F.; Yousef, N.S.; Mazrou, Y.S.A.; Elkholy, W.A.E.S.; El-Refaiy, A.I.; Elfeky, F.A.; Albadrani, M.; El-Tokhy, A.I.; Abdelaal, K. Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System. Cells 2021, 10, 2419. https://doi.org/10.3390/cells10092419
Abd El-Hamed WF, Yousef NS, Mazrou YSA, Elkholy WAES, El-Refaiy AI, Elfeky FA, Albadrani M, El-Tokhy AI, Abdelaal K. Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System. Cells. 2021; 10(9):2419. https://doi.org/10.3390/cells10092419
Chicago/Turabian StyleAbd El-Hamed, Wafaa Fayez, Nahed Samy Yousef, Yasser S. A. Mazrou, Walaa A. E. S. Elkholy, Amal I. El-Refaiy, Faten A. Elfeky, Muayad Albadrani, Ahmed I. El-Tokhy, and Khaled Abdelaal. 2021. "Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System" Cells 10, no. 9: 2419. https://doi.org/10.3390/cells10092419
APA StyleAbd El-Hamed, W. F., Yousef, N. S., Mazrou, Y. S. A., Elkholy, W. A. E. S., El-Refaiy, A. I., Elfeky, F. A., Albadrani, M., El-Tokhy, A. I., & Abdelaal, K. (2021). Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System. Cells, 10(9), 2419. https://doi.org/10.3390/cells10092419