Impact of Microorganisms and Parasites on Neuronally Controlled Drosophila Behaviours
Abstract
:1. Introduction
2. A Brief Resumé of the Drosophila Immune System and its Players
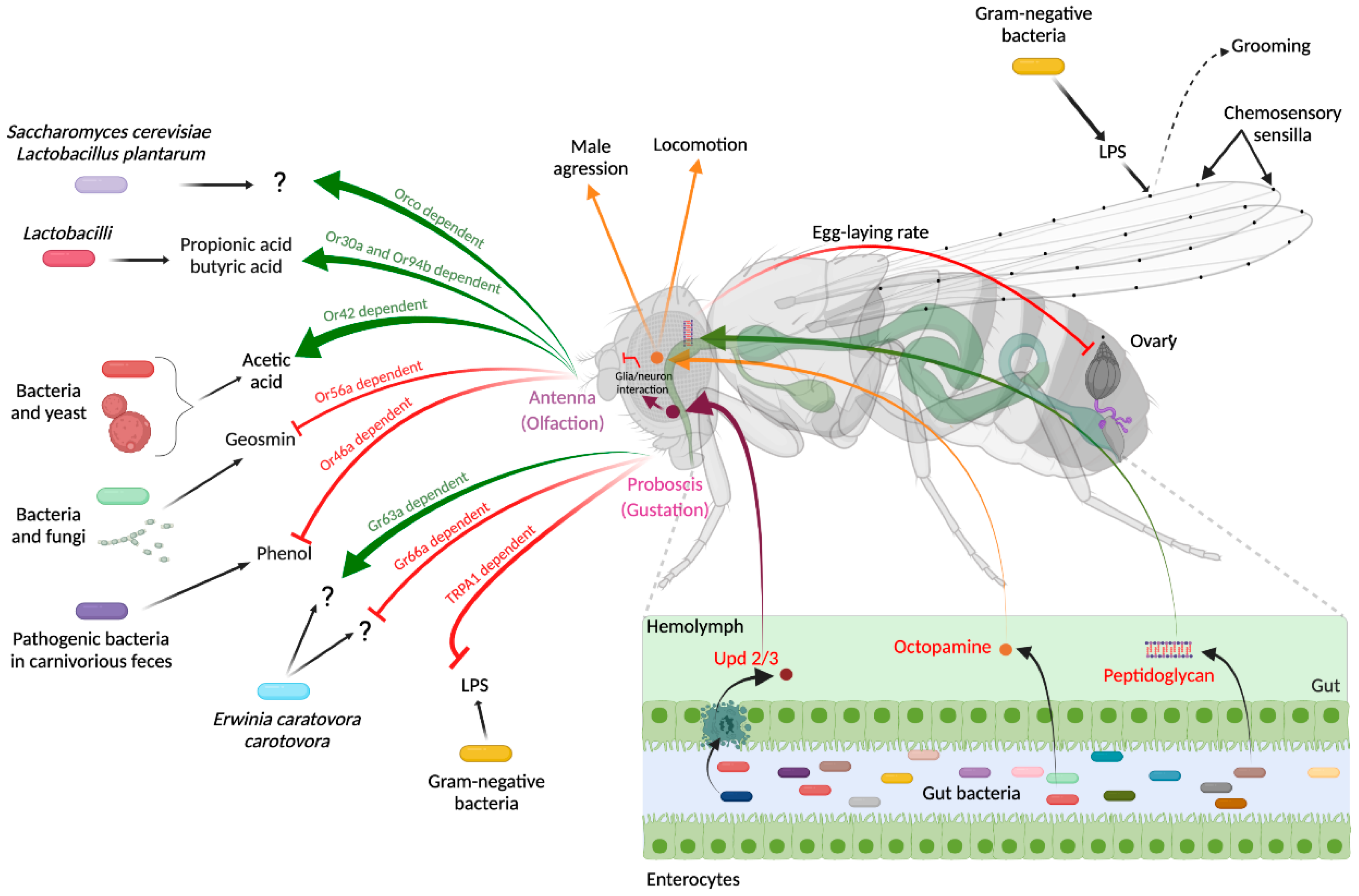
3. How Are Bacteria Sensed by the Nervous System?
4. The Immune PGRP/NF-κB Module Plays an Important Role in Bacteria–Neuron Interactions
5. The NF-κB Pathway and Its Transcriptional Targets, the AMP, Regulate Other Behaviors in Flies
6. Octopamine, a Neuromodulator Implicated in Many Bacteria Drosophila Nervous System Interactions
7. When Blood Cells and Neurons Communicate to Respond to External Cues
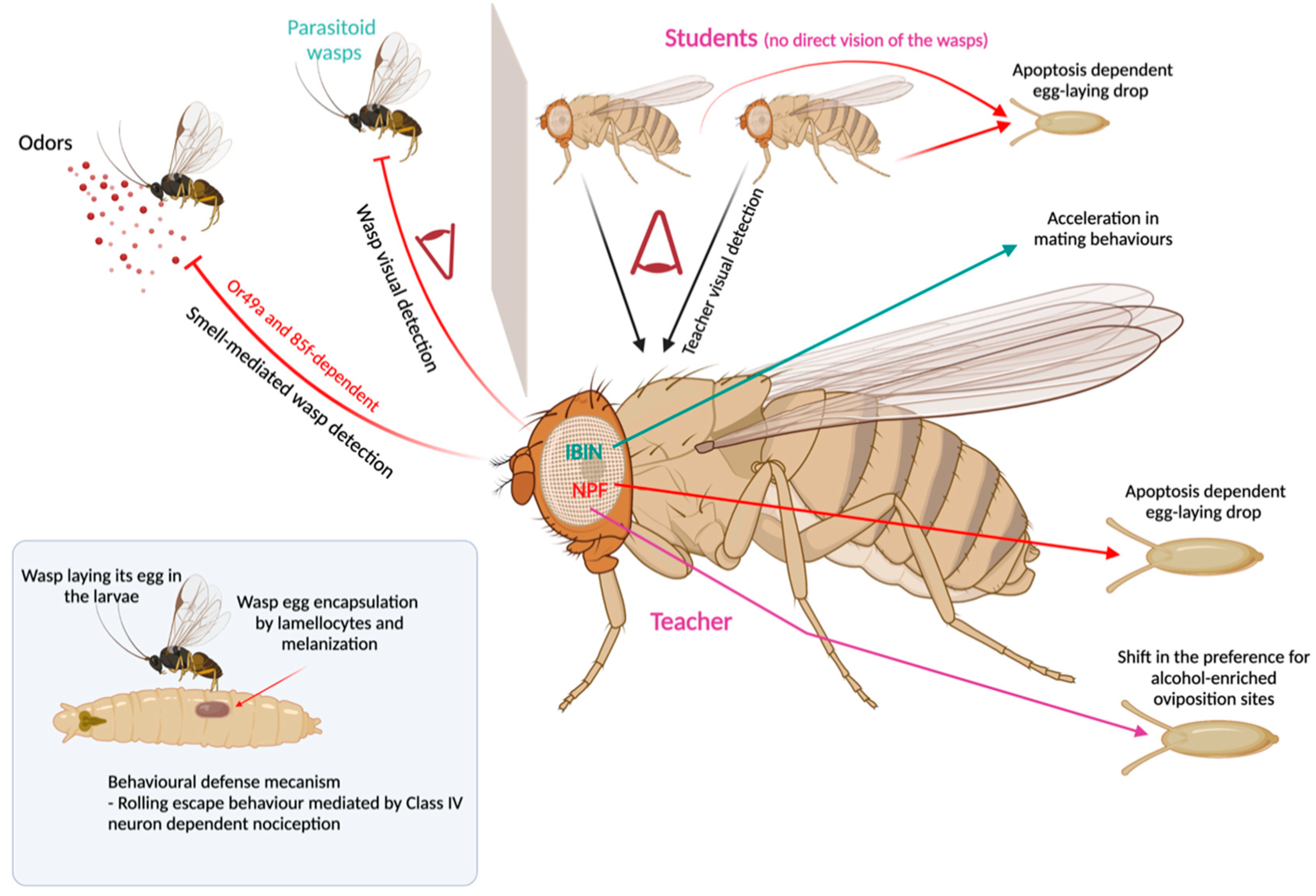
8. Behavioral Immunity toward Parasitoid Wasps
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota–host interactions in insects. J. Exp. Biol. 2021, 224 Pt 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, K.A.; Lee, W.J. Drosophila as a model system for deciphering the ’host physiology-nutrition-microbiome’ axis. Curr. Opin. Insect Sci. 2020, 41, 112–119. [Google Scholar] [CrossRef]
- Grenier, T.; Leulier, F. How commensal microbes shape the physiology of Drosophila melanogaster. Curr. Opin. Insect Sci. 2020, 41, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Liberti, J.; Engel, P. The gut microbiota—Brain axis of insects. Curr. Opin. Insect Sci. 2020, 39, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Foley, E. Microbial recognition regulates intestinal epithelial growth in homeostasis and disease. FEBS J. 2021, 1–26. [Google Scholar] [CrossRef]
- Rosendo Machado, S.; Van der Most, T.; Miesen, P. Genetic determinants of antiviral immunity in dipteran insects—Compiling the experi-mental evidence. Dev. Comp. Immunol. 2021, 119, 104010. [Google Scholar] [CrossRef] [PubMed]
- Lye, S.H.; Chtarbanova, S. Drosophila as a Model to Study Brain Innate Immunity in Health and Disease. Int. J. Mol. Sci. 2018, 19, 3922. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.; Huang, X.; Yin, Y. Beyond immunity: The Imd pathway as a coordinator of host defense, organismal physiology and be-havior. Dev. Comp. Immunol. 2018, 83, 51–59. [Google Scholar] [CrossRef]
- Kraus, A.; Buckley, K.M.; Salinas, I. Sensing the world and its dangers: An evolutionary perspective in neuroimmunology. eLife 2021, 10. [Google Scholar] [CrossRef]
- Shakhar, K. The Inclusive Behavioral Immune System. Front. Psychol. 2019, 10, 1004. [Google Scholar] [CrossRef]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the immune system: A focus on cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster—From microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef]
- Ferrandon, D.; Imler, J.-L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Royet, J.; Charroux, B. Mechanisms and consequence of bacteria detection by the Drosophila gut epithelium. Gut Microbes 2013, 4, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kleino, A.; Silverman, N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2014, 42, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Michel, T.; Reichhart, J.-M.; Hoffmann, J.A.; Royet, J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nat. Cell Biol. 2001, 414, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Yano, T.; Aggarwal, K.; Lim, J.-H.; Ueda, K.; Oshima, Y.; Peach, C.; Erturk-Hasdemir, D.; Goldman, W.; Oh, B.-H.; et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 2006, 7, 715–723. [Google Scholar] [CrossRef]
- Bosco-Drayon, V.; Poidevin, M.; Boneca, I.G.; Narbonne-Reveau, K.; Royet, J.; Charroux, B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune re-sponses to infectious bacteria and tolerance to microbiota. Cell Host Microbe 2012, 12, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Gottar, M.; Gobert, V.; Michel, T.; Belvin, M.; Duyk, G.; Hoffmann, J.A.; Ferrandon, D.; Royet, J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nat. Cell Biol. 2002, 416, 640–644. [Google Scholar] [CrossRef]
- Schneider, J.; Imler, J.-L. Sensing and signalling viral infection in drosophila. Dev. Comp. Immunol. 2021, 117, 103985. [Google Scholar] [CrossRef]
- Parsons, B.; Foley, E. Cellular immune defenses of Drosophila melanogaster. Dev. Comp. Immunol. 2016, 58, 95–101. [Google Scholar] [CrossRef]
- Gold, K.; Brückner, K. Macrophages and cellular immunity in Drosophila melanogaster. Semin. Immunol. 2015, 27, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansourian, S.; Stensmyr, M.C. The chemical ecology of the fly. Curr. Opin. Neurobiol. 2015, 34, 95–102. [Google Scholar] [CrossRef]
- Venu, I.; Durisko, Z.; Xu, J.; Dukas, R. Social attraction mediated by fruit flies’ microbiome. J. Exp. Biol. 2014, 217, 1346–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, H.; Keesey, I.W.; Hansson, B.S.; Knaden, M. Gut microbiota affects development and olfactory behavior in Drosophila melanogaster. J. Exp. Biol. 2019, 222, jeb192500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keita, S.; Masuzzo, A.; Royet, J.; Kurz, C.L. Drosophila larvae food intake cessation following exposure to Erwinia contaminated media requires odor perception, Trpa1 channel and evf virulence factor. J. Insect Physiol. 2017, 99, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Depetris-Chauvin, A. Galagovsky, D.; Chevalier, C.; Maniere, G.; Grosjean, Y. Olfactory detection of a bacterial short-chain fatty acid acts as an orexigenic signal in Dro-sophila melanogaster larvae. Sci. Rep. 2017, 7, 14230. [Google Scholar] [CrossRef] [PubMed]
- Stensmyr, M.C.; Dweck, H.K.; Farhan, A.; Ibba, I.; Strutz, A.; Mukunda, L.; Linz, J.; Grabe, V.; Steck, K.; Lavista-Llanos, S.; et al. A Conserved Dedicated Olfactory Circuit for Detecting Harmful Microbes in Drosophila. Cell 2012, 151, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Mansourian, S.; Corcoran, J.; Enjin, A.; Löfstedt, C.; Dacke, M.; Stensmyr, M.C. Fecal-Derived Phenol Induces Egg-Laying Aversion in Drosophila. Curr. Biol. 2016, 26, 2762–2769. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.N.; Trautman, E.P.; Crawford, J.M.; Stabb, E.V.; Handelsman, J.; Broderick, N. Metabolite exchange between microbiome members produces compounds that influence Drosophila behavior. eLife 2017, 6, e18855. [Google Scholar] [CrossRef]
- Wong, A.C.N.; Wang, Q.P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut Microbiota Modifies Olfactory-Guided Microbial Preferences and Foraging Decisions in Dro-sophila. Curr. Biol. 2017, 27, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Soldano, A.; Alpizar, Y.; Boonen, B.; Franco, L.; López-Requena, A.; Liu, G.; Mora, N.; Yaksi, E.; Voets, T.; Vennekens, R.; et al. Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila. eLife 2016, 5, e13133. [Google Scholar] [CrossRef]
- Yanagawa, A.; Couto, A.; Sandoz, J.-C.; Hata, T.; Mitra, A.; Agha, M.A.; Marion-Poll, F. LPS perception through taste-induced reflex in Drosophila melanogaster. J. Insect Physiol. 2019, 112, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Charroux, B.; Daian, F.; Royet, J. Drosophila Aversive Behavior toward Erwinia carotovora carotovora Is Mediated by Bitter Neu-rons and Leukokinin. iScience 2020, 23, 101152. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, G.; Foldi, I.; Aurikko, J.; Wentzell, J.S.; Lim, M.A.; Fenton, J.C.; Gay, N.J.; Hidalgo, A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 2013, 16, 1248–1256. [Google Scholar] [CrossRef] [Green Version]
- Shmueli, A.; Shalit, T.; Okun, E.; Shohat-Ophir, G. The Toll Pathway in the Central Nervous System of Flies and Mammals. Neuro Mol. Med. 2018, 20, 419–436. [Google Scholar] [CrossRef]
- Cantera, R.; Roos, E.; Engström, Y. Dif and cactus are colocalized in the larval nervous system of Drosophila melanogaster. J. Neurobiol. 1999, 38, 16–26. [Google Scholar] [CrossRef]
- Kurz, C.L.; Charroux, B.; Chaduli, D.; Viallat-Lieutaud, A.; Royet, J. Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. eLife 2017, 6, e21937. [Google Scholar] [CrossRef]
- Masuzzo, A.; Manière, G.; Viallat-Lieutaud, A.; Avazeri, É.; Zugasti, O.; Grosjean, Y.; Kurz, L.; Royet, J. Peptidoglycan-dependent NF-kappaB activation in a small subset of brain octopaminergic neurons controls female oviposition. eLife 2019, 8, e50559. [Google Scholar] [CrossRef]
- Harris, N.; Braiser, D.J.; Dickman, D.K.; Fetter, R.D.; Tong, A.; Davis, G.W. The Innate Immune Receptor PGRP-LC Controls Presynaptic Homeostatic Plasticity. Neuron 2015, 88, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Harris, N.; Fetter, R.D.; Brasier, D.J.; Tong, A.; Davis, G.W. Molecular Interface of Neuronal Innate Immunity, Synaptic Vesicle Stabilization, and Presynaptic Homeostatic Plasticity. Neuron 2018, 100, 1163–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobler, J.M.; Jimenez, F.J.R.; Petcu, I.; Kadow, I.C.G. Immune Receptor Signaling and the Mushroom Body Mediate Post-ingestion Pathogen Avoidance. Curr. Biol. 2020, 30, 4693–4709. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Li, H.; Borch Jensen, M.; Maksoud, E.; Borneo, J.; Liang, Y.; Quake, S.R.; Luo, L.; Haghigh, P.; Jasper, H. Gut cytokines modulate olfaction through metabolic reprogramming of glia. Nature 2021, 596, 97–102. [Google Scholar] [CrossRef]
- Williams, J.A.; Sathyanarayanan, S.; Hendricks, J.C.; Sehgal, A. Interaction between sleep and the immune response in Drosophila: A role for the NFkappaB relish. Sleep 2007, 30, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.H.; Pike, D.H.; Beizaeipour, Z.; Williams, J.A. Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NFkappaB Relish. BMC Neurosci. 2010, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Toda, H.; Williams, J.A.; Gulledge, M.; Sehgal, A. A sleep-inducing gene, nemuri, links sleep and immune function in Drosophila. Science 2019, 363, 509–515. [Google Scholar] [CrossRef]
- Dissel, S.; Seugnet, L.; Thimgan, M.S.; Silverman, N.; Angadi, V.; Thacher, P.V.; Burnham, M.; Shaw, P.J. Differential activation of immune factors in neurons and glia contribute to individual differences in resilience/vulnerability to sleep disruption. Brain Behav. Immun. 2015, 47, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Terry, E.E.; Fejer, E.; Gamba, D.; Hartmann, N.; Logsdon, J.; Michalski, D.; Rois, L.E.; Scuderi, M.J.; Kunst, M.; et al. Achilles is a circadian clock-controlled gene that regulates immune function in Drosophila. Brain Behav. Immun. 2017, 61, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Azpeleta, R.B.; Wu, J.; Gill, J.; Welte, R.; Seidel, C.; McKinney, S.; Dissel, S.; Si, K. Antimicrobial peptides modulate long-term memory. PLoS Genet. 2018, 14, e1007440. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M.A.; Lemaitre, B. New insights on Drosophila antimicrobial peptide function in host defense and be-yond. Curr. Opin. Immunol. 2020, 62, 22–30. [Google Scholar] [CrossRef]
- Cao, Y.; Chtarbanova, S.; Petersen, A.J.; Ganetzky, B. Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain. Proc. Natl. Acad. Sci. USA 2013, 110, E1752–E1760. [Google Scholar] [CrossRef] [Green Version]
- Kounatidis, I.; Chtarbanova, S. Role of Glial Immunity in Lifespan Determination: A Drosophila Perspective. Front. Immunol. 2018, 9, 1362. [Google Scholar] [CrossRef]
- Kounatidis, I.; Chtarbanova, S.; Cao, Y.; Hayne, M.; Jayanth, D.; Ganetzky, B.; Ligoxygakis, P. NF-kappaB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep. 2017, 19, 836–848. [Google Scholar] [CrossRef]
- Roeder, T. The control of metabolic traits by octopamine and tyramine in invertebrates. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef]
- Rohrscheib, C.E.; Bondy, E.; Josh, P.; Riegler, M.; Eyles, D.; van Swinderen, B.; Weible, M.W.; Brownlie, J.C. Wolbachia Influences the Production of Octopamine and Affects Drosophila Male Aggression. Appl. Environ. Microbiol. 2015, 81, 4573–4580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schretter, C.E.; Vielmetter, J.; Bartos, I.; Marka, Z.; Marka, S.; Argade, S.; Mazmanian, S.K. A gut microbial factor modulates locomotor behaviour in Drosophila. Nat. Cell Biol. 2018, 563, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Selkrig, J.; Farhan, M.; Ng, S.H.; Chua, J.Y.; Tumkaya, T.; Ho, J.; Chiang, Y.N.; Rieger, D.; Pettersson, S.; Helfrich-Förster, C.; et al. The Drosophila microbiome has a limited influence on sleep, activity, and courtship behaviors. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Luan, X.; Liu, Q.; Wang, J.; Chang, X.; Snijders, A.M.; Mao, J.-H.; Secombe, J.; Dan, Z.; Chen, J.-H.; et al. Drosophila Histone Demethylase KDM5 Regulates Social Behavior through Immune Control and Gut Microbiota Maintenance. Cell Host Microbe 2019, 25, 537–552. [Google Scholar] [CrossRef] [Green Version]
- Letourneau, M.; Lapraz, F.; Sharma, A.; Vanzo, N.; Waltzer, L.; Crozatier, M. Drosophila hematopoiesis under normal conditions and in response to immune stress. FEBS Lett. 2016, 590, 4034–4051. [Google Scholar] [CrossRef] [PubMed]
- Mase, A.; Augsburger, J.; Brückner, K. Macrophages and Their Organ Locations Shape Each Other in Development and Homeostasis—A Drosophila Perspective. Front. Cell Dev. Biol. 2021, 9, 630272. [Google Scholar] [CrossRef]
- Csordás, G.; Gábor, E.; Honti, V. There and back again: The mechanisms of differentiation and transdifferentiation in Drosophila blood cells. Dev. Biol. 2021, 469, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Makhijani, K.; Alexander, B.; Rao, D.; Petraki, S.; Herboso, L.; Kukar, K.; Batool, I.; Wachner, S.; Gold, K.S.; Wong, C.; et al. Regulation of Drosophila hematopoietic sites by Activin-beta from active sensory neurons. Nat. Commun. 2017, 8, 15990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.; Mukherjee, T.; Mondal, B.C.; Liu, T.; Young, G.C.; Wijewarnasuriya, D.P.; Banerjee, U. Olfactory Control of Blood Progenitor Maintenance. Cell 2013, 155, 1141–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, B.; Spratford, C.M.; Yoon, S.; Cha, N.; Banerjee, U.; Shim, J. Systemic control of immune cell development by integrated carbon dioxide and hypoxia chemosensation in Drosophila. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Fleury, F.; Ris, N.; Allemand, R.; Fouillet, P.; Carton, Y.; Boulétreau, M. Ecological and genetic interactions in Drosophila-parasitoids communities: A case study with D. mela-nogaster, D. simulans and their common Leptopilina parasitoids in south-eastern France. Genetica 2004, 120, 181–194. [Google Scholar] [CrossRef]
- Russo, J.; Dupas, S.; Frey, F.; Carton, Y.; Brehelin, M. Insect immunity: Early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitol. 1996, 112, 135–142. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reac-tions. Pigment Cell Res. 1993, 6, 117–126. [Google Scholar] [CrossRef]
- Robertson, J.L.; Tsubouchi, A.; Tracey, W.D. Larval Defense against Attack from Parasitoid Wasps Requires Nociceptive Neurons. PLoS ONE 2013, 8, e78704. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.A.M.; Dweck, H.K.M.; Stökl, J.; Hofferberth, J.E.; Trona, F.; Weniger, K.; Rybak, J.; Seki, Y.; Stensmyr, M.; Sachse, S.; et al. Drosophila Avoids Parasitoids by Sensing Their Semiochemicals via a Dedicated Olfactory Circuit. PLoS Biol. 2015, 13, e1002318. [Google Scholar] [CrossRef] [Green Version]
- Kacsoh, B.Z.; Bozler, J.; Ramaswami, M.; Bosco, G. Social communication of predator-induced changes in Drosophila behavior and germ line physiol-ogy. eLife 2015, 4, e07423. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; De Roode, J.C.; Kacsoh, B.Z.; Schlenke, T. Defence strategies against a parasitoid wasp in Drosophila: Fight or flight? Biol. Lett. 2011, 8, 230–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kacsoh, B.Z.; Lynch, Z.R.; Mortimer, N.T.; Schlenke, T.A. Data from: Fruit flies medicate offspring after seeing parasites. Science 2013, 339, 947–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadanandappa, M.K.; Sathyanarayana, S.H.; Kondo, S.; Bosco, G. Neuropeptide F signaling regulates parasitoid-specific germline development and egg-laying in Drosophila. PLoS Genet. 2021, 17, e1009456. [Google Scholar] [CrossRef]
- Milan, N.F.; Kacsoh, B.; Schlenke, T.A. Alcohol Consumption as Self-Medication against Blood-Borne Parasites in the Fruit Fly. Curr. Biol. 2012, 22, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Lynch, Z.R.; Schlenke, T.; Morran, L.T.; De Roode, J.C. Ethanol confers differential protection against generalist and specialist parasitoids of Drosophila melanogaster. PLoS ONE 2017, 12, e0180182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozler, J.; Kacsoh, B.Z.; Bosco, G. Transgeneratonal inheritance of ethanol preference is caused by maternal NPF repression. eLife 2019, 8, e45391. [Google Scholar] [CrossRef]
- Bozler, J.; Kacsoh, B.Z.; Bosco, G. Maternal Priming of Offspring Immune System in Drosophila. G3 Genes Genomes Genet. 2020, 10, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Ebrahim, S.A.M.; Talross, G.J.S.; Carlson, J.R. Sight of parasitoid wasps accelerates sexual behavior and upregulates a micropeptide gene in Drosophila. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Kacsoh, B.; Bozler, J.; Hodge, S.; Ramaswami, M.; Bosco, G. A Novel Paradigm for Nonassociative Long-Term Memory in Drosophila: Predator-Induced Changes in Oviposition Behavior. Genetics 2015, 199, 1143–1157. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montanari, M.; Royet, J. Impact of Microorganisms and Parasites on Neuronally Controlled Drosophila Behaviours. Cells 2021, 10, 2350. https://doi.org/10.3390/cells10092350
Montanari M, Royet J. Impact of Microorganisms and Parasites on Neuronally Controlled Drosophila Behaviours. Cells. 2021; 10(9):2350. https://doi.org/10.3390/cells10092350
Chicago/Turabian StyleMontanari, Martina, and Julien Royet. 2021. "Impact of Microorganisms and Parasites on Neuronally Controlled Drosophila Behaviours" Cells 10, no. 9: 2350. https://doi.org/10.3390/cells10092350
APA StyleMontanari, M., & Royet, J. (2021). Impact of Microorganisms and Parasites on Neuronally Controlled Drosophila Behaviours. Cells, 10(9), 2350. https://doi.org/10.3390/cells10092350





