The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival
Abstract
1. Heme Oxygenases (HOs) and Oxidative Stress
2. The Metabolism of Heme
2.1. Heme
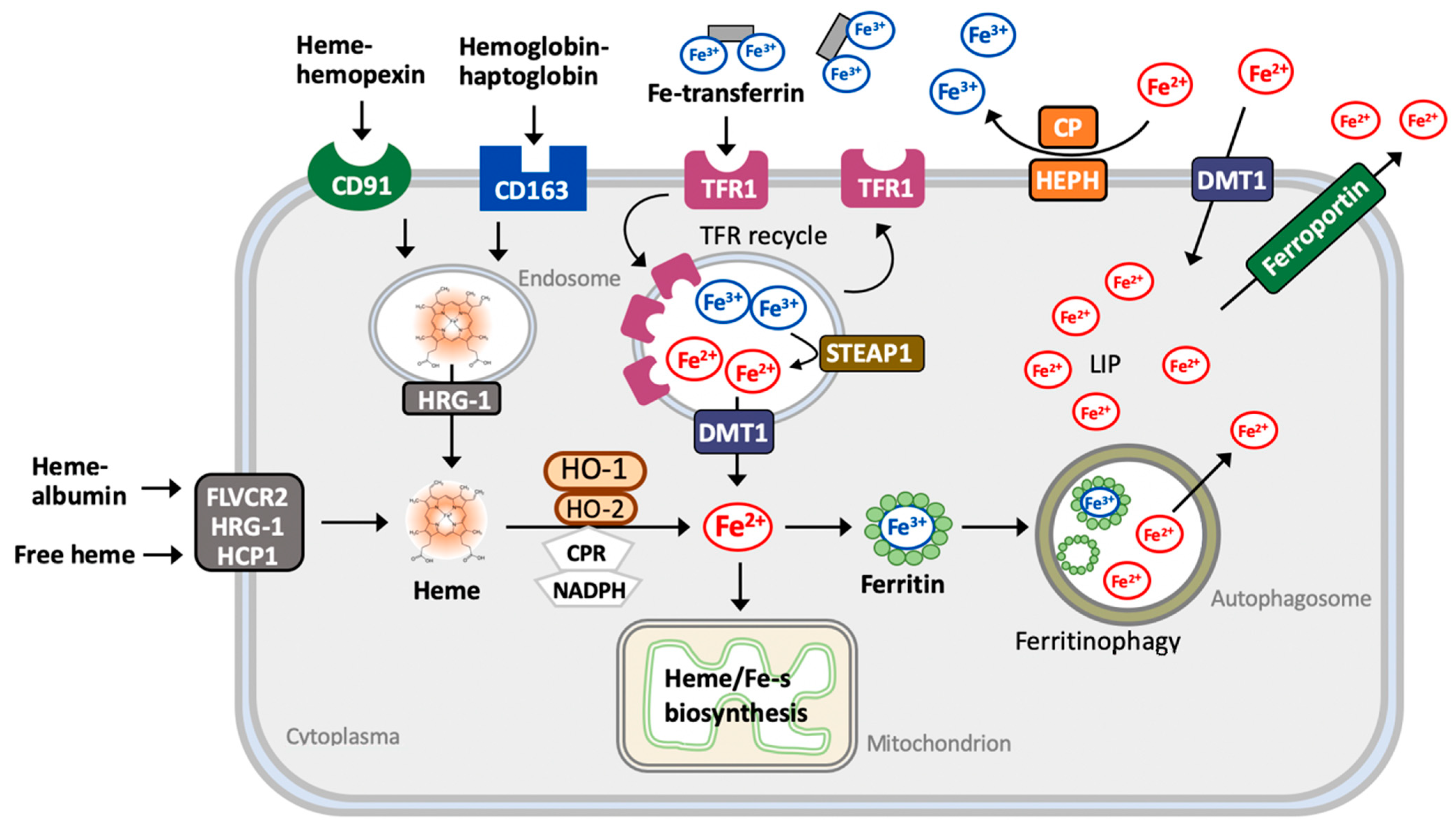
2.2. Iron
2.3. Carbon Monoxide (CO)
2.4. Bilirubin and Biliverdin
3. The Regulation of HO-1 Expression and Activity
3.1. Transcriptional Regulation of HO-1
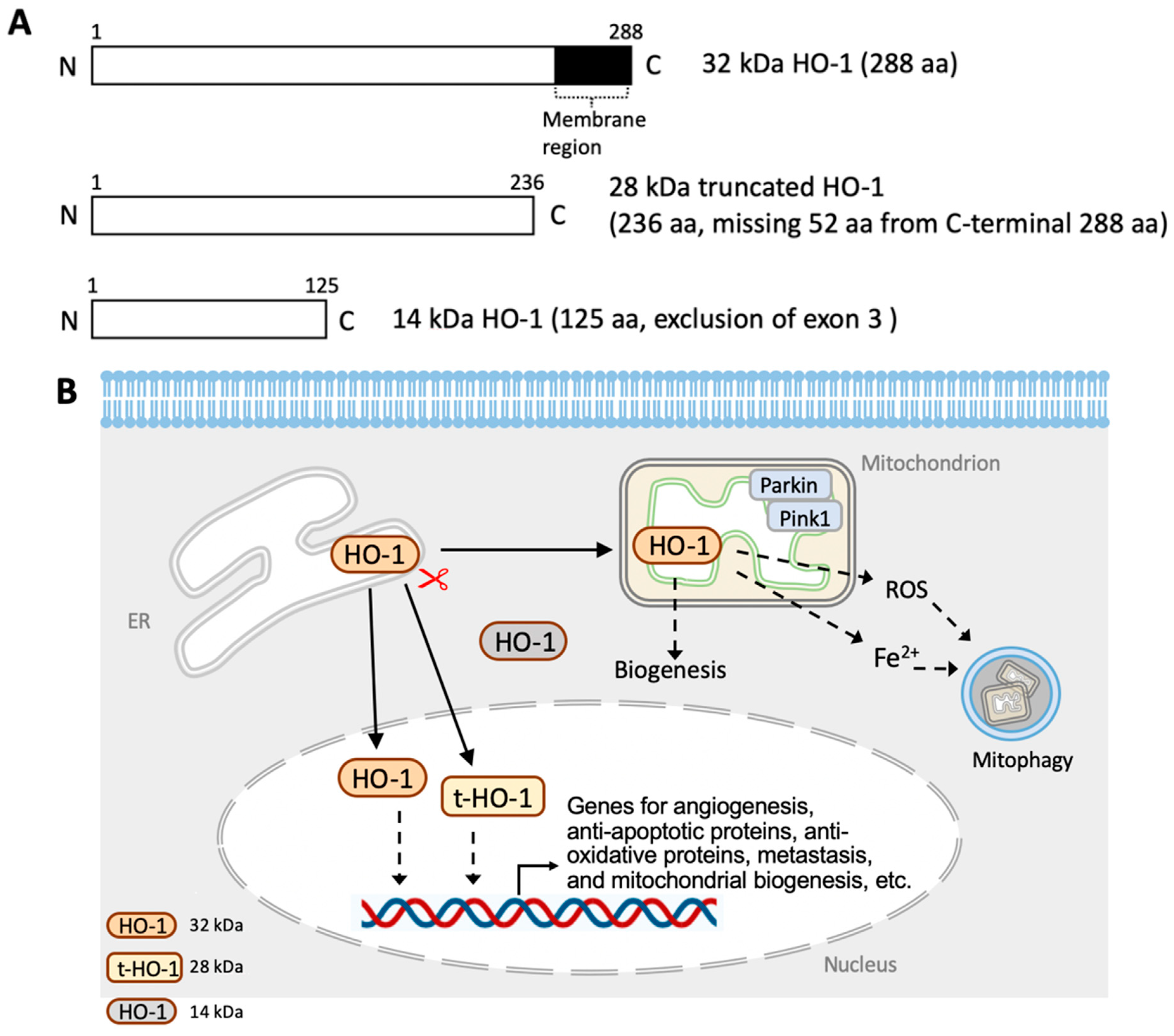
3.2. Translational and Post-Translational Regulation of HO-1
4. The Crosstalk between HO-1 and Redox Signaling
4.1. HO-1 in the Endoplasmic Reticulum
4.2. HO-1 in Mitochondria
4.3. HO-1 in the Nucleus
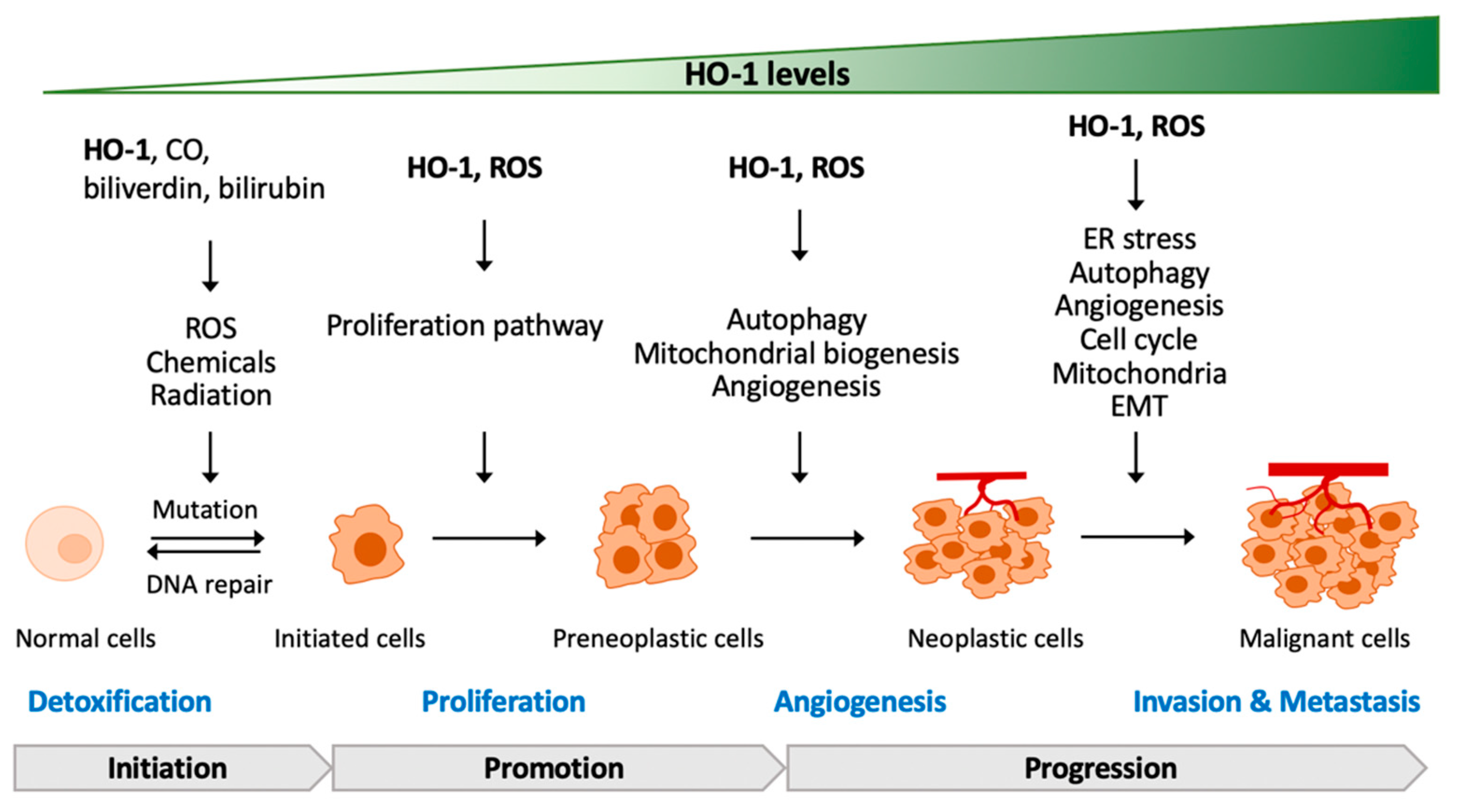
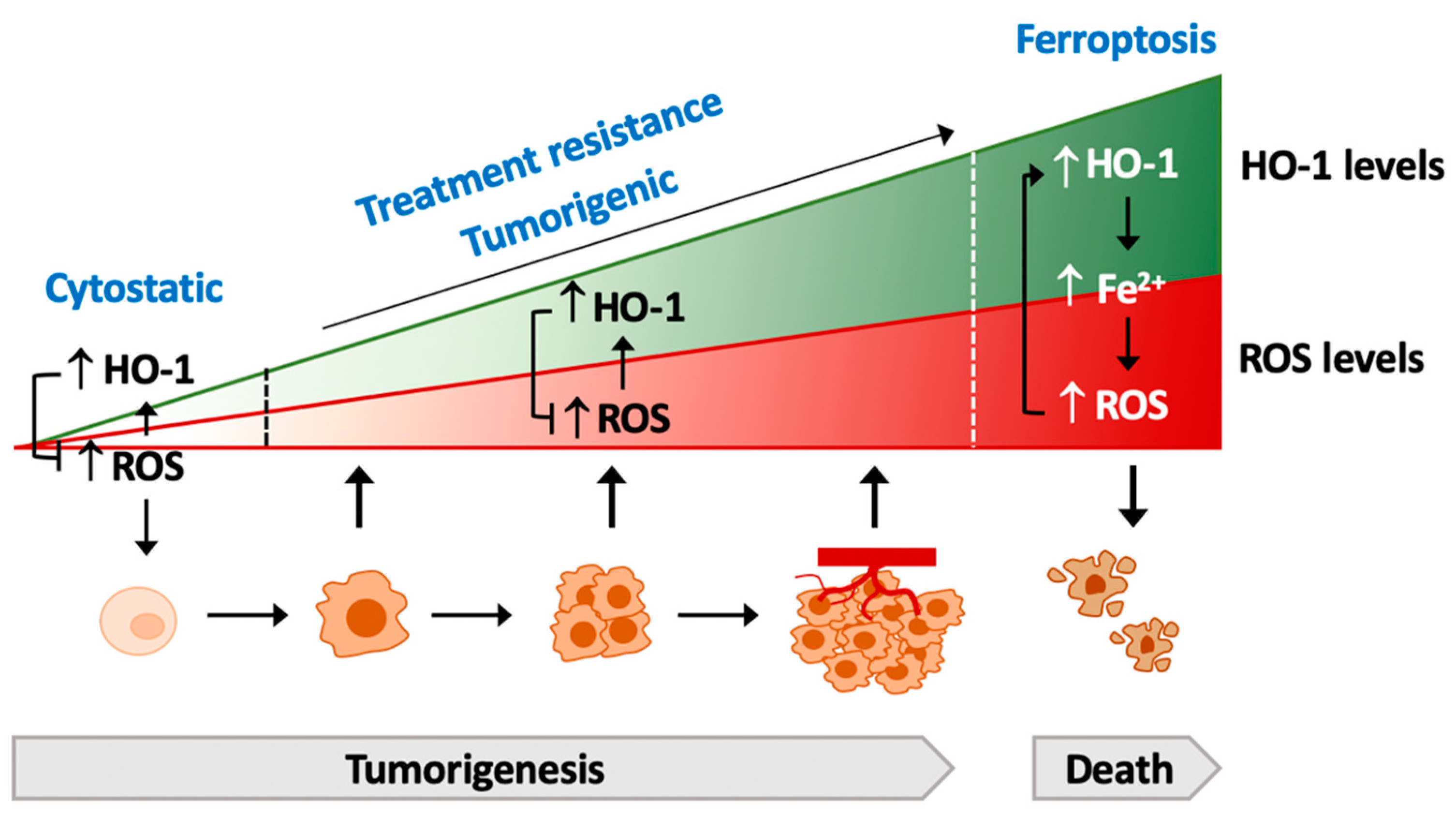
5. The Contradictory Role of HO-1 in Tumorigenesis
5.1. HO-1 Deficiency or Mutation in Tumorigenesis
5.2. HO-1-Regulated Proliferation and Development of Cancer Cells
5.3. HO-1-Regulated Angiogenesis of Cancer Cells
5.4. HO-1-Regulated Metastasis of Cancer Cells
6. HO-1-Drived Resistance against Therapy
| Therapeutic Treatment | Cancer Types | HO-1 Status | Resolution or HO-1 Regulator | Reference |
|---|---|---|---|---|
| BIX-01204 (G9a inhibitor) | KG leukemia stem cells | Induced PERK–autophagy–ROS–HO-1 | PERK inhibition (shRNA, GSK2606414) or autophagy inhibition (Bafilomycin A1) to inhibit Nrf2/HO-1 and increase ROS | [127] |
| Bortezomib (BTZ) | Neuroblastoma: HTLA-231 and MDA | Increased Nrf2 and HO-1 | ZnPP co-treatment enhanced sensitivity of BTZ-mediated apoptosis | [128] |
| Bortezomib (BTZ) | Neuroblastoma: HTLA-230 | Activated Nrf2 to increase HO-1 gCLM, xCT, and GSH | HO-1 siRNS sensitized BTZ-induced cell death, all-trans retinoic acid (Nrf2 inhibitor) reversed BTZ-increased HO-1, gCLM, xCT, and GSH, and sensitized Bortezomib-induced cytotoxicity | [129] |
| Bortezomib (BTZ) | Multiple myeloma (U266, KMS26 SKM-M1, and MM1S) | Induced ER stress, ROS generation, and upregulated nuclear HO-1 | E64d prevented nuclear localization of HO-1 and increased BTZ sensitivity | [78] |
| Bortezomib (BTZ) | Parent U226 and bortezomib-resistant U266 | Theioredoxin reductase regulated Nrf2 and HO-1 | ZnPP restored BTZ-mediated apoptosis | [130] |
| Cisplatin | Ovarian cancer: SKOV-3 and CAOV-3 Human ovarian cancer tissues | Induced Sirtuin 5–Nrf2–HO1 pathway to inhibit ROS generation Higher Sirtuin-5 expression | Sirtuin 5 siRNA sensitized cisplatin-induced ROS and DNA damage | [126] |
| Cisplatin | Hepatoma cells: HepG2, 97H, and SMMC7721 HepG2 xenograft | Increased HO-1 expression | ZnPP co-treatment increased ROS, caspase-3 activity, and apoptosis ZnPP enhanced cisplatin-inhibited tumor growth | [131] |
| Cisplatin and pisrarubicin | Hepatoblastoma: HepG2 Human hepatoblastoma specimens (cisplatin and pirarubicin) | Induced EGFR–AKT/ERK–HO-1 | EGFR inhibitor (AG1478) and siHO-1 sensitized cisplatin and pirarubicin-induced cell death | [132] |
| Cytarabine | Leukemia HL-60 and cytarabine-resistant HL-60 (HL-60R) Chemotherapy relapsed samples | HL-60R cells have higher HO-1 expression compared to parental HL-60 Higher HO-1 and HIF-α expression | HO-1 siRNA sensitized cytarabine-induced apoptosis in HL-60R cells | [133] |
| Doxorubicin (DOX) | Breast cancer: MDA-MB-231, and -MB-231 | Induced Src–STAT3–HO-1 Increased HO-1 induced a cytoprotective autophagic flux and increased both Beclin-1 and LC3-I/II | SiRNA of Src and STAT3 sensitized DOX-induced cell death and DOX-increased HO-1, and prevented HO-1-upregulated Beclin-1 and LC3-I/II | [121] |
| Doxorubicin Vinblastine Radiation | Lung adenocarcinoma cells: A549 | HRP-3–Nrf2–HO-1–ROS–p53–PUMA pathway mediated chemoresistance and radioresistance | HRP3 siRNA enhanced sensitivity of doxorubicin, vinblastine, and radiation-induced apoptosis | [134] |
| 5-Fluoracil (5-FU) | MDR1-overexpressed colon carcinoma (HCT-116/R) | HCT-116/R cells expressed higher expression of HIF-1F, Nrf2, and HO-1, as well as increased NOX2 activity and ROS compared to parental cells | NOX inhibitor (HDC) and Nrf2 inhibitor (ML-385) enhanced 5-FU-induced apoptosis | [135] |
| 5-Fluorouracil (5-FU) | Pancreatic cancer, CPFAC and BxPC-3 | Increased HO-1 (higher NQO1 and SOD2) Higher EMT marker (Nanog, Oct4, CD133, and ABCG2) | Nrf2 siRNA increased sensitivity of 5-FU-mediated cytotoxicity | [136] |
| 5-Fluorouracil (5-FU) | Colorectal cancer: SNUC5 and 5-FU-resistant SUNC5 (SNUC5-5-FUR) | ISNUC5-5-FUR exhibited increased ROS–Nrf2–HO-1 compared to parental cells | shRNA of Nrf2 or HO-1 enhanced sensitivity of 5-FU-mediated apoptosis of SNUC5-5-FUR cells and tumor inhibition in SNUC5-5-FUR xenograft mouse | [137] |
| Gemcitabine Radiation | Pancreatic cancer cells: Panc-1, Mla PaCa-2, SU8686, and Colo 357 | Increased HO-1 expression | HO-1 siRNA enhanced sensitivity to Gemcitabine and radiation-mediated cell death | [119] |
| Gemcitabine Radiation | Urothelial carcinoma: T24 and MGHU3 | Increased HO-1 expression | ZnPP co-treatment enhanced sensitivity of gemcitabine or radiation-mediated apoptosis | [120] |
| NMS E793 | A375 melanoma cells | Upregulated ER stress response protein IRE1α, ERO-1, GRP78, and CHOP Upregulated HO-1 | SnMP (HO-1 inhibitor) co-treatment induced higher ER stress, increased ROS, and promoted apoptosis | [138] |
| Pharmorubicin | MDA-MB-231, MCF-7 breast cancer cells | Induced PI3K-AKT-HO-1-autophpagy (LC3-I/II) | HO-1 siRNA sensitized pharmorubicin-mediated reduced chemoresistance | [124] |
| Radiation | Lung adenocarcinoma cells: A549 | Increased HO-1 and ROS levels | HRP-3 knockdown Inhibited Nrf2/HO-1 Enhanced ROS | [134] |
| Low-dose radiation | Lung adenocarcinoma cells: A549 | Induced ROS–autophagy–Nrf2-HO-1 | NAC (ROS scavenger) blocked autophagy and Nrf2/HO-1; Nrf2 knockdown or ZnPP treatment reversed resistance to radiation | [125] |
7. HO-1 Commands the Lifespan of Cancer Cells
7.1. HO-1 and Apoptosis
7.2. HO-1 and Ferroptosis
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Tenhunen, R.; Marver, H.; Pimstone, N.R.; Trager, W.F.; Cooper, D.Y.; Schmid, R. Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450. Biochemistry 1972, 11, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Kutty, R.K.; Kutty, G.; Rodriguez, I.R.; Chader, G.J.; Wiggert, B. Chromosomal localization of the human heme oxygenase genes: Heme oxygenase-1 (HMOX1) maps to chromosome 22q12 and heme oxygenase-2 (HMOX2) maps to chromosome 16p13.3. Genomics 1994, 20, 513–516. [Google Scholar] [CrossRef]
- Munoz-Sanchez, J.; Chanez-Cardenas, M.E. A review on hemeoxygenase-2: Focus on cellular protection and oxygen response. Oxid. Med. Cell Longev. 2014, 2014, 604981. [Google Scholar] [CrossRef]
- Hayashi, S.; Omata, Y.; Sakamoto, H.; Higashimoto, Y.; Hara, T.; Sagara, Y.; Noguchi, M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004, 336, 241–250. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999, 103, 129–135. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Yadav, S.P.; Sachdeva, A.; Pruthi, P.K.; Sawhney, S.; Piplani, T.; Wada, T.; Yachie, A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J. Pediatr. Hematol. Oncol. 2011, 33, 74–78. [Google Scholar] [CrossRef]
- Yachie, A. Heme oxygenase-1 deficiency and oxidative stress: A review of 9 independent human cases and animal models. Int. J. Mol. Sci. 2021, 22, 1514. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Tonegawa, S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. USA 1997, 94, 10919–10924. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.Y.; Park, E.; Lee, S.J.; Chung, S.W. Heme oxygenase-accelerates Erastin-induced ferroptotic cell death. Oncotarget 2015, 6, 24393–24403. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Chiang, S.K.; Chen, S.E.; Yu, Y.L.; Chou, R.H.; Chang, W.C. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018, 416, 124–137. [Google Scholar] [CrossRef]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayır, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M.; et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018, 128, 3341–3355. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; Furfaro, A.L. HO-1 induction in cancer progression: A matter of cell adaptation. Antioxidants 2017, 6, 29. [Google Scholar] [CrossRef]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A dual role of heme oxygenase-1 in cancer cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef]
- Gozzelino, R.; Soares, M.P. Coupling heme and iron metabolism via ferritin H chain. Antioxid. Redox Signal. 2014, 20, 1754–1768. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Gouveia, Z.; Sorares, M.P.; Gozzelino, R. Heme cytotoxicity and the pathogenesis of immune-mediated inflammatory diseases. Front Pharmacol. 2012, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Cooper, S.; Levi, S.; Arosio, P. Mutated recombinant human heavy-chain ferritins and myelosuppression in vitro and in vivo: A link between ferritin ferroxidase activity and biological function. Proc. Natl. Acad. Sci. USA 1991, 88, 770–774. [Google Scholar] [CrossRef]
- Jeney, V.; Balla, J.; Yachie, A.; Varga, Z.; Vercellotti, G.M.; Eaton, J.W.; Balla, G. Pro-oxidant and cytoxic effects of circulating heme. Blood 2002, 100, 879–887. [Google Scholar] [CrossRef]
- Gatica, D.; Lahiri, V.; Klionsky, D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018, 20, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, V.G.; Sharma, V.S.; Pilz, R.B.; Magde, D.; Koesling, D. Basis of guanylate cyclase activation by carbon monoxide. Proc. Natl. Acad. Sci. USA 1995, 92, 2568–2571. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Pae, H.O.; Zheng, M.; Park, R.; Kim, Y.M.; Chung, H.T. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ. Res. 2007, 101, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, P.; Alam, J.; Fu, X.Y.; Lee, P.J. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J. Biol. Chem. 2005, 280, 8714–8721. [Google Scholar] [CrossRef]
- Al-Owais, M.M.; Scragg, J.L.; Dallas, M.L.; Boycott, H.E.; Warburton, P.; Chakrabarty, A.; Boyle, J.P.; Peers, C. Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. J. Biol. Chem. 2012, 287, 24754–24764. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen- activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Boczkowski, J.; Poderoso, J.J.; Motterlini, R. CO-metal interaction: Vital signaling from a lethal gas. Trends Biochem. Sci. 2006, 31, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Choi, A.M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl. Res. 2016, 167, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Ma, K.C.; Choi, A.M.K. Carbon monoxide in lung cell physiology and disease, Am. J. Physiol. Cell Physiol. 2018, 314, C211–C227. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.S.; Figueiredo-Pereira, C.; Vieira, H.L.A. Carbon monoxide and mitochondria—Modulation of cell metabolism, redox response and cell death. Front. Physiol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Snyder, S.H. Bilirubin benefits: Cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 2004, 113, 1776–1782. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Takeshige, K.; Cheung, B.S.; Minakami, S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim. Biophys. Acta 1991, 1076, 369–373. [Google Scholar] [CrossRef]
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taille, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Dureuil, B.; et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: Implications for protection against endotoxic shock in rats. FASEB J. 2005, 19, 1890–1892. [Google Scholar] [CrossRef]
- Jansen, T.; Daiber, A. Direct antioxidant properties of bilirubin and biliverdin. Is there a role for biliverdin reductase? Front. Pharmacol. 2012, 3, 30. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef]
- Dey, S.; Sayers, C.M.; Verginadis, I.I.; Lehman, S.L.; Cheng, Y.; Cerniglia, G.J.; Tuttle, S.W.; Feldman, M.D.; Zhang, P.J.; Fuchs, S.Y.; et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J. Clin. Investig. 2015, 125, 2592–2608. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Perrella, M.A. Heme oxygenase-1: A novel drug target for atherosclerotic diseases? Circulation 2006, 114, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.S.; Weis, S.; Yang, G.; Zhuang, T.; Abate, A.; Dennery, P.A. Catalytic inactive heme oxygenase-1 protein regulates its own expression in oxidative stress. Free Radic. Biol. Med. 2008, 44, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Ogawa, K.; Sun, J.; Taketani, S.; Nakajima, O.; Nishitani, C.; Sassa, S.; Hayashi, N.; Yamamoto, M.; Shibahara, S.; Fujita, H.; et al. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001, 20, 2835–2843. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hoshino, H.; Takaku, K.; Nakajima, O.; Muto, A.; Suzuki, H.; Tashiro, S.; Takahashi, S.; Shibabara, S.; Alam, J.; et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002, 21, 5216–5224. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Jain, A.K.; Bloom, D.A.; Jaiswal, A.K. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J. Biol. Chem. 2005, 280, 16891–16900. [Google Scholar] [CrossRef]
- Beckman, J.D.; Chen, C.; Nguyen, J.; Thayanithy, V.; Subramanian, S.; Steer, C.J.; Vercellotti, G.M. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J. Biol. Chem. 2011, 286, 3194–3202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, W.; Fan, Z.; Yang, C.; Wang, W.; Xiong, M.; Ma, C.; Yang, J. MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis 2019, 290, 9–18. [Google Scholar] [CrossRef]
- Reziwan, K.; Sun, D.; Zhang, B.; Zhao, Z. MicroRNA-1225 activates Keap1-Nrf2-HO-1 signalling to inhibit TNFalpha-induced osteoclastogenesis by mediating ROS generation. Cell Biochem. Funct. 2019, 37, 256–265. [Google Scholar] [CrossRef]
- Skrzypek, K.; Tertil, M.; Golda, S.; Ciesla, M.; Weglarczyk, K.; Collet, G.; Guichard, A.; Kozakowska, M.; Boczkowski, J.; Was, H.; et al. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid. Redox Signal. 2013, 19, 644–660. [Google Scholar] [CrossRef]
- Li, H.; Di, G.; Zhang, Y.; Xue, R.; Zhang, J.; Liang, J. MicroRNA-155 and microRNA-181a, via HO-1, participate in regulating the immunotoxicity of cadmium in the kidneys of exposed Cyprinus carpio. Fish Shellfish Immunol. 2019, 95, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Piras, S.; Furfaro, A.L.; Caggiano, R.; Brondolo, L.; Garibaldi, S.; Ivaldo, C.; Marinari, U.M.; Pronzato, M.A.; Faraonio, R.; Nitti, M. microRNA-494 favors ho-1 expression in neuroblastoma cells exposed to oxidative stress in a bach1-independent way. Front. Oncol. 2018, 8, 199. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, S.J.; Lin, M.W.; Tsai, H.L.; Kuo, S.S.; Chen, J.W.; Charng, M.J.; Wu, T.C.; Chen, L.C.; Ding, Y.D.; et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Zhong, M.; Nisar, M.F.; Wu, Y.; Ouyang, M.; Bartsch, J.W.; Zhong, J.L. A novel heme oxygenase-1 splice variant, 14 kDa HO-1, promotes cell proliferation and increases relative telomere length. Biochem. Biophys. Res. Commun. 2018, 500, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Chiang, M.T.; Li, F.A.; Yeh, C.T.; Lee, W.H.; Chau, L.Y. Acetylation is essential for nuclear heme oxygenase-1-enhanced tumor growth and invasiveness. Oncogene 2017, 36, 6805–6814. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Di Domenico, F.D.; Sultana, R.; Coccia, R.; Mancuso, C.; Perluigi, M.; Butterfied, D.A. Heme oxygenase-1 post-translational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic. Biol. Med. 2012, 52, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; Midwinter, R.G.; Ni, J.; Hamid, H.A.; Parish, C.R.; Stocker, R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid. Redox Signal. 2014, 20, 1723–1742. [Google Scholar] [CrossRef] [PubMed]
- Chillappagari, S.; Belapurkar, R.; Moller, A.; Molenda, N.; Kracht, M.; Rohrbach, S.; Schmitz, M.L. SIAH2-mediated and organ-specific restriction of HO-1 expression by a dual mechanism. Sci. Rep. 2020, 10, 2268. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Lan, W.M.; Chau, L.Y. TRC8 suppresses tumorigenesis through targeting heme oxygenase-1 for ubiquitination and degradation. Oncogene 2013, 32, 2325–2334. [Google Scholar] [CrossRef]
- Dwyer, B.E.; Nishimura, R.N.; De Vellis, J.; Yoshida, T. Heme oxygenase is a heat shock protein and PEST protein in rat astroglial cells. Glia 1992, 5, 300–305. [Google Scholar] [CrossRef]
- Shibahara, S.; Muller, R.; Taguchi, H.; Yoshida, T. Cloning and expression of cDNA for rat heme oxygenase. Proc. Natl. Acad. Sci. USA 1985, 82, 7865–7869. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, M.; Alonso, E.N.; Alonso, E.G.; Lacunza, E.; Curino, A.C.; Facchinetti, M.M. Nuclear localization of heme oxygenase-1 in pathophysiological conditions: Does it explain the dual role in cancer? Antioxidants 2021, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F.; et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef] [PubMed]
- Converso, D.P.; Taille, C.; Carreras, M.C.; Jaitovich, A.; Poderoso, J.J.; Boczkowski, J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006, 20, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Slebos, D.J.; Ryter, S.W.; van der Toorn, M.; Liu, F.; Guo, F.; Baty, C.J.; Karlsson, J.M.; Watkins, S.C.; Kim, H.P.; Wang, X.; et al. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am. J. Respir. Cell Mol. Biol. 2007, 36, 409–417. [Google Scholar] [CrossRef]
- Bansal, S.; Biswas, G.; Avadhani, N.G. Mitochondria-targeted heme oxygenase-1 induces oxidative stress and and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity. Redox Biol. 2013, 2, 273–283. [Google Scholar] [CrossRef]
- Hull, T.D.; Boddu, R.; Guo, L.; Tisher, C.C.; Traylor, A.M.; Patel, B.; Joseph, R.; Prabhu, S.D.; Suliman, H.B.; Piantadosi, C.A.; et al. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight 2016, 1, e85817. [Google Scholar] [CrossRef]
- Suliman, H.B.; Keenan, J.E.; Piantadosi, C.A. Mitochondrial quality-control dysregulation in conditional HO-1−/− mice. JCI Insight 2017, 2, e89676. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Ye, Z.; Manevich, Y.; Ball, L.E.; Bethard, J.R.; Jiang, Y.L.; Broome, A.M.; Dalton, A.C.; Wang, G.Y.; et al. Isoflavone ME-344 disrupts redox homeostasis and mitochondrial function by targeting heme oxygenase. Cancer Res. 2019, 79, 4072–4085. [Google Scholar] [CrossRef]
- Hwang, H.W.; Lee, J.R.; Chou, K.Y.; Suen, C.S.; Hwang, M.J.; Chen, C.; Shieh, R.C.; Chau, L.Y. Oligomerization is crucial for the stability and function of heme oxygenase-1 in the endoplasmic reticulum. J. Biol. Chem. 2009, 284, 22672–22679. [Google Scholar] [CrossRef]
- Linnenbaum, M.; Busker, M.; Kraehling, J.R.; Behrends, S. Heme oxygenase isoforms differ in their subcellular trafficking during hypoxia and are differentially modulated by cytochrome P450 reductase. PLoS ONE 2012, 7, e35483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Yeh, C.T.; Sun, Y.J.; Chiang, M.T.; Lan, W.M.; Chau, L.Y. Signal peptide peptidase-mediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene 2015, 34, 2360–2370. [Google Scholar] [CrossRef]
- Gandini, N.A.; Fermento, M.E.; Salomon, D.G.; Blasco, J.; Patel, V.; Gutkind, J.S.; Molinolo, A.A.; Facchinetti, M.M.; Curino, A.C. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp. Mol. Pathol. 2012, 93, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Gallo, D.; Csizmadia, E.; Harris, C.; Belcher, J.; Vercellotti, G.M.; Penacho, N.; Seth, P.; Sukhatme, V.; Ahmed, A.; et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013, 73, 7009–7021. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Behrends, S. Translocation of heme oxygenase-1 contributes to imatinib resistance in chronic myelogenous leukemia. Oncotarget 2017, 8, 67406–67421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; Vanella, L.; Conticello, C.; Romano, A.; Saccone, S.; Godos, J.; Di Raimondo, F.; Li Volti, G. Heme oxygenase-1 nuclear translocation regulates bortezomibinduced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget 2016, 7, 28868–28880. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive oxygen species in the tumor microenvironment: An overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Jozkowicz, A.; Was, H.; Dulak, J. Heme oxygenase-1 in tumors: Is it a false friend? Antioxid. Redox. Signal. 2007, 9, 2099–2117. [Google Scholar] [CrossRef]
- Luu Hoang, K.N.; Anstee, J.E.; Arnold, J.N. The diverse roles of heme oxygenase-1 in tumor progression. Front. Immunol. 2021, 12, 658315. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Hedblom, A.; Harris, C.; Csizmadia, E.; Gallo, D.; Wegiel, B. Heme oxygenase-1 and carbon monoxide modulate DNA repair through ataxia- telangiectasia mutated (ATM) protein. Proc. Natl. Acad. Sci USA 2011, 108, 14491–14496. [Google Scholar] [CrossRef] [PubMed]
- Barikbin, R.; Berkhout, L.; Bolik, J.; Schmidt-Arras, D.; Ernst, T.; Ittrich, H.; Adam, G.; Parplys, A.; Casar, C.; Krech, T.; et al. Early heme oxygenase 1 induction delays tumour initiation and enhances DNA damage repair in liver macrophages of Mdr2(−/−) mice. Sci. Rep. 2018, 8, 16238. [Google Scholar] [CrossRef]
- Seiwert, N.; Wecklein, S.; Demuth, P.; Hasselwander, S.; Kemper, T.A.; Schwerdtle, T.; Brunner, T.; Fahrer, J. Heme oxygenase 1 protects human colonocytes against ROS formation, oxidative DNA damage and cytotoxicity induced by heme iron, but not inorganic iron. Cell Death Dis. 2020, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, J.; Ge, Y.; Cao, H.; Ge, X.; Luo, J.; Xue, J.; Yang, H.; Zhang, S.; Cao, J. Epigallocatechin-3-gallate (EGCG) protects skin cells from ionizing radiation via heme oxygenase-1 (HO-1) overexpression. J. Radiat. Res. 2014, 55, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, D.; Xiao, H.; Wei, H.; Matunda, C.; Zhang, H.; Li, X.; Wang, C.; Zou, C.; Gao, X.; et al. Enhancement of DEN-induced liver tumorigenesis in heme oxygenase-1 G143H mutant transgenic mice. Biochem. Biophys. Res. Commun. 2016, 481, 169–175. [Google Scholar] [CrossRef] [PubMed]
- He, J.Z.; Ho, J.J.D.; Gingerich, S.; Courtman, D.W.; Marsden, P.A.; Ward, M.E. Enhanced translation of heme oxygenase-2 preserves human endothelial cell viability during hypoxia. J. Biol. Chem. 2010, 285, 9452–9461. [Google Scholar] [CrossRef]
- Sacca, P.; Meiss, R.; Casas, G.; Mazza, O.; Calvo, J.C.; Navone, N.; Vazquez, E. Nuclear translocation of haeme oxygenase-1 is associated to prostate cancer. Br. J. Cancer. 2007, 97, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Talieri, M.; Papadopoulou, S.; Scorilas, A.; Xynopoulos, D.; Arnogianaki, N.; Plataniotis, G.; Yotis, J.; Agnanti, N. Cathepsin B and cathepsin D expression in the progression of colorectal adenoma to carcinoma. Cancer Lett. 2004, 205, 97–106. [Google Scholar] [CrossRef]
- Chan, A.T.; Baba, Y.; Shima, K.; Nosho, K.; Chung, D.C.; Hung, K.E.; Mahmood, U.; Madden, K.; Poss, K.; Ranieri, A.; et al. Cathepsin B expression and survival in colon cancer: Implications for molecular detection of neoplasia. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2777–2785. [Google Scholar] [CrossRef]
- Gandini, N.A.; Fermento, M.E.; Salomon, D.G.; Obiol, D.J.; Andres, N.C.; Zenklusen, J.C.; Arevalo, J.; Blasco, J.; Lopez Romero, A.; Facchinetti, M.M.; et al. Heme oxygenase-1 expression in human gliomas and its correlation with poor prognosis in patients with astrocytoma. Tumour Biol. 2014, 35, 2803–2815. [Google Scholar] [CrossRef]
- Mayerhofer, M.; Florian, S.; Krauth, M.T.; Aichberger, K.J.; Bilban, M.; Marculescu, R.; Printz, D.; Fritsch, G.; Wagner, O.; Selzer, E.; et al. Identification of heme oxygenase-1 as a novel BCR/ABL-dependent survival factor in chronic myeloid leukemia. Cancer Res. 2004, 64, 3148–3154. [Google Scholar] [CrossRef]
- Balan, M.; Chakraborty, S.; Flynn, E.; Zurakowski, D.; Pal, S. Honokiol inhibits c-Met-HO-1 tumor-promoting pathway and its cross-talk with calcineurin inhibitor-mediated renal cancer growth. Sci. Rep. 2017, 7, 5900. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.S.; Wu, M.S.; Bien, M.Y.; Chen, C.H.; Lin, C.H.; Chen, B.C. Epidermal growth factor stimulates nuclear factor-kappaB activation and heme oxygenase-1 expression via c-Src, NADPH oxidase, PI3K, and Akt in human colon cancer cells. PLoS ONE 2014, 9, e104891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alaluf, E.; Vokaer, B.; Detavernier, A.; Azouz, A.; Splittgerber, M.; Carrette, A.; Boon, L.; Libert, F.; Soares, M.; Le Moine, A.; et al. Heme oxygenase 1 orchestrates the immunosuppressive program of tumor-associated macrophages. JCI Insight 2020, 5, e133929. [Google Scholar] [CrossRef]
- Sacco, A.; Battaglia, A.M.; Botta, C.; Aversa, I.; Mancuso, S.; Costanzo, F.; Biamonte, F. Iron metabolism in the tumor microenvironment-implications for anti-cancer immune response. Cells 2021, 10, 303. [Google Scholar] [CrossRef]
- Nemeth, Z.; Li, M.; Csizmadia, E.; Dome, B.; Johansson, M.; Persson, J.L.; Seth, P.; Otterbein, L.; Wegiel, B. Heme oxygenase-1 in macrophages controls prostate cancer progression. Oncotarget 2015, 6, 33675–33688. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 7, 1745–1770. [Google Scholar] [CrossRef]
- Sunamura, M.; Duda, D.G.; Ghattas, M.H.; Lozonschi, L.; Motoi, F.; Yamauchi, J.; Matsuno, S.; Shibahara, S.; Abraham, N.G. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis 2003, 6, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Deshane, J.; Chen, S.; Caballero, S.; Grochot-Przeczek, A.; Was, H.; Li Calzi, S.; Lach, R.; Hock, T.D.; Chen, B.; Hill-Kapturczak, N.; et al. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J. Exp. Med. 2007, 204, 605–618. [Google Scholar] [CrossRef]
- Loboda, A.; Jazwa, A.; Grochot-Przeczek, A.; Rutkowski, A.J.; Cisowski, J.; Agarwal, A.; Jozkowicz, A.; Dulak, J. Heme oxygenase-1 and the vascular bed: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 2008, 10, 1767–1812. [Google Scholar] [CrossRef]
- Birrane, G.; Li, H.; Yang, S.; Tahado, S.D.; Seng, S. Cigarette smoke induces nuclear translocation of heme oxygenase 1 (HO-1) in prostate cancer cells: Nuclear HO-1 promotes vascular endothelial growth factor secretion. Int. J. Oncol. 2013, 42, 1919–1928. [Google Scholar] [CrossRef]
- Cheng, C.C.; Guan, S.S.; Yang, H.J.; Chang, C.C.; Luo, T.Y.; Chang, J.; Ho, A.S. Blocking heme oxygenase-1 by zine protoporphyrin reduces tumor hypoxia-mediated VEGF release and inhibits tumor angiogenesis as a potential therapeutic agent against colorectal cancer. J. Biomed. Sci. 2016, 23, 18. [Google Scholar] [CrossRef]
- Bauer, A.; Mylroie, H.; Thornton, C.C.; Calay, D.; Birdsey, G.M.; Kiprianos, A.P.; Wilson, G.K.; Soares, M.P.; Yin, X.; Mayr, M.; et al. Identification of cyclins A1, E1 and vimentin as downstream targets of heme oxygenase-1 in vascular endothelial growth factor-mediated angiogenesis. Sci. Rep. 2016, 6, 29417. [Google Scholar] [CrossRef]
- Maamoun, H.; Zachariah, M.; McVey, J.H.; Green, F.R.; Agouni, A. Heme oxygenase (HO)-1 induction prevents endoplasmic reticulum stress-mediated endothelial cell death and impaired angiogenic capacity. Biochem. Pharmacol. 2017, 127, 46–59. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, C.K.; Lee, H.; Jeoung, D.; Ha, K.S.; Kwon, Y.G.; Kim, K.W.; Kim, Y.M. Carbon monoxide promotes VEGF expression by increasing HIF-1alpha protein level via two distinct mechanisms, translational activation and stabilization of HIF-1alpha protein. J. Biol. Chem. 2010, 285, 32116–32125. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Chiang, M.T.; Chang, P.C.; Chau, L.Y. Myeloid heme oxygenase-1 promotes metastatic tumor colonization in mice. Cancer Sci. 2015, 106, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Cichon, T.; Smolarczyk, R.; Rudnicka, D.; Stopa, M.; Chevalier, C.; Leger, J.J.; Lackowska, B.; Grochot, A.; Bojkowska, K.; et al. Overexpression of heme oxygenase-1 in murine melanoma: Increased proliferation and viability of tumor cells, decreased survival of mice. Am. J. Pathol. 2006, 169, 2181–2198. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Angela, N.; Weinberg, R.A. EMT in cancer. Nature Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Castruccio Castracani, C.; Longhitano, L.; Distefano, A.; Di Rosa, M.; Pittala, V.; Lupo, G.; Caruso, M.; Corona, D.; Tibullo, D.; Li Volti, G. Heme oxygenase-1 and carbon monoxide regulate growth and progression in glioblastoma cells. Mol. Neurobiol. 2020, 57, 2436–2446. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Xue, J.; Zhao, X.; Liu, P. Autophagy inhibition promotes epithelial-mesenchymal transition through ROS/HO-1 pathway in ovarian cancer cells. Am. J. Cancer Res. 2016, 6, 2162–2177. [Google Scholar]
- Chang, Y.J.; Chen, W.Y.; Huang, C.Y.; Liu, H.H.; Wei, P.L. Glucose-regulated protein 78 (GRP78) regulates colon cancer metastasis through EMT biomarkers and the NRF-2/HO-1 pathway. Tumour Biol. 2015, 36, 1859–1869. [Google Scholar] [CrossRef]
- Wang, X.; Ye, T.; Xue, B.; Yang, M.; Li, R.; Xu, X.; Zeng, X.; Tian, N.; Bao, L.; Huang, Y. Mitochondrial GRIM-19 deficiency facilitates gastric cancer metastasis through oncogenic ROS-NRF2-HO-1 axis via a NRF2-HO-1 loop. Gastric Cancer 2021, 24, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.S.; Hsu, Y.C.; Lee, J.J.; Chen, M.J.; Huang, S.Y.; Cheng, S.P. Heme Oxygenase-1 inhibitors induce cell cycle arrest and suppress tumor growth in thyroid cancer cells. Int. J. Mol. Sci. 2018, 19, 2502. [Google Scholar] [CrossRef]
- Tertil, M.; Golda, S.; Skrzypek, K.; Florczyk, U.; Weglarczyk, K.; Kotlinowski, J.; Maleszewska, M.; Czauderna, S.; Pichon, C.; Kieda, C.; et al. Nrf2-heme oxygenase-1 axis in mucoepidermoid carcinoma of the lung: Antitumoral effects associated with down-regulation of matrix metalloproteinases. Free Radic. Biol. Med. 2015, 89, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Q.; Cheng, W.; Wei, H.; Jiang, W.E.F.; Yu, Y.; Jin, J.; Zou, C. Heme oxygenase-1 inhibits tumor metastasis mediated by notch1 pathway in murine mammary carcinoma. Oncol. Res. 2019, 27, 643–651. [Google Scholar] [CrossRef]
- Brenneisen, P.; Reichert, A.S. Nanotherapy and reactive oxygen species (ROS) in cancer: A novel perspective. Antioxidants 2018, 7, 31. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Berberat, P.O.; Dambrauskas, Z.; Gulbinas, A.; Giese, T.; Giese, N.; Kunzli, B.; Autschbach, F.; Meuer, S.; Buchler, M.W.; Friess, H. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin. Cancer Res. 2005, 11, 3790–3798. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Fujimoto, K.; Anai, S.; Ohnishi, S.; Nakai, Y.; Inoue, T.; Matsumura, Y.; Tomioka, A.; Ikeda, T.; Okajima, E.; et al. Inhibition of heme oxygenase-1 enhances the cytotoxic effect of gemcitabine in urothelial cancer cells. Anticancer Res. 2010, 30, 2145–2152. [Google Scholar] [PubMed]
- Tan, Q.; Wang, H.; Hu, Y.; Hu, M.; Li, X.; Aodengqimuge; Ma, Y.; Wei, C.; Song, L. Src/STAT3-dependent heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to doxorubicin by promoting autophagy. Cancer Sci. 2015, 106, 1023–1032. [Google Scholar] [CrossRef]
- Tang, Q.F.; Sun, J.; Yu, H.; Shi, X.J.; Lv, R.; Wei, H.C.; Yin, P.H. The Zuo Jin Wan formula induces mitochondrial apoptosis of cisplatin-resistant gastric cancer cells via cofilin-1. Evid.-Based Complement. Altern. Med. 2016, 2016, 8203789. [Google Scholar] [CrossRef]
- Tracey, N.; Creedon, H.; Kemp, A.J.; Culley, J.; Muir, M.; Klinowska, T.; Brunton, V.G. HO-1 drives autophagy as a mechanism of resistance against HER2-targeted therapies. Breast Cancer Res. Treat. 2020, 179, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Kong, Y.; Shao, C.; Yue, X.; Wang, Z.; Zhang, N. Heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to pharmorubicin by promoting autophagy via PI3K/Akt pathway. J. Cell Mol. Med. 2018, 22, 5311–5321. [Google Scholar] [CrossRef]
- Chen, N.; Wu, L.; Yuan, H.; Wang, J.I. ROS/Autophagy/Nrf2 pathway mediated low-dose radiation induced radio-resistance in human lung adenocarcinoma A549 cell. Int. J. Biol. Sci. 2015, 11, 833–844. [Google Scholar]
- Sun, X.; Wang, S.; Gai, J.; Guan, J.; Li, J.; Li, Y.; Zhao, J.; Zhao, C.; Fu, L.; Li, Q. SIRT5 promotes cisplatin resistance in ovarian cancer by suppressing DNA damage in a ROS-dependent manner via regulation of the Nrf2/HO-1 pathway. Front. Oncol. 2019, 9, 754. [Google Scholar] [CrossRef]
- Jang, J.E.; Eom, J.I.; Jeung, H.K.; Chung, H.; Kim, Y.R.; Kim, J.S.; Cheong, J.W.; Min, Y.H. PERK/NRF2 and autophagy form a resistance mechanism against G9a inhibition in leukemia stem cells. J. Exp. Clin. Cancer Res. 2020, 39, 66. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Piras, S.; Passalacqua, M.; Domenicotti, C.; Parodi, A.; Fenoglio, D.; Pronzato, M.A.; Marinari, U.M.; Moretta, L.; Traverso, N.; et al. HO-1 up-regulation: A key point in high-risk neuroblastoma resistance to bortezomib. Biochim. Biophys. Acta 2014, 1842, 613–622. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Piras, S.; Domenicotti, C.; Fenoglio, D.; De Luigi, A.; Salmona, M.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; et al. Role of Nrf2, HO-1 and GSH in Neuroblastoma Cell Resistance to Bortezomib. PLoS ONE 2016, 11, e0152465. [Google Scholar] [CrossRef]
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Tonissen, K.F. Cross-talk between two antioxidants, thioredoxin reductase and heme oxygenase-1, and therapeutic implications for multiple myeloma. Redox Biol. 2016, 8, 175–185. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, H.S.; Qi, D.F.; Zhang, J.; Jiang, X.C.; Shi, K.; Zhang, X.J.; Zhang, X.H. Zinc protoporphyrin IX enhances chemotherapeutic response of hepatoma cells to cisplatin. World J. Gastroenterol. 2014, 20, 8572–8582. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kubota, M.; Kinoshita, Y.; Arai, Y.; Oyama, T.; Yokota, N.; Saito, K.; Matsuda, Y.; Osawa, M. Epidermal growth factor receptor/heme oxygenase-1 axis is involved in chemoresistance to cisplatin and pirarubicin in HepG2 cell lines and hepatoblastoma specimens. Pediatr. Surg. Int. 2019, 35, 1369–1378. [Google Scholar] [CrossRef]
- Zhe, N.; Wang, J.; Chen, S.; Lin, X.; Chai, Q.; Zhang, Y.; Zhao, J.; Fang, Q. Heme oxygenase-1 plays a crucial role in chemoresistance in acute myeloid leukemia. Hematology 2015, 20, 384–891. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.S.; Hong, E.H.; Lee, S.J.; Baek, J.H.; Lee, C.W.; Yim, J.H.; Um, H.D.; Hwang, S.G. Depletion of hepatoma-derived growth factor-related protein-3 induces apoptotic sensitization of radioresistant A549 cells via reactive oxygen species-dependent p53 activation. Biochem. Biophys. Res. Commun. 2013, 439, 333–339. [Google Scholar] [CrossRef]
- Waghela, B.N.; Vaidya, F.U.; Pathak, C. Upregulation of NOX-2 and Nrf-2 promotes 5-fluorouracil resistance of human colon carcinoma (HCT-116) cells. Biochemistry 2021, 86, 262–274. [Google Scholar]
- Kim, E.J.; Kim, Y.J.; Lee, H.I.; Jeong, S.H.; Nam, H.J.; Cho, J.H. NRF2 knockdown resensitizes 5-fluorouracil-resistant pancreatic cancer cells by suppressing HO-1 and ABCG2 expression. Int. J. Mol. Sci. 2020, 21, 4646. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Kim, K.C.; Kang, H.K.; Chang, W.Y.; Park, I.C.; Keum, Y.S.; Surh, Y.J.; Hyun, J.W. Epigenetic modification of Nrf2 in 5-fluorouracil-resistant colon cancer cells: Involvement of TET-dependent DNA demethylation. Cell Death Dis. 2014, 5, e1183. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, I.; Parenti, R.; Zappala, A.; Vanella, L.; Tibullo, D.; Pepe, F.; Onni, T.; Li Volti, G. Combined inhibition of Hsp90 and heme oxygenase-1 induces apoptosis and endoplasmic reticulum stress in melanoma. Acta Histochem. 2015, 117, 705–711. [Google Scholar] [CrossRef]
- Gao, C.; Peng, F.H.; Peng, L.K. MiR-200c sensitizes clear-cell renal cell carcinoma cells to sorafenib and imatinib by targeting heme oxygenase-1. Neoplasma 2014, 61, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dang, J.; Liang, Q.; Yin, L. Thermal-responsive carbon monoxide (CO) delivery expedites metabolic exhaustion of cancer cells toward reversal of chemotherapy resistance. ACS Cent. Sci. 2019, 5, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 2, 205–218. [Google Scholar] [CrossRef]
- Sharpe, J.C.; Arnoult, D.; Woule, R.J. Control of mitochondrial permeability by Bcl-2 family members. Biochim. Biophys. Acta. 2004, 1644, 107–113. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Chanvorachote, P.; Stehlik, C.; Tse, W.; Callery, P.S.; Wang, L.; Rojanasakul, Y. Regulation of apoptosis by Bcl-2 cysteine oxidation in human lung epithelial cells. Mol. Biol. Cell 2013, 24, 858–869. [Google Scholar] [CrossRef]
- Banerjee, P.; Basu, A.; Datta, D.; Gasser, M.; Waaga-Gasser, A.M.; Pal, S. The heme oxygenase-1 protein is overexpressed in human renal cancer cells following activation of the Ras-Raf-ERK pathway and mediates anti-apoptotic signal. J. Biol. Chem. 2011, 286, 33580–33590. [Google Scholar] [CrossRef]
- Ren, Q.G.; Yang, S.L.; Hu, J.L.; Li, P.D.; Chen, Y.S.; Wang, Q.S. Evaluation of HO-1 expression, cellular ROS production, cellular proliferation and cellular apoptosis in human esophageal squamous cell carcinoma tumors and cell lines. Oncol. Rep. 2016, 35, 2270–2276. [Google Scholar] [CrossRef]
- Chakraborty, S.; Balan, M.; Flynn, E.; Zurakowski, D.; Choueiri, T.K.; Pal, S. Activation of c-Met in cancer cells mediates growth-promoting signals against oxidative stress through Nrf2-HO-1. Oncogenesis 2019, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Su, W.; Jiao, Q. NGF protects neuroblastoma cells against beta-amyloid-induced apoptosis via the Nrf2/HO-1 pathway. FEBS Open Bio 2019, 9, 2063–2071. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Li, Y.; Wang, S.; Li, Q. Apatinib, a novel tyrosine kinase inhibitor, Promotes ROS-dependent apoptosis and autophagy via the Nrf2/HO-1 pathway in ovarian cancer cells. Oxid. Med. Cell Longev. 2020, 2020, 314518. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Cieslar-Pobuda, A.; Saenko, Y.; Foksinski, M.; Olinski, R.; Rzeszowska-Wolny, J.; Wiechec, E. The impact of DIDS-induced inhibition of voltage-dependent anion channels (VDAC) on cellular response of lymphoblastoid cells to ionizing radiation. Med. Chem. 2017, 13, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Blankstein, A.R.; Konzelman, C.; Gibson, S.B. Lysosomal destabilizing drug siramesine and the dual tyrosine kinase inhibitor lapatinib induce a synergistic ferroptosis through reduced heme oxygenase-1(HO-1) levels. Oxid. Med. Cell. Longev. 2019, 2019, 9561281. [Google Scholar] [CrossRef] [PubMed]
- Podkalicka, P.; Mucha, O.; Jozkowicz, A.; Dulak, J.; Loboda, A. Heme oxygenase inhibition in cancers: Possible tools and targets. Contemp. Oncol. 2018, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells 2021, 10, 2401. https://doi.org/10.3390/cells10092401
Chiang S-K, Chen S-E, Chang L-C. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells. 2021; 10(9):2401. https://doi.org/10.3390/cells10092401
Chicago/Turabian StyleChiang, Shih-Kai, Shuen-Ei Chen, and Ling-Chu Chang. 2021. "The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival" Cells 10, no. 9: 2401. https://doi.org/10.3390/cells10092401
APA StyleChiang, S.-K., Chen, S.-E., & Chang, L.-C. (2021). The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells, 10(9), 2401. https://doi.org/10.3390/cells10092401






