Effects of Obesogenic Feeding and Free Fatty Acids on Circadian Secretion of Metabolic Hormones: Implications for the Development of Type 2 Diabetes
Abstract
:1. Introduction
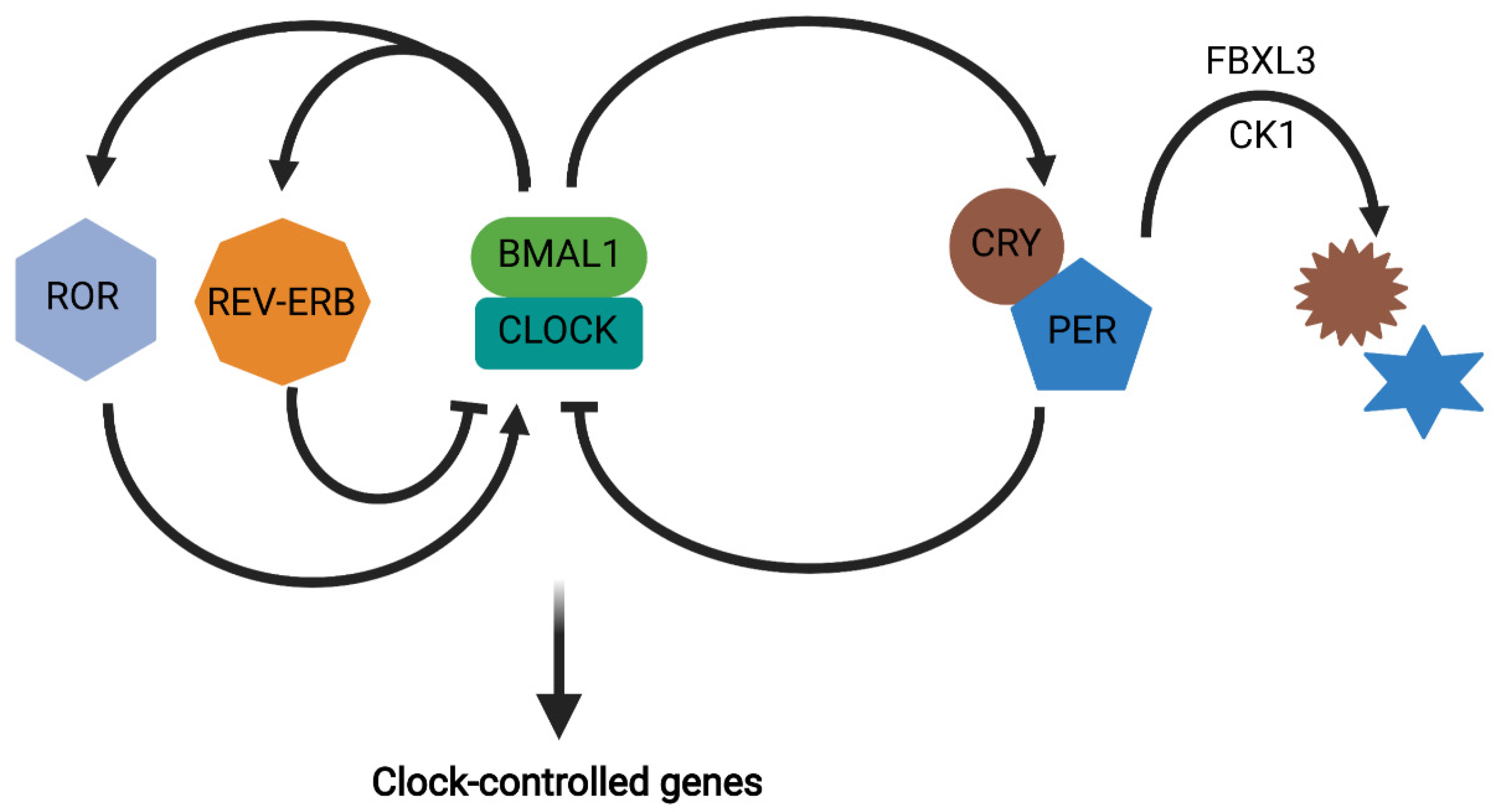
2. Circadian Rhythms
3. Circadian Regulation of Metabolic Function
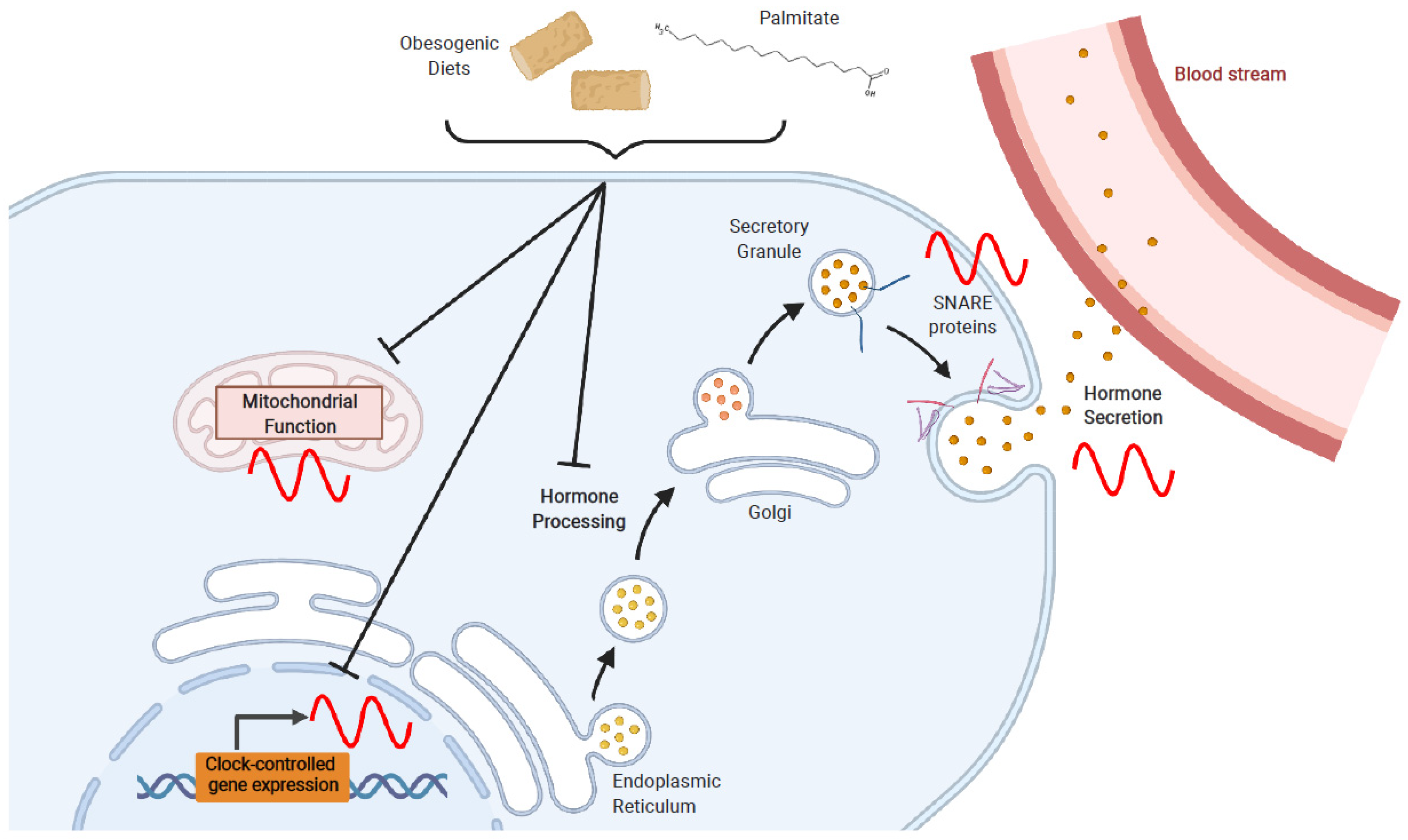
4. Effects of Obesogenic Feeding and Free Fatty Acid Exposure on Circadian Secretion of L Cell and β Cell Hormones
4.1. Obesogenic Diets
4.2. Fatty Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasuga, M. Insulin resistance and pancreatic beta cell failure. J. Clin. Investig. 2006, 116, 1756–1760. [Google Scholar] [CrossRef]
- Sun, X.; Li, P.; Yang, X.; Li, W.; Qiu, X.; Zhu, S. From genetics and epigenetics to the future of precision treatment for obesity. Gastroenterol. Rep. 2017, 5, 266–270. [Google Scholar] [CrossRef] [Green Version]
- Gerhart-Hines, Z.; Lazar, M.A. Circadian metabolism in the light of evolution. Endocr. Rev. 2015, 36, 289–304. [Google Scholar] [CrossRef]
- Sookoian, S.; Gemma, C.; Gianotti, T.F.; Burgueño, A.; Castaño, G.; Pirola, C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008, 87, 1606–1615. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Lee, Y.C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.Q.; Ordovas, J.M. Clock genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Lee, Y.C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.Q.; Ordovas, J.M. Genetic variants in human clock associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population). Eur. J. Hum. Genet. 2010, 18, 364–369. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Sánchez-Moreno, C.; Smith, C.E.; Lee, Y.C.; Nicolás, F.; Ordovas, J.M. Ghrelin, sleep reduction and evening preference: Relationships to clock 3111 T/C SNP and weight loss. PLoS ONE 2011, 6, e17435. [Google Scholar] [CrossRef] [Green Version]
- Woon, P.Y.; Kaisaki, P.J.; Bragança, J.; Bihoreau, M.T.; Levy, J.C.; Farrall, M.; Gauguier, D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 14412–14417. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, J.; Langenberg, C.; Prokopenko, I.; Saxena, R.; Soranzo, N.; Jackson, A.U.; Wheeler, E.; Glazer, N.L.; Bouatia-Naji, N.; Gloyn, A.L.; et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010, 42, 105–116. [Google Scholar] [CrossRef]
- Englund, A.; Kovanen, L.; Saarikoski, S.T.; Haukka, J.; Reunanen, A.; Aromaa, A.; Lönnqvist, J.; Partonen, T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J. Circadian Rhythm. 2009, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garaulet, M.; Corbalán-Tutau, M.D.; Madrid, J.A.; Baraza, J.C.; Parnell, L.D.; Lee, Y.C.; Ordovas, J.M. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J. Am. Diet. Assoc. 2010, 110, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Nieto, F.J. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch. Intern. Med. 2005, 165, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Donga, E.; van Dijk, M.; van Dijk, J.G.; Biermasz, N.R.; Lammers, G.J.; van Kralingen, K.W.; Corssmit, E.P.; Romijn, J.A. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J. Clin. Endocrinol. Metab. 2010, 95, 2963–2968. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef] [Green Version]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudic, R.D.; McNamara, P.; Curtis, A.M.; Boston, R.C.; Panda, S.; Hogenesch, J.B.; Fitzgerald, G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004, 2, e377. [Google Scholar] [CrossRef] [Green Version]
- Marcheva, B.; Ramsey, K.M.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Lamia, K.A.; Storch, K.F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamia, K.A.; Papp, S.J.; Yu, R.T.; Barish, G.D.; Uhlenhaut, N.H.; Jonker, J.W.; Downes, M.; Evans, R.M. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 2011, 480, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; Nusinow, D.A.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Yeh, B.; Rakshit, K.; Colwell, C.S.; Matveyenko, A.V. Circadian disruption and diet-induced obesity synergize to promote development of β-cell failure and diabetes in male rats. Endocrinology 2015, 156, 4426–4436. [Google Scholar] [CrossRef] [Green Version]
- Clemenzi, M.N.; Martchenko, A.; Loganathan, N.; Tse, E.K.; Brubaker, P.L.; Belsham, D.D. Analysis of Western diet, palmitate and BMAL1 regulation of neuropeptide Y expression in the murine hypothalamus and BMAL1 knockout cell models. Mol. Cell. Endocrinol. 2020, 507, 110773. [Google Scholar] [CrossRef]
- Thomas, A.P.; Hoang, J.; Vongbunyong, K.; Nguyen, A.; Rakshit, K.; Matveyenko, A.V. Administration of melatonin and metformin prevents deleterious effects of circadian disruption and obesity in male rats. Endocrinology 2016, 157, 4720–4731. [Google Scholar] [CrossRef] [Green Version]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [Green Version]
- Gil-Lozano, M.; Wu, W.K.; Martchenko, A.; Brubaker, P.L. High-fat diet and palmitate alter the rhythmic secretion of glucagon-like peptide-1 by the rodent L-cell. Endocrinology 2016, 157, 586–599. [Google Scholar] [CrossRef] [Green Version]
- Martchenko, A.; Oh, R.H.; Wheeler, S.E.; Gurges, P.; Chalmers, J.A.; Brubaker, P.L. Suppression of circadian secretion of glucagon-like peptide-1 by the saturated fatty acid, palmitate. Acta Physiol. 2017, 222, e13007. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, S.E.; Martchenko, A.; Cox, B.J.; Naismith, K.; Waller, A.; Gurges, P.; Sweeney, M.E.; Philpott, D.J.; Brubaker, P.L. Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes 2020, 69, 2589–2602. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, D.; Arthurs, B.; Li, P.; Durudogan, L.; Gupta, N.; Yin, L. Palmitate inhibits SIRT1-dependent BMAL1/CLOCK interaction and disrupts circadian gene oscillations in hepatocytes. PLoS ONE 2015, 10, e0130047. [Google Scholar]
- Tal, Y.; Chapnik, N.; Froy, O. Non-obesogenic doses of fatty acids modulate the functionality of the circadian clock in the liver. Cell. Mol. Life Sci. 2019, 76, 1795–1806. [Google Scholar] [CrossRef]
- Tal, Y.; Chapnik, N.; Froy, O. Non-obesogenic doses of palmitate disrupt circadian metabolism in adipocytes. Adipocyte 2019, 8, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Bass, J.; Lazar, M.A. Circadian time signatures of fitness and disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Ann. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [Green Version]
- Maury, E.; Hong, H.K.; Bass, J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. 2014, 40, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals RORα as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lahens, N.F.; Balance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [Green Version]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Ann. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [Green Version]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, K.; Nishio, T.; Nakagawa, H.; Nakamura, S.; Fukuda, Y. Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res. 1978, 142, 384–389. [Google Scholar] [CrossRef]
- Stephan, F.K.; Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 1972, 69, 1583–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tousson, E.; Meissl, H. Suprachiasmatic nuclei grafts restore the circadian rhythm in the paraventricular nucleus of the hypothalamus. J. Neurosci. 2004, 24, 2983–2988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistlberger, R.E. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994, 18, 171–195. [Google Scholar] [CrossRef]
- Koike, N.; Yoo, S.H.; Huang, H.C.; Kumar, V.; Lee, C.; Kim, T.K.; Takahashi, J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Hoogerwerf, W.A.; Hellmich, H.L.; Cornélissen, G.; Halberg, F.; Shahinian, V.B.; Bostwick, J.; Savidge, T.C.; Cassone, V.M. Clock gene expression in the murine gastrointestinal tract: Endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 2007, 133, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Rakshit, K.; Qian, J.; Ernst, J.; Matveyenko, A.V. Circadian variation of the pancreatic islet transcriptome. Physiol. Genom. 2016, 48, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, V.; Saini, C.; Giovannoni, L.; Gobet, C.; Sage, D.; Unser, M.; Heddad Masson, M.; Gu, G.; Bosco, D.; Gachon, F.; et al. Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev. 2017, 31, 383–398. [Google Scholar] [CrossRef]
- Rey, G.; Cesbron, F.; Rougemont, J.; Reinke, H.; Brunner, M.; Naef, F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011, 9, e1000595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zvonic, S.; Ptitsyn, A.A.; Conrad, S.A.; Scott, L.K.; Floyd, Z.E.; Kilroy, G.; Wu, X.; Goh, B.C.; Mynatt, R.L.; Gimble, J.M. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 2006, 55, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, J.J.; Andrews, J.L.; McDearmon, E.L.; Campbell, K.S.; Barber, B.K.; Miller, B.H.; Walker, J.R.; Hogenesch, J.B.; Takahashi, J.S.; Esser, K.A. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol. Genom. 2007, 31, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gachon, F.; Loizides-Mangold, U.; Petrenko, V.; Dibner, C. Glucose homeostasis: Regulation by peripheral circadian clocks in rodents and humans. Endocrinology 2017, 158, 1074–1084. [Google Scholar] [CrossRef]
- Martchenko, A.; Martchenko, S.E.; Biancolin, A.D.; Brubaker, P.L. Circadian rhythms and the gastrointestinal tract: Relationship to metabolism and gut hormones. Endocrinology 2020, 161, bqaa167. [Google Scholar] [CrossRef]
- Eissele, R.; Göke, R.; Willemer, S.; Harthus, H.P.; Vermeer, H.; Arnold, R.; Göke, B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Investig. 1992, 22, 283–291. [Google Scholar] [CrossRef]
- Dong, C.X.; Brubaker, P.L. Ghrelin, the proglucagon-derived peptides and peptide YY in nutrient homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 705–715. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [Green Version]
- Brubaker, P.L. Glucagon-like peptide-2 and the regulation of intestinal growth and function. Compr. Physiol. 2018, 8, 1185–1210. [Google Scholar]
- Lindgren, O.; Mari, A.; Deacon, C.F.; Carr, R.D.; Winzell, M.S.; Vikman, J.; Ahren, B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J. Clin. Endocrinol. Metab. 2009, 94, 2887–2892. [Google Scholar] [CrossRef] [Green Version]
- Gil-Lozano, M.; Hunter, P.M.; Behan, L.-A.; Gladanac, B.; Casper, R.F.; Brubaker, P.L. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am. J. Physiol. Endocrinol. Metab. 2016, 310, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Santiago, J.; Muñoz, G.; Jiménez Rodríguez, D.; José, J.; Morante, H. Diurnal rhythms of plasma GLP-1 levels in normal and overweight/obese subjects: Lack of effect of weight loss. J. Physiol. Biochem. 2015, 71, 17–28. [Google Scholar]
- Mingrone, G.; Nolfe, G.; Gissey, G.C.; Iaconelli, A.; Leccesi, L.; Guidone, C.; Nanni, G.; Holst, J.J. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia 2009, 52, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Gonnissen, H.K.; Rutters, F.; Mazuy, C.; Martens, E.A.; Adam, T.C.; Westerterp-Plantenga, M.S. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am. J. Clin. Nutr. 2012, 96, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Gil-Lozano, M.; Mingomataj, E.L.; Wu, W.K.; Ridout, S.A.; Brubaker, P.L. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 2014, 63, 3674–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancolin, A.D.; Martchenko, A.; Mitova, E.; Gurges, P.; Michalchyshyn, E.; Chalmers, J.A.; Doria, A.; Mychaleckyj, J.C.; Adriaenssens, A.E.; Reimann, F.; et al. The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol. Metab. 2020, 31, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Strubbe, J.H. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol. Behav. 1998, 63, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, V.; Dibner, C. Cell-specific resetting of mouse islet cellular clocks by glucagon, glucagon-like peptide 1 and somatostatin. Acta Physiol. 2018, 222, e13021. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [Green Version]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, J.; Xiang, D.; Li, G.; Liu, D.; Zhang, C. Circadian rhythms and bile acid homeostasis: A comprehensive review. Chronobiol. Int. 2020, 37, 618–628. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Brighton, C.A.; Rievaj, J.; Kuhre, R.E.; Glass, L.L.; Schoonjans, K.; Holst, J.J.; Gribble, F.M.; Reimann, F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology 2015, 156, 3961–3970. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.R.; Martchenko, A.; Sweeney, M.E.; Maalouf, M.F.; Psichas, A.; Gribble, F.M.; Reimann, F.; Brubaker, P.L. Essential role of syntaxin-binding protein-1 in the regulation of glucagon-like peptide-1 secretion. Endocrinology 2020, 161, bqaa039. [Google Scholar] [CrossRef] [Green Version]
- Perelis, M.; Marcheva, B.; Ramsey, K.M.; Schipma, M.J.; Hutchison, A.L.; Taguchi, A.; Peek, C.B.; Hong, H.; Huang, W.; Omura, C.; et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 2015, 350, aac4250. [Google Scholar] [CrossRef] [Green Version]
- Panaro, B.L.; Yusta, B.; Matthews, D.; Koehler, J.A.; Song, Y.; Sandoval, D.A.; Drucker, D.J. Intestine-selective reduction of Gcg expression reveals the importance of the distal gut for GLP-1 secretion. Mol. Metab. 2020, 37, 100990. [Google Scholar] [CrossRef]
- Galvin, S.G.; Kay, R.G.; Foreman, R.; Larraufie, P.; Meek, C.L.; Biggs, E.; Ravn, P.; Jermutus, L.; Reimann, F.; Gribble, F.M. The human and mouse islet peptidome: Effects of obesity and type 2 diabetes, and assessment of intraislet production of glucagon-like peptide-1. J. Proteome Res. 2021. [Google Scholar] [CrossRef]
- Sadacca, L.A.; Lamia, K.A.; deLemos, A.S.; Blum, B.; Weitz, C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 2011, 54, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Rakshit, K.; Qian, J.; Gaonkar, K.S.; Dhawan, S.; Colwell, C.S.; Matveyenko, A.V. Postnatal ontogenesis of the islet circadian clock plays a contributory role in β-cell maturation process. Diabetes 2018, 67, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Rakshit, K.; Matveyenko, A.V. Induction of core circadian clock transcription factor Bmal1 enhances β-cell function and protects against obesity-induced glucose intolerance. Diabetes 2021, 70, 143–154. [Google Scholar] [CrossRef] [PubMed]
- McCommis, K.S.; Butler, A.A. The importance of keeping time in the liver. Endocrinology 2021, 162, bqaa230. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Zeitgebers of skeletal muscle and implications for metabolic health. J. Physiol. 2021, 1–10. [Google Scholar] [CrossRef]
- Heyde, I.; Begemann, K.; Oster, H. Contributions of white and brown adipose tissues to the circadian regulation of energy metabolism. Endocrinology 2021, 162, bqab009. [Google Scholar] [CrossRef]
- Blancas-Velazquez, A.; la Fleur, S.E.; Mendoza, J. Effects of a free-choice high-fat high-sugar diet on brain PER2 and BMAL1 protein expression in mice. Appetite 2017, 117, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Velazquez, A.S.; Unmehopa, U.A.; Eggels, L.; Koekkoek, L.; Kalsbeek, A.; Mendoza, J.; la Fleur, S.E. A free-choice high-fat high-sugar diet alters day-night Per2 gene expression in reward-related brain areas in rats. Front. Endocrinol. 2018, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Rakshit, K.; Hsu, T.W.; Matveyenko, A.V. Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia 2016, 59, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Fukuchi, S.; Hamaguchi, K.; Seike, M.; Himeno, K.; Sakata, T.; Yoshimatsu, H. Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats. Exp. Biol. Med. 2004, 229, 486–493. [Google Scholar] [CrossRef]
- Rocca, A.; Brubaker, P.L. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology 1995, 136, 5593–5599. [Google Scholar] [CrossRef]
- Iakoubov, R.; Izzo, A.; Yeung, A.; Whiteside, C.I.; Brubaker, P.L. Protein kinase Czeta is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 2007, 148, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, H.; Yamada, R.; Das, S.S.; Sato, T.; Takahashi, A.; Hiratsuka, M.; Hirasawa, N. Glucagon-like peptide-1 production in the GLUTag cell line is impaired by free fatty acids via endoplasmic reticulum stress. Metabolism 2014, 63, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.; Holst, J.J.; Zhang, Q.; Sjöholm, A. Molecular mechanisms of lipoapoptosis and metformin protection in GLP-1 secreting cells. Biochem. Biophys. Res. Commun. 2012, 427, 91–95. [Google Scholar] [PubMed] [Green Version]
- Cunha, D.A.; Hekerman, P.; Ladrière, L.; Bazarra-Castro, A.; Ortis, F.; Wakeham, M.C.; Moore, F.; Rasschaert, J.; Cardozo, A.K.; Bellomo, E.; et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J. Cell Sci. 2008, 121, 2308–2318. [Google Scholar]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Nemecz, M.; Constantin, A.; Dumitrescu, M.; Alexandru, N.; Filippi, A.; Tanko, G.; Georgescu, A. The distinct effects of palmitic and oleic acid on pancreatic beta cell function: The elucidation of associated mechanisms and effector molecules. Front. Pharmacol. 2019, 9, 1554. [Google Scholar]
- Natalicchio, A.; Biondi, G.; Marrano, N.; Labarbuta, R.; Tortosa, F.; Spagnuolo, R.; D’Oria, R.; Carchia, E.; Leonardini, A.; Cignarelli, A.; et al. Long-term exposure of pancreatic β-cells to palmitate results in SREBP-1C-dependent decreases in GLP-1 receptor signaling via CREB and AKT and insulin secretory response. Endocrinology 2016, 157, 2243–2258. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [Green Version]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Fick, L.J.; Fick, G.H.; Belsham, D.D. Palmitate alters the rhythmic expression of molecular clock genes and orexigenic neuropeptide Y mRNA levels within immortalized, hypothalamic neurons. Biochem. Biophys. Res. Commun. 2011, 413, 414–419. [Google Scholar] [CrossRef]
- Stamenkovic, J.A.; Olsson, A.H.; Nagorny, C.L.; Malmgren, S.; Dekker-Nitert, M.; Ling, C.; Mulder, H. Regulation of core clock genes in human islets. Metabolism 2012, 61, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhou, L.; Lu, Y.; Zhang, J.; Jian, F.; Liu, Y.; Li, F.; Li, W.; Wang, X.; Li, G. Activation of SIRT1 protects pancreatic β-cells against palmitate-induced dysfunction. Biochim. Biophys. Acta 2012, 1822, 1815–1825. [Google Scholar] [CrossRef] [Green Version]
- Desai, T.; Koulajian, K.; Ivovic, A.; Breen, D.M.; Luu, L.; Tsiani, E.L.; Wheeler, M.B.; Giacca, A. Pharmacologic or genetic activation of SIRT1 attenuates the fat-induced decrease in beta-cell function in vivo. Nutr. Diabetes 2019, 9, 11. [Google Scholar] [CrossRef]
- Petrenko, V.; Gandasi, N.R.; Sage, D.; Tengholm, A.; Barg, S.; Dibner, C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2484–2495. [Google Scholar] [CrossRef] [Green Version]
- Javeed, N.; Brown, M.R.; Rakshit, K.; Her, T.; Sen, S.K.; Matveyenko, A.V. Pro-inflammatory cytokine interleukin 1β disrupts β cell circadian clock function and regulation of insulin secretion. Endocrinology 2021, 162, bqaa084. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martchenko, A.; Brubaker, P.L. Effects of Obesogenic Feeding and Free Fatty Acids on Circadian Secretion of Metabolic Hormones: Implications for the Development of Type 2 Diabetes. Cells 2021, 10, 2297. https://doi.org/10.3390/cells10092297
Martchenko A, Brubaker PL. Effects of Obesogenic Feeding and Free Fatty Acids on Circadian Secretion of Metabolic Hormones: Implications for the Development of Type 2 Diabetes. Cells. 2021; 10(9):2297. https://doi.org/10.3390/cells10092297
Chicago/Turabian StyleMartchenko, Alexandre, and Patricia Lee Brubaker. 2021. "Effects of Obesogenic Feeding and Free Fatty Acids on Circadian Secretion of Metabolic Hormones: Implications for the Development of Type 2 Diabetes" Cells 10, no. 9: 2297. https://doi.org/10.3390/cells10092297
APA StyleMartchenko, A., & Brubaker, P. L. (2021). Effects of Obesogenic Feeding and Free Fatty Acids on Circadian Secretion of Metabolic Hormones: Implications for the Development of Type 2 Diabetes. Cells, 10(9), 2297. https://doi.org/10.3390/cells10092297





