Elongational Stresses and Cells
Abstract
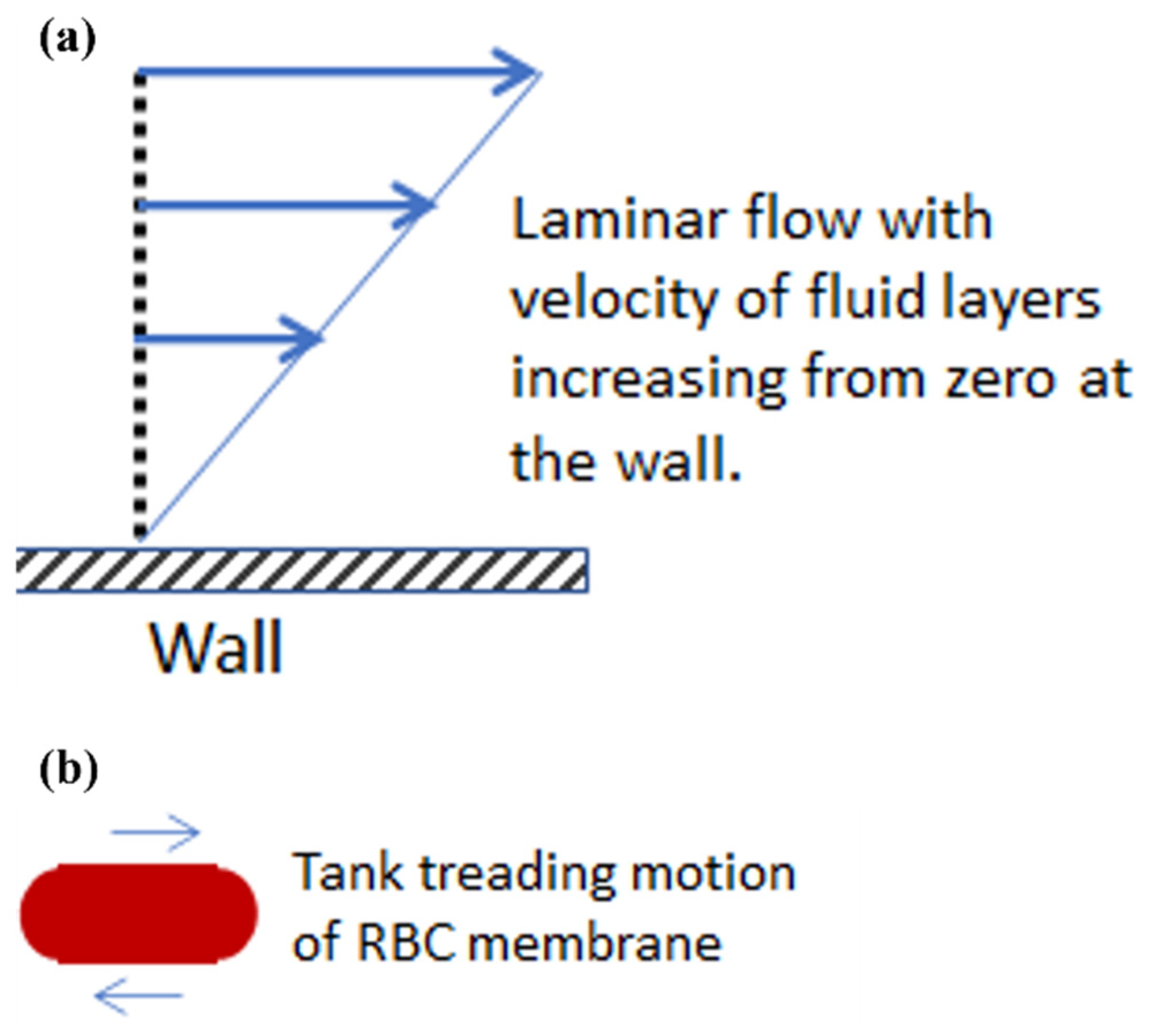
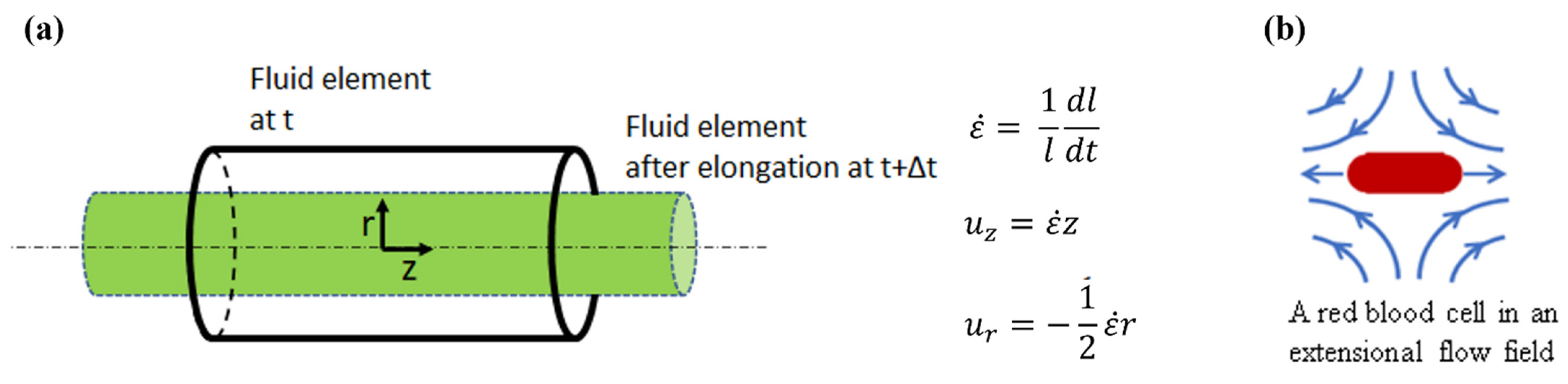
:1. Introduction
2. Experimental Methods for Extensional Stresses on Cells
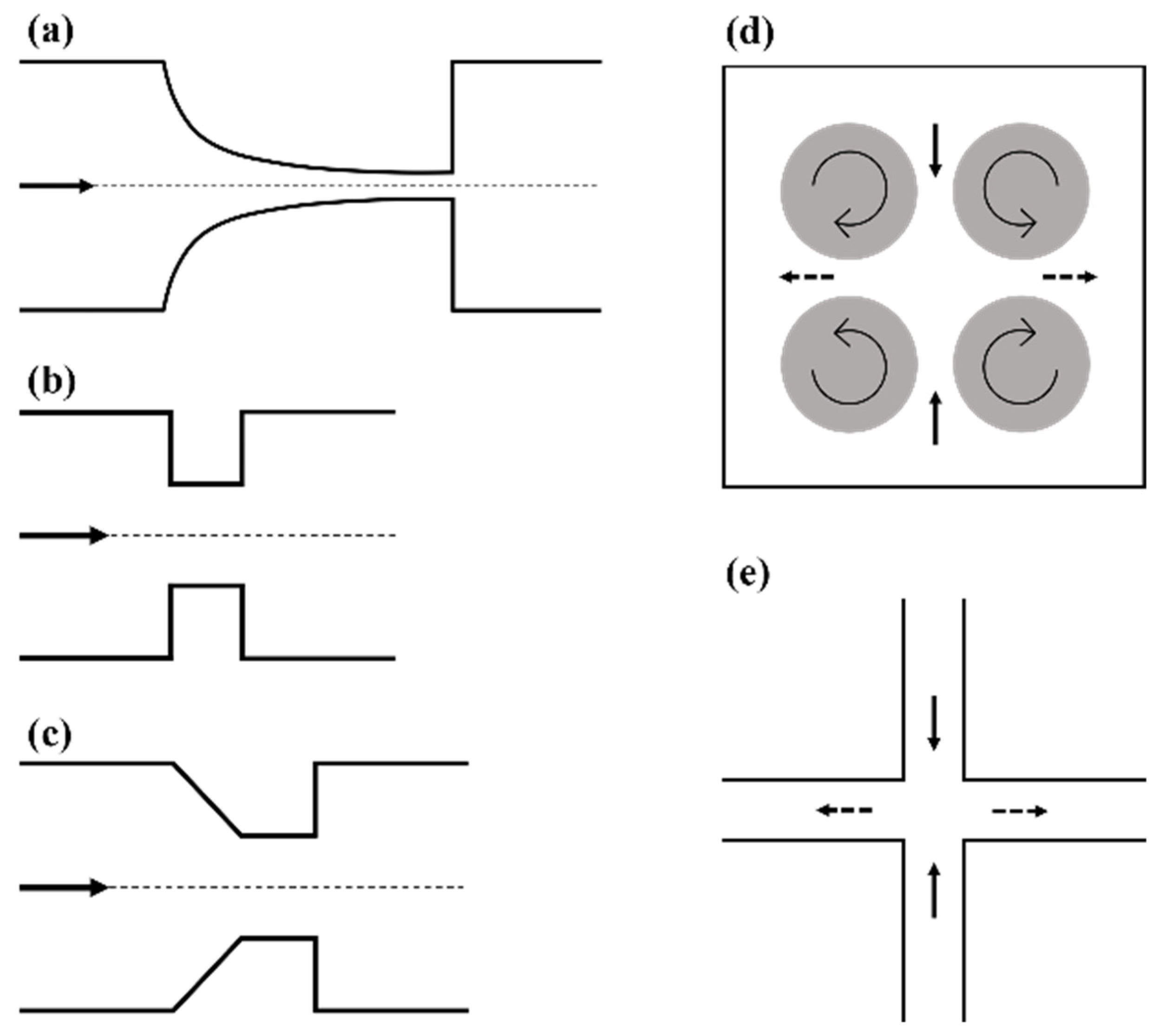
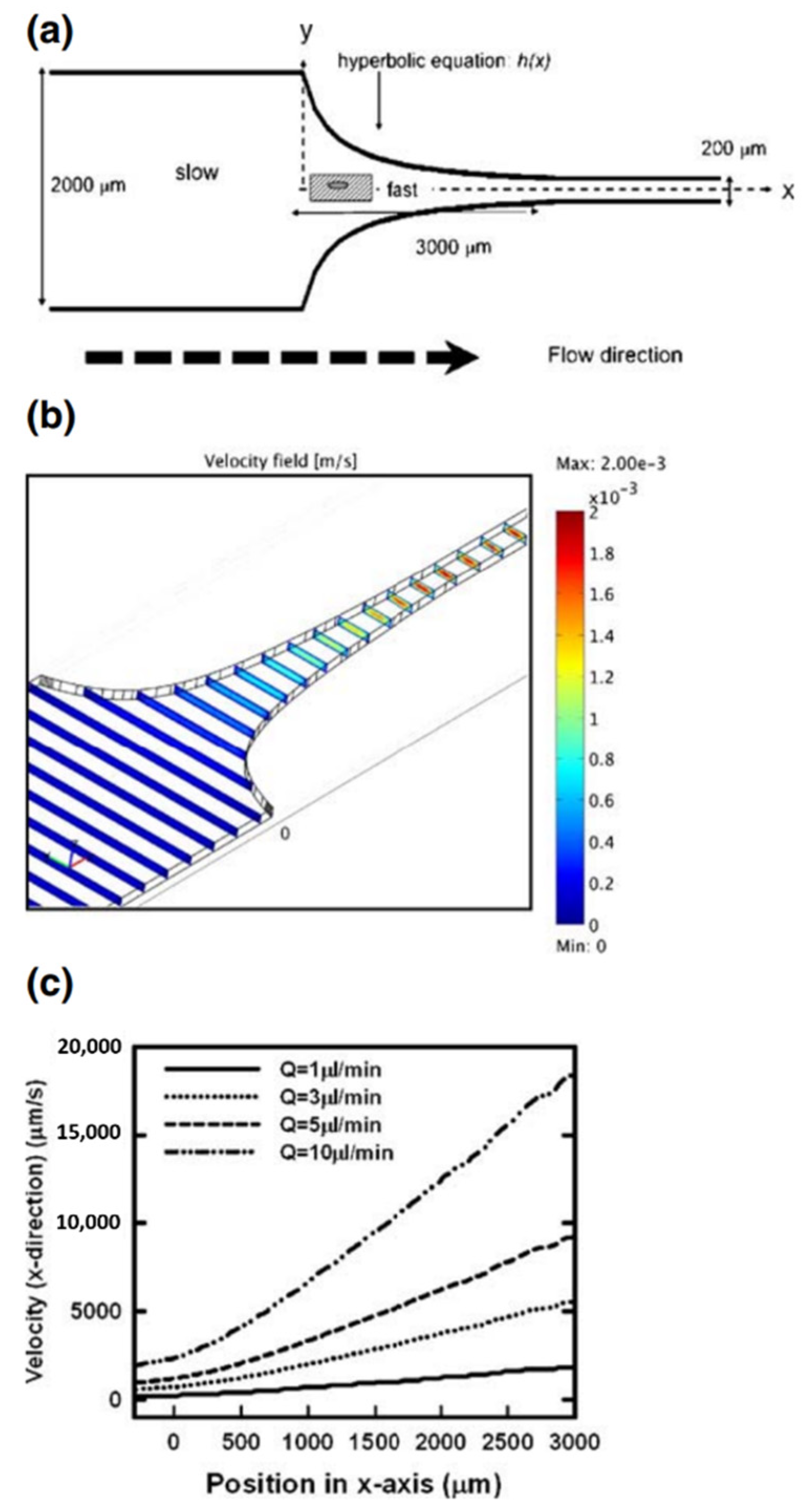
2.1. Microfluidic Systems Based on Flow through a Contraction
2.2. Taylor’s Four-Roll Mill
2.3. Cross-Flow Microfluidic Systems
2.4. Optical Tweezers
3. Cell Deformability and Extensional Stresses
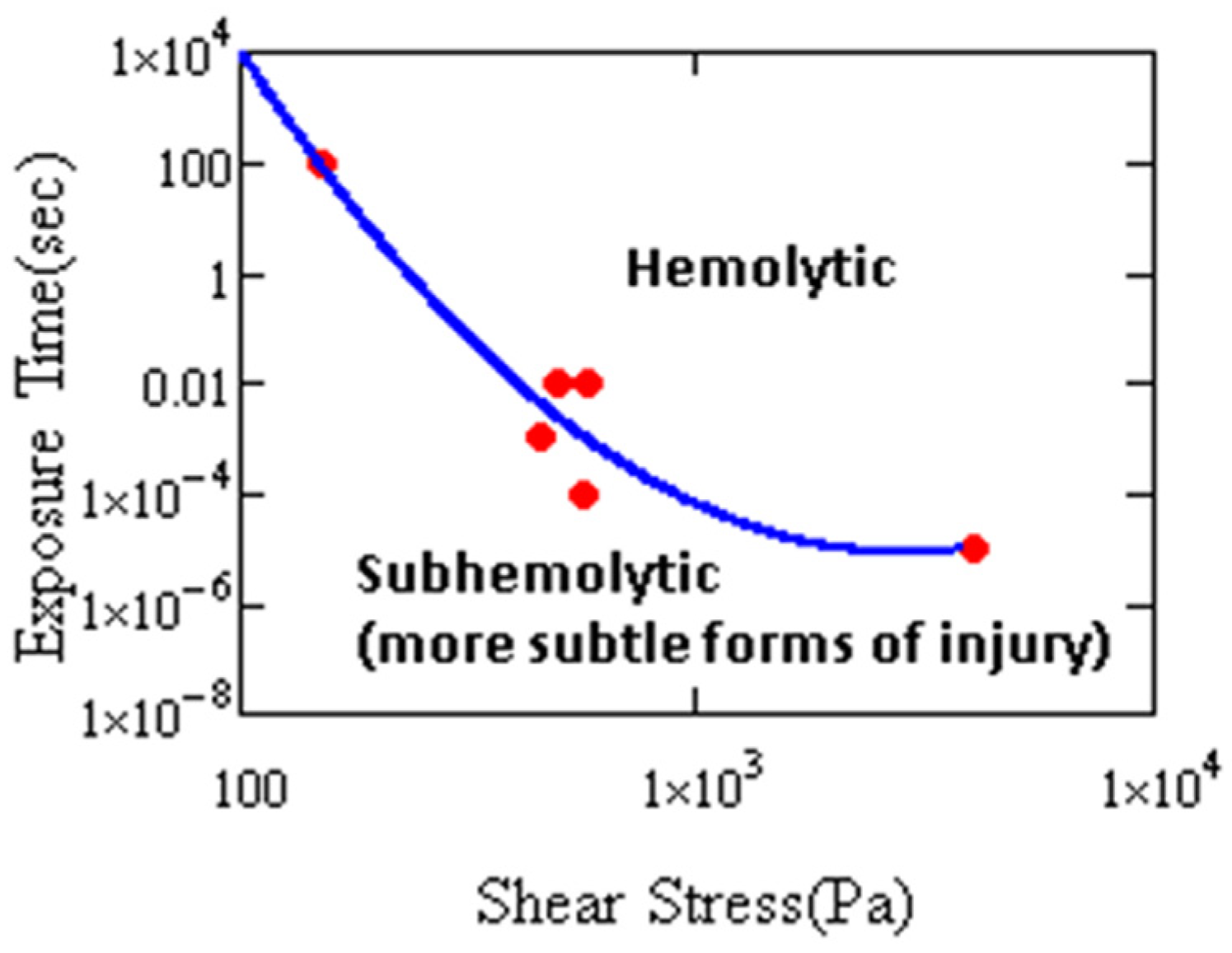
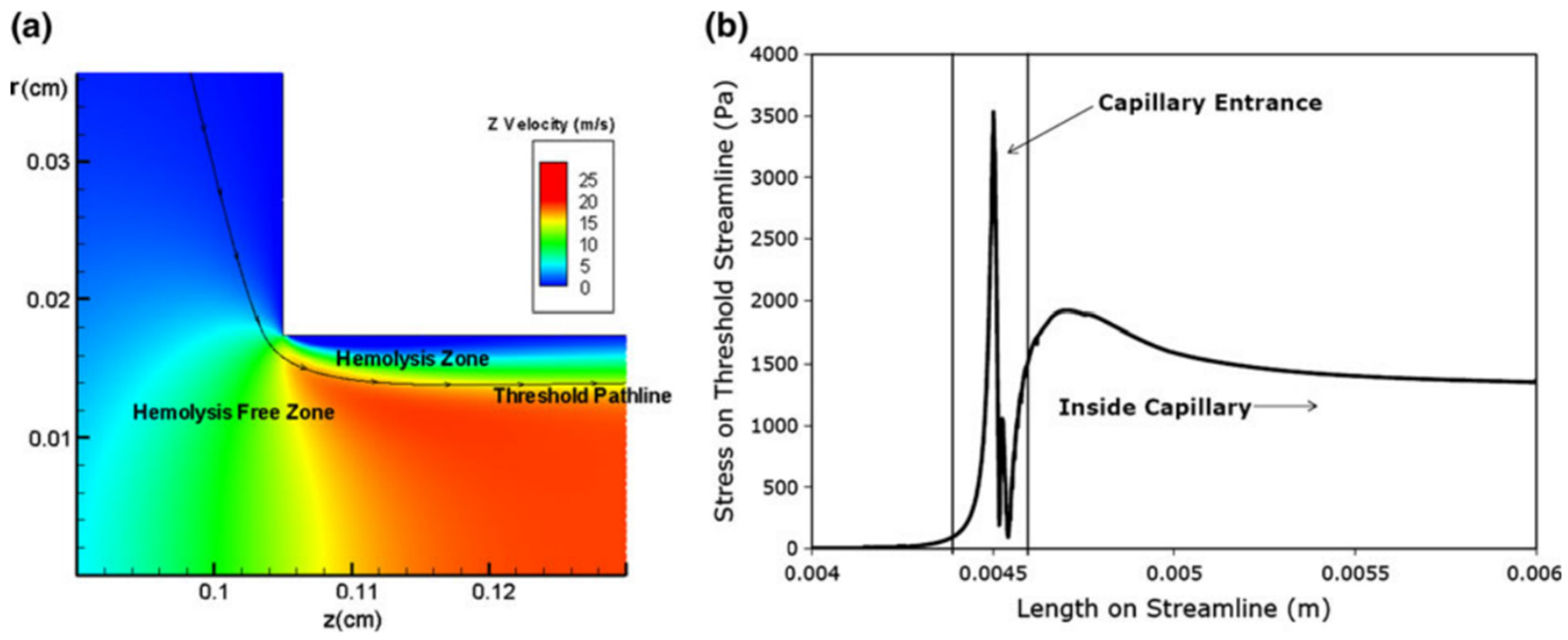
4. Cell Injury by Hydrodynamic Stress
5. Extensional Stresses and Cell Disruption
6. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Korenaga, R.; Ando, J.; Tsuboi, H.; Yang, W.D.; Sakuma, I.; Toyooka, T.; Kamiya, A. Laminar-Flow Stimulates Atp- and Shear Stress-Dependent Nitric-Oxide Production in Cultured Bovine Endothelial-Cells. Biochem. Biophys. Res. Commun. 1994, 198, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kuchan, M.J.; Frangos, J.A. Role of Calcium and Calmodulin in Flow-Induced Nitric-Oxide Production in Endothelial-Cells. Am. J. Physiol. 1994, 266, C628–C636. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Groot, A.C.G.D.; Hierck, B.P. The development of the heart and microcirculation: Role of shear stress. Med. Biol. Eng. Comput. 2008, 46, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntosh, W.H.; Ozturk, M.; Down, L.A.; Papavassiliou, D.V.; O’Rear, E.A. Hemodynamics of the renal artery ostia with implications for their structural development and efficiency of flow. Biorheology 2015, 52, 257–268. [Google Scholar] [CrossRef]
- Fraser, K.H.; Zhang, T.; Taskin, M.E.; Griffith, B.P.; Wu, Z.J. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: Shear stress, exposure time and hemolysis index. J. Biomech. Eng. 2012, 134, 081002. [Google Scholar] [CrossRef]
- Federici, A.B.; Budde, U.; Castaman, G.; Rand, J.H.; Tiede, A. Current diagnostic and therapeutic approaches to patients with acquired von Willebrand syndrome: A 2013 update. Semin. Thromb. Hemost. 2013, 39, 191–201. [Google Scholar] [CrossRef]
- Heck, M.L.; Yen, A.; Snyder, T.A.; O’Rear, E.A.; Papavassiliou, D.V. Flow-Field Simulations and Hemolysis Estimates for the Food and Drug Administration Critical Path Initiative Centrifugal Blood Pump. Artif. Organs 2017, 41, E129–E140. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Schmidschonbein, H. Tank Tread Motion of Red-Cell Membranes in Viscometric Flow-Behavior of Intracellular and Extracellular Markers (with Film). Blood Cells 1977, 3, 351–365. [Google Scholar]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Yen, J.H.; Chen, S.F.; Chern, M.K.; Lu, P.C. The effects of extensional stress on red blood cell hemolysis. Biomed. Eng. Appl. Basis Commun. 2015, 27, 1550042. [Google Scholar] [CrossRef]
- Balasundaram, B.; Harrison, S.T.L. Study of physical and biological factors involved in the disruption of E. coli by hydrodynamic cavitation. Biotechnol. Progr. 2006, 22, 907–913. [Google Scholar] [CrossRef]
- Kubo, M.T.K.; Augusto, P.E.D.; Cristianini, M. Effect of high pressure homogenization (HPH) on the physical stability of tomato juice. Food Res. Int. 2013, 51, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Trouton, F.T. On the coefficient of viscous traction and its relation to that of viscosity. Proc. Math. Phys. Eng. 1906, 77, 426–440. [Google Scholar] [CrossRef]
- Petrie, C.J.S. One hundred years of extensional flow. J. Non-Newton. Fluid Mech. 2006, 137, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Blair, G.W.; Folley, S.J.; Malpress, F.H.; Coppen, F.M. Variations in certain properties of bovine cervical mucus during the oestrous cycle. Biochem. J. 1941, 35, 1039–1049. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.G. The rheology of dilute solutions of flexible polymers: Progress and problems. J. Rheol. 2005, 49, 1–70. [Google Scholar] [CrossRef]
- Perkins, T.T.; Smith, D.E.; Chu, S. Single polymer dynamics in an elongational flow. Science 1997, 276, 2016–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kania, S.; Webb, E.B.; Oztekin, A.; Cheng, X.H.; Zhang, X.H. Rare Event Prediction of Von Willebrand Factor Multimer Unfolding in Extensional Flow. Biophys. J. 2021, 120, 297a. [Google Scholar] [CrossRef]
- Dobson, J.; Kumar, A.; Willis, L.F.; Tuma, R.; Higazi, D.R.; Turner, R.; Lowe, D.C.; Ashcroft, A.E.; Radford, S.E.; Kapur, N.; et al. Inducing protein aggregation by extensional flow. Proc. Natl. Acad. Sci. USA 2017, 114, 4673–4678. [Google Scholar] [CrossRef] [Green Version]
- Zhussupbekov, M.; Méndez Rojano, R.; Wu, W.T.; Massoudi, M.; Antaki, J.F. A Continuum Model for the Unfolding of von Willebrand Factor. Ann. Biomed. Eng. 2021, 16, 1–13. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Atac, N.; Baskurt, O.K.; Meiselman, H.J.; Yalcin, O. Erythrocyte deformability responses to intermittent and continuous subhemolytic shear stress. Biorheology 2014, 51, 171–185. [Google Scholar] [CrossRef]
- Ma, N.N.; Koelling, K.W.; Chalmers, J.J. Fabrication and use of a transient contractional flow device to quantify the sensitivity of mammalian and insect cells to hydrodynamic forces. Biotechnol. Bioeng. 2002, 80, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Buerck, J.P.; Burke, D.K.; Schmidtke, D.W.; Snyder, T.A.; Papavassiliou, D.; O’Rear, E.A. A Flow Induced Autoimmune Response and Accelerated Senescence of Red Blood Cells in Cardiovascular Devices. Sci. Rep. 2019, 9, 19443. [Google Scholar] [CrossRef] [PubMed]
- Ober, T.J.; Haward, S.J.; Pipe, C.J.; Soulages, J.; McKinley, G.H. Microfluidic extensional rheometry using a hyperbolic contraction geometry. Rheol. Acta 2013, 52, 529–546. [Google Scholar] [CrossRef] [Green Version]
- Faghih, M.M.; Sharp, M.K. Deformation of human red blood cells in extensional flow through a hyperbolic contraction. Biomech. Modeling Mechanobiol. 2020, 19, 251–261. [Google Scholar] [CrossRef]
- Mancuso, J.E.; Ristenpart, W.D. Stretching of red blood cells at high strain rates. Phys. Rev. Fluids 2017, 2. [Google Scholar] [CrossRef]
- Henon, Y.; Sheard, G.J.; Fouras, A. Erythrocyte deformation in a microfluidic cross-slot channel. Rsc Adv. 2014, 4, 36079–36088. [Google Scholar] [CrossRef]
- Akbaridoust, F.; Philip, J.; Marusic, I. Assessment of a miniature four-roll mill and a cross-slot microchannel for high-strain-rate stagnation point flows. Meas. Sci. Technol. 2018, 29, 045302. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.; Lim, C.T.; Suresh, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Yaginuma, T.; Oliveira, M.S.N.; Lima, R.; Ishikawa, T.; Yamaguchi, T. Human red blood cell behavior under homogeneous extensional flow in a hyperbolic-shaped microchannel. Biomicrofluidics 2013, 7, 054110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.S.; Yim, Y.; Ahn, K.H.; Lee, S.J. Extensional flow-based assessment of red blood cell deformability using hyperbolic converging microchannel. Biomed. Microdevices 2009, 11, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A Microfluidic Deformability Assessment of Pathological Red Blood Cells Flowing in a Hyperbolic Converging Microchannel. Micromachines 2019, 10, 645. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zografos, K.; Fidalgo, J.; Duchêne, C.; Quintard, C.; Darnige, T.; Filipe, V.; Huille, S.; Du Roure, O.; Oliveira, M.S.N.; et al. Optimised hyperbolic microchannels for the mechanical characterisation of bio-particles. Soft Matter 2020, 16, 9844–9856. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Galli, V.; Holzner, G.; Stavrakis, S.; DeMello, A.; Dubini, G. Deformation of leukaemia cell lines in hyperbolic microchannels: Investigating the role of shear and extensional components. Lab Chip 2020, 20, 2539–2548. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Pinho, D.; Faustino, V.; Lima, R. A simple microfluidic device for the deformability assessment of blood cells in a continuous flow. Biomed. Microdevices 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Oliveira, M.S.N.; Alves, M.A.; Pinho, F.T.; McKinley, G.H. Viscous flow through microfabricated hyperbolic contractions. Exp. Fluids 2007, 43, 437–451. [Google Scholar] [CrossRef]
- Gregoriades, N.; Clay, J.; Ma, N.; Koelling, K.; Chalmers, J.J. Cell damage of microcarrier cultures as a function of local energy dissipation created by a rapid extensional flow. Biotechnol. Bioeng. 2000, 69, 171–182. [Google Scholar] [CrossRef]
- Urbanska, M.; Muñoz, H.E.; Shaw Bagnall, J.; Otto, O.; Manalis, S.R.; Di Carlo, D.; Guck, J. A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat. Methods 2020, 17, 587–593. [Google Scholar] [CrossRef]
- Taylor, G.I. The formation of emulsions in definable fields of flow. Proc. Math. Phys. Eng. 1934, 146, 0501–0523. [Google Scholar] [CrossRef] [Green Version]
- Higdon, J.J.L. The kinematics of the four-roll mill. Phys. Fluids A Fluid Dyn. 1993, 5, 274–276. [Google Scholar] [CrossRef]
- Lagnado, R.R.; Leal, L.G. Visualization of three-dimensional flow in a four-roll mill. Exp. Fluids 1990, 9, 25–32. [Google Scholar] [CrossRef]
- Andreotti, B.; Douady, S.; Couder, Y. An experiment on two aspects of the interaction between strain and vorticity. J. Fluid Mech. 2001, 444, 151–174. [Google Scholar] [CrossRef] [Green Version]
- Haward, S.J. Microfluidic extensional rheometry using stagnation point flow. Biomicrofluidics 2016, 10, 043401. [Google Scholar] [CrossRef] [Green Version]
- Dylla-Spears, R.; Townsend, J.E.; Jen-Jacobson, L.; Sohn, L.L.; Muller, S.J. Single-molecule sequence detection via microfluidic planar extensional flow at a stagnation point. Lab Chip 2010, 10, 1543. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M. Kinematic analyses of a cross-slot microchannel applicable to cell deformability measurement under inertial or viscoelastic flow. Korean J. Chem. Eng. 2015, 32, 2406–2411. [Google Scholar] [CrossRef]
- Coventry, K.D.; Mackley, M.R. Cross-slot extensional flow birefringence observations of polymer melts using a multi-pass rheometer. J. Rheol. 2008, 52, 401–415. [Google Scholar] [CrossRef]
- Pathak, J.A.; Hudson, S.D. Rheo-optics of Equilibrium Polymer Solutions: Wormlike Micelles in Elongational Flow in a Microfluidic Cross-Slot. Macromolecules 2006, 39, 8782–8792. [Google Scholar] [CrossRef]
- Poole, R.J.; Alves, M.A.; Oliveira, P.J. Purely Elastic Flow Asymmetries. Phys. Rev. Lett. 2007, 99, 164503. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Kim, Y.; Lee, S.J.; Kim, J.M. Shape Measurement of Ellipsoidal Particles in a Cross-Slot Microchannel Utilizing Viscoelastic Particle Focusing. Anal. Chem. 2017, 89, 8662–8666. [Google Scholar] [CrossRef]
- Cha, S.; Shin, T.; Lee, S.S.; Shim, W.; Lee, G.; Lee, S.J.; Kim, Y.; Kim, J.M. Cell Stretching Measurement Utilizing Viscoelastic Particle Focusing. Anal. Chem. 2012, 84, 10471–10477. [Google Scholar] [CrossRef]
- Lim, C.T.; Dao, M.; Suresh, S.; Sow, C.H.; Chew, K.T. Large deformation of living cells using laser traps. Acta Mater. 2004, 52, 1837–1845. [Google Scholar] [CrossRef]
- Gossett, D.R.; Tse, H.T.K.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.Y.; Clark, A.T.; Di Carlo, D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tse, H.T.K.; Gossett, D.R.; Moon, Y.S.; Masaeli, M.; Sohsman, M.; Ying, Y.; Mislick, K.; Adams, R.P.; Rao, J.Y.; Di Carlo, D. Quantitative Diagnosis of Malignant Pleural Effusions by Single-Cell Mechanophenotyping. Sci. Transl. Med. 2013, 5, 212ra163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, G.M.; Schlappritzi, E.; Straub, P.W.; Reinhart, W.H. Erythrocyte deformability has no influence on the rate of erythrophagocytosis in vitro by autologous human monocytes/macrophages. Br. J. Haematol. 1994, 86, 629–634. [Google Scholar] [CrossRef]
- Meinderts, S.M.; Oldenborg, P.A.; Beuger, B.M.; Klei, T.R.L.; Johansson, J.; Kuijpers, T.W.; Matozaki, T.; Huisman, E.J.; De Haas, M.; van den Berg, T.K.; et al. Human and murine splenic neutrophils are potent phagocytes of IgG-opsonized red blood cells. Blood Adv. 2017, 1, 875–886. [Google Scholar] [CrossRef] [Green Version]
- Mohandas, N.; Clark, M.R.; Jacobs, M.S.; Shohet, S.B. Analysis of Factors Regulating Erythrocyte Deformability. J. Clin. Investig. 1980, 66, 563–573. [Google Scholar] [CrossRef]
- Barber, B.E.; Russell, B.; Grigg, M.J.; Zhang, R.; William, T.; Amir, A.; Lau, Y.L.; Chatfield, M.D.; Dondorp, A.M.; Anstey, N.M.; et al. Reduced red blood cell deformability in Plasmodium knowlesi malaria. Blood Adv. 2018, 2, 433. [Google Scholar] [CrossRef] [Green Version]
- Connes, P.; Renoux, C.; Romana, M.; Abkarian, M.; Joly, P.; Martin, C.; Hardy-Dessources, M.D.; Ballas, S.K. Blood rheological abnormalities in sickle cell anemia. Clin. Hemorheol. Microcirc. 2018, 68, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Huisjes, R.; Makhro, A.; Llaudet-Planas, E.; Hertz, L.; Petkova-Kirova, P.; Verhagen, L.P.; Pignatelli, S.; Rab, M.A.E.; Schiffelers, R.M.; Seiler, E.; et al. Density, heterogeneity and deformability of red cells as markers of clinical severity in hereditary spherocytosis. Haematologica 2020, 105, 338–347. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Undar, A.; Antaki, J.F.; Watach, M.J.; Calhoon, J.H.; Borovetz, H.S. Decrease in red blood cell deformability caused by hypothermia, hemodilution, and mechanical stress: Factors related to cardiopulmonary bypass. Asaio J. 1999, 45, 307–310. [Google Scholar] [CrossRef]
- Velker, J.A.; Mcintire, L.V.; Lynch, E.C. Alteration of Erythrocyte Deformability Due to Shear-Stress as Assessed by Nuclepore Filters. ASAIO J. 1977, 23, 732–735. [Google Scholar] [CrossRef]
- Orear, E.A.; Udden, M.M.; Mcintire, L.V.; Lynch, E.C. Reduced Erythrocyte Deformability Associated with Calcium Accumulation. Biochim. Biophys. Acta 1982, 691, 274–280. [Google Scholar] [CrossRef]
- Musielak, M. Red blood cell-deformability measurement: Review of techniques. Clin. Hemorheol. Microcirc. 2009, 42, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Faghih, M.M.; Sharp, M.K. Characterization of erythrocyte membrane tension for hemolysis prediction in complex flows. Biomech. Model. Mechanobiol. 2018, 17, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.M.; Haest, C.W.M.; Stohr, M.; Kamp, D.; Deuticke, B. Selective Alteration of Erythrocyte Deformability by Sh-Reagents-Evidence for an Involvement of Spectrin in Membrane Shear Elasticity. Biochim. Biophys. Acta 1978, 510, 270–282. [Google Scholar] [CrossRef]
- Renoux, C.; Faivre, M.; Bessaa, A.; Da Costa, L.; Joly, P.; Gauthier, A.; Connes, P. Impact of surface-area-to-volume ratio, internal viscosity and membrane viscoelasticity on red blood cell deformability measured in isotonic condition. Sci. Rep. 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- McGraw, L.A. Blood Cell Deformability in Uniaxial Extensional Flow. Ph.D. Thesis, Carnegie Mellon University, Pittsburgh, PA, USA, 1992. [Google Scholar]
- Evans, E.; Fung, Y.C. Improved measurements of the erythrocyte geometry. Microvasc. Res. 1972, 4, 335–347. [Google Scholar] [CrossRef]
- Linderkamp, O.; Wu, P.Y.; Meiselman, H.J. Geometry of neonatal and adult red blood cells. Pediatr. Res. 1983, 17, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Faghih, M.M.; Sharp, M.K. Modeling and prediction of flow-induced hemolysis: A review. Biomech. Model. Mechanobiol. 2019, 18, 845–881. [Google Scholar] [CrossRef]
- Evans, E.A.; La Celle, P.L. Intrinsic material properties of the erythrocyte membrane indicated by mechanical analysis of deformation. Blood 1975, 45, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.; Behr, M.; Pasquali, M. A tensor-based measure for estimating blood damage. Artif. Organs 2004, 28, 1002–1015. [Google Scholar] [CrossRef]
- Bae, Y.B.; Jang, H.K.; Shin, T.H.; Phukan, G.; Tran, T.T.; Lee, G.; Hwang, W.R.; Kim, J.M. Microfluidic assessment of mechanical cell damage by extensional stress. Lab Chip 2016, 16, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Liang, M. Single-Cell Stretching in Viscoelastic Fluids with Electronically Triggered Imaging for Cellular Mechanical Phenotyping. Anal. Chem. 2021, 93, 4567–4575. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Park, I.; Lim, K.M.; Doh, J.; Cho, S.G.; Chung, A.J. Microfluidic Cell Stretching for Highly Effective Gene Delivery into Hard-to-Transfect Primary Cells. ACS Nano 2020, 14, 15094–15106. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, P.L.; Dorman, F.D.; Steinbach, J.H. Some Mechanical Effects That Influence Hemolysis. ASAIO J. 1965, 11, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Leverett, L.B.; Hellums, J.D.; Alfrey, C.P.; Lynch, E.C. Red blood cell damage by shear stress. Biophys. J. 1972, 12, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Herbertson, L.H.; Olia, S.E.; Daly, A.; Noatch, C.P.; Smith, W.A.; Kameneva, M.V.; Malinauskas, R.A. Multilaboratory Study of Flow-Induced Hemolysis Using the FDA Benchmark Nozzle Model. Artif. Organs 2015, 39, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Keshaviah, P. Hemolysis in the Accelerated Flow Region Ofan Abrupt Contraction. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 1974. [Google Scholar]
- Khoo, D.P.Y.; Cookson, A.N.; Gill, H.S.; Fraser, K.H. Normal fluid stresses are prevalent in rotary ventricular assist devices: A computational fluid dynamics analysis. Int. J. Artif. Organs 2018, 41, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Down, L.A.; Papavassiliou, D.V.; O’Rear, E.A. Significance of Extensional Stresses to Red Blood Cell Lysis in a Shearing Flow. Ann. Biomed. Eng. 2011, 39, 1632–1642. [Google Scholar] [CrossRef]
- Purvis, N.B.; Giorgio, T.D. The Effects of Elongational Stress Exposure on the Activation and Aggregation of Blood-Platelets. Biorheology 1991, 28, 355–367. [Google Scholar] [CrossRef]
- Aloi, L.E.; Cherry, R.S. Cellular response to agitation characterized by energy dissipation at the impeller tip. Chem. Eng. Sci. 1996, 51, 1523–1529. [Google Scholar] [CrossRef]
- Kresta, S. Turbulence in stirred tanks: Anisotropic, approximate, and applied. Can. J. Chem. Eng. 1998, 76, 563–576. [Google Scholar] [CrossRef]
- Kunas, K.T.; Papoutsakis, E.T. Damage Mechanisms of Suspended Animal-Cells in Agitated Bioreactors with and without Bubble Entrainment. Biotechnol. Bioeng. 1990, 36, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A. A Relationship between Reynolds Stresses and Viscous Dissipation-Implications to Red-Cell Damage. Ann. Biomed. Eng. 1995, 23, 21–28. [Google Scholar] [CrossRef]
- Ozturk, M.; O’Rear, E.A.; Papavassiliou, D.V. Hemolysis Related to Turbulent Eddy Size Distributions Using Comparisons of Experiments to Computations. Artif. Organs 2015, 39, E227–E239. [Google Scholar] [CrossRef]
- Ozturk, M.; Papavassiliou, D.V.; O’Rear, E.A. An Approach for Assessing Turbulent Flow Damage to Blood in Medical Devices. J. Biomech. Eng. 2017, 139, 011008. [Google Scholar] [CrossRef]
- Avci, M.; Heck, M.; O’Rear, E.A.; Papavassiliou, D.V. Hemolysis estimation in turbulent flow for the FDA critical path initiative centrifugal blood pump. Biomech. Model. Mechanobiol. 2021, 1–14. [Google Scholar] [CrossRef]
- Chalmers, J.J. Mixing, aeration and cell damage, 30+ years later: What we learned, how it affected the cell culture industry and what we would like to know more about. Curr. Opin. Chem. Eng. 2015, 10, 94–102. [Google Scholar] [CrossRef]
- Alrubeai, M.; Singh, R.P.; Goldman, M.H.; Emery, A.N. Death Mechanisms of Animal-Cells in Conditions of Intensive Agitation. Biotechnol. Bioeng. 1995, 45, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Alrubeai, M.; Thomas, C.R. Estimation of Disruption of Animal-Cells by Turbulent Capillary-Flow. Biotechnol. Bioeng. 1993, 42, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Silva, R.; Chalmers, J.J.; Casnocha, S.A.; Bass, L.A.; Ma, N.N. Physiological Responses of CHO Cells to Repetitive Hydrodynamic Stress. Biotechnol. Bioeng. 2009, 103, 1103–1117. [Google Scholar] [CrossRef]
- Garciabriones, M.A.; Chalmers, J.J. Flow Parameters Associated with Hydrodynamic Cell Injury. Biotechnol. Bioeng. 1994, 44, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Lee, E.G.; Lee, I.S.; Woo, N.S.; Han, S.M.; Kim, Y.J.; Hwang, W.R. Hydrodynamic extensional stress during the bubble bursting process for bioreactor system design. Korea-Aust. Rheol. J. 2016, 28, 315–326. [Google Scholar] [CrossRef]
- Godoy-Silva, R.; Berdugo, C.; Chalmers, J.J. Aeration, Mixing, and Hydrodynamics, Animal Cell Bioreactors. In Encyclopedia of Industrial Biotechnology; Flickinger, M.C., Ed.; Wiley: New York, NY, USA, 2010; pp. 1–27. [Google Scholar] [CrossRef]
- Chisti, Y. Animal-cell damage in sparged bioreactors. Trends Biotechnol. 2000, 18, 420–432. [Google Scholar] [CrossRef]
- Wu, J.Y.; Goosen, M.F.A. Evaluation of the killing volume of gas bubbles in sparged animal cell culture bioreactors. Enzym. Microb Tech. 1995, 17, 1036–1042. [Google Scholar] [CrossRef]
- Garciabriones, M.A.; Brodkey, R.S.; Chalmers, J.J. Computer-Simulations of the Rupture of a Gas Bubble at a Gas-Liquid Interface and Its Implications in Animal-Cell Damage. Chem. Eng. Sci. 1994, 49, 2301–2320. [Google Scholar] [CrossRef]
- Handa, A.; Emery, A.N.; Spier, R.E. On the evaluation of gas-liquid interfacial effects on hybridoma viability in bubble column bioreactors. Dev. Biol. Stand. 1987, 66, 241–253. [Google Scholar]
- Oh, S.K.W.; Nienow, A.W.; Alrubeai, M.; Emery, A.N. Further-Studies of the Culture of Mouse Hybridomas in an Agitated Bioreactor with and without Continuous Sparging. J. Biotechnol. 1992, 22, 245–270. [Google Scholar] [CrossRef]
- Mcqueen, A.; Meilhoc, E.; Bailey, J.E. Flow Effects on the Viability and Lysis of Suspended Mammalian-Cells. Biotechnol. Lett. 1987, 9, 831–836. [Google Scholar] [CrossRef]
- Mcqueen, A.; Bailey, J.E. Influence of Serum Level, Cell-Line, Flow Type and Viscosity on Flow-Induced Lysis of Suspended Mammalian-Cells. Biotechnol. Lett. 1989, 11, 531–536. [Google Scholar] [CrossRef]
- Tanzeglock, T.; Soos, M.; Stephanopoulos, G.; Morbidelli, M. Induction of Mammalian Cell Death by Simple Shear and Extensional Flows. Biotechnol. Bioeng. 2009, 104, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.Q.; Yang, B.W.; Mohabatpour, F.; Betancourt, N.; Sarker, M.D.; Papagerakis, P.; Chen, X.B. Process-induced cell damage: Pneumatic versus screw-driven bioprinting. Biofabrication 2020, 12, 025011. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.Q.; Chen, X.B. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Coccaro, N.; Ferrari, G.; Donsi, F. Understanding the break-up phenomena in an orifice-valve high pressure homogenizer using spherical bacterial cells (Lactococcus lactis) as a model disruption indicator. J. Food Eng. 2018, 236, 60–71. [Google Scholar] [CrossRef]
- Pathanibul, P.; Taylor, T.M.; Davidson, P.M.; Harte, F. Inactivation of Escherichia coli and Listeria innocua in apple and carrot juices using high pressure homogenization and nisin. Int. J. Food Microbiol. 2009, 129, 316–320. [Google Scholar] [CrossRef]
- Ayazi Shamlou, P.; Siddiqi, S.F.; Titchener-Hooker, N.J. A physical model of high-pressure disruption of bakers’ yeast cells. Chem. Eng. Sci. 1995, 50, 1383–1391. [Google Scholar] [CrossRef]
- Panozzo, A.; Manzocco, L.; Calligaris, S.; Bartolomeoli, I.; Maifreni, M.; Lippe, G.; Nicoli, M.C. Effect of high pressure homogenisation on microbial inactivation, protein structure and functionality of egg white. Food Res. Int. 2014, 62, 718–725. [Google Scholar] [CrossRef]
- Briñez, W.J.; Roig-Sagués, A.X.; Herrero, M.M.H.; López, B.G. Inactivation of Staphylococcus spp. strains in whole milk and orange juice using ultra high pressure homogenisation at inlet temperatures of 6 and 20 °C. Food Control. 2007, 18, 1282–1288. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Toniazzo, T.; Boulet, C.; Oner, M.E.; Sablani, S.S.; Tang, J.; Barbosa-Cánovas, G.V. Inactivation of Listeria innocua and Escherichia coli in carrot juice by combining high pressure processing, nisin, and mild thermal treatments. Innov. Food Sci. Emerg. Technol. 2019, 54, 93–102. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Zhou, W.; Yuan, W.; Wang, D. Algal cell lysis by bacteria: A review and comparison to conventional methods. Algal Res. 2020, 46, 101794. [Google Scholar] [CrossRef]


| Method | Pros | Cons | Key Results |
|---|---|---|---|
| Hyperbolic Microfluidic Devices |
|
| |
| Abrupt/Tapered Constriction Microfluidic Devices |
|
| |
| Cross-Flow Microfluidic Devices |
|
| |
| Optical Tweezers |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, K.M.; Papavassiliou, D.V.; O’Rear, E.A. Elongational Stresses and Cells. Cells 2021, 10, 2352. https://doi.org/10.3390/cells10092352
Foster KM, Papavassiliou DV, O’Rear EA. Elongational Stresses and Cells. Cells. 2021; 10(9):2352. https://doi.org/10.3390/cells10092352
Chicago/Turabian StyleFoster, Kylie M., Dimitrios V. Papavassiliou, and Edgar A. O’Rear. 2021. "Elongational Stresses and Cells" Cells 10, no. 9: 2352. https://doi.org/10.3390/cells10092352
APA StyleFoster, K. M., Papavassiliou, D. V., & O’Rear, E. A. (2021). Elongational Stresses and Cells. Cells, 10(9), 2352. https://doi.org/10.3390/cells10092352









