Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration
Abstract
1. Introduction
2. Intervertebral Disc Disease
2.1. Structure of the IVD
2.2. Pathogenesis of IVDD
3. Current Treatment of IVDD
4. Biological Therapies for IVDD
5. Cell-Based Therapies for IVDD
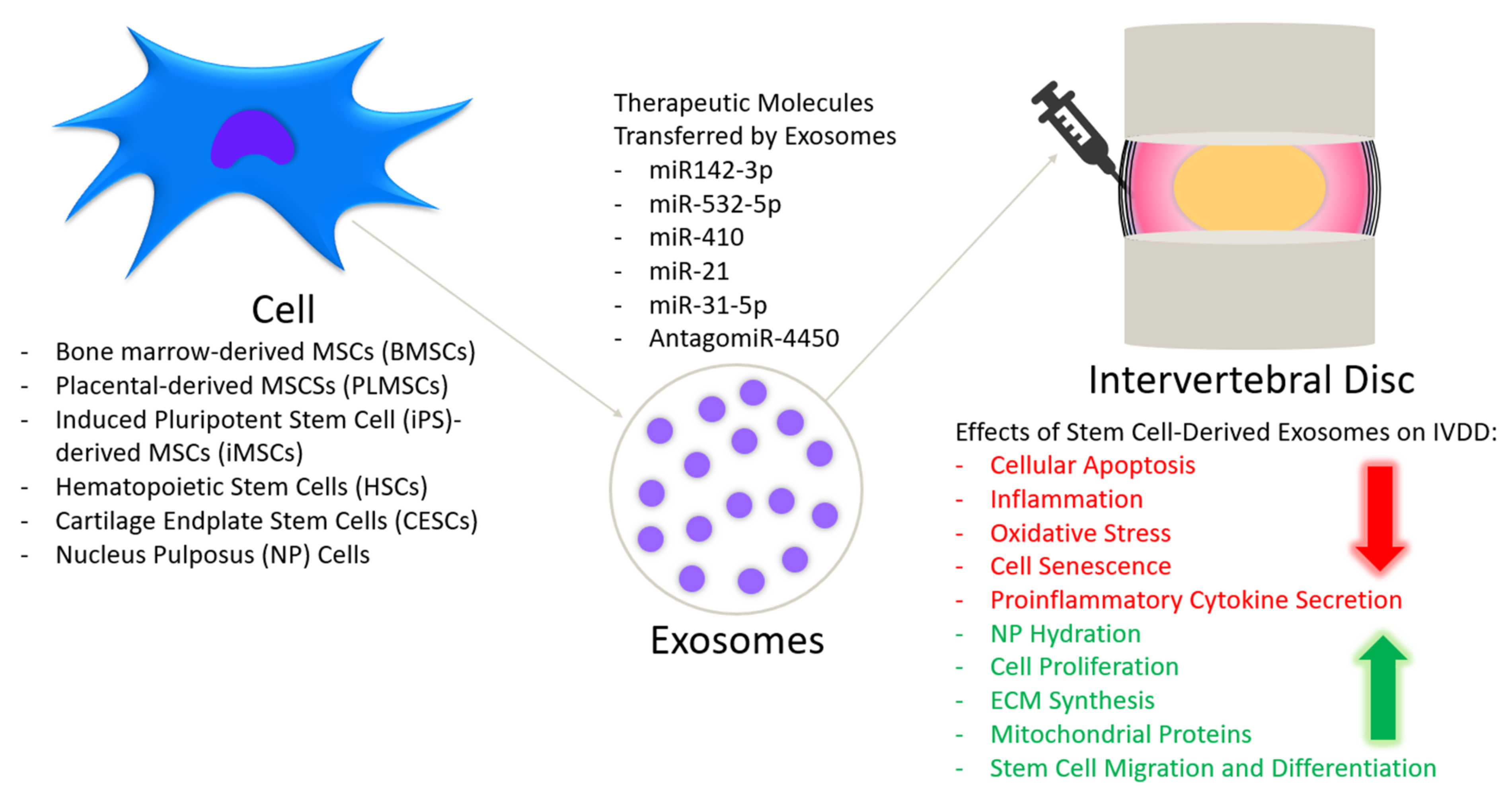
6. Stem Cell-Derived Exosomes as a Treatment for IVDD
6.1. Overview of Exosomes
6.2. Overview of Stem Cell-Derived Exosomes
6.3. Effect of Stem Cell-Derived Exosomes on IVDD
| Cell Type | Cell Origin | Scope of Study | Reference |
|---|---|---|---|
| Mesenchymal Stem Cells (MSCs) | Human | In vitro and in vivo | [24,137] |
| Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) | Rat | In vitro | [123] |
| Mouse | In vitro | [124] | |
| Human | In vitro | [119,125,127] | |
| Human | In vitro and in vivo | [134,135] | |
| Placental-Derived Mesenchymal Stem Cells (PLMSCs) | Human | In vitro and in vivo | [136] |
| Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells (iMSCs) | Human | In vitro | [120] |
| Human | In vitro and In vivo | [138] | |
| Embryonic Stem Cell (ESC)-Derived Hematopoietic Stem Cells (HSCs) | Mouse | In vitro | [121] |
| Chondrocytes | Rabbit | In vitro | [122] |
| Fibroblasts | Human | In vitro and in vivo | [24] |
| Cartilage Endplate Stem Cells (CESCs) | Rat | In vitro and in vivo | [139] |
| Nucleus Pulposus (NP) Cells | Rat | In vitro | [126] |
| Human | In vitro | [127] | |
| Notochordal Cells | Pig | In vitro | [131] |
| Origin of Exosomes | Author | Experimental Objective | Animal Model | Results | Reference |
|---|---|---|---|---|---|
| MSCs | Zhang et al., 2020 | To demonstrate NLRP3-mediated NP cell pyroptosis induction in IVDD mice model and identify regulators of this process | Mouse | Treatment with MSC-Exos and miR-410 reversed the increased protein levels of NLRP3 and alleviated the severity degree of IVDD | [24] |
| BMSCs | Xia et al., 2019 | To investigate the therapeutic effect of exosomes via a reduction in NLRP3 inflammasome expression | Rabbit | Treatment with BMSC-Exos attenuates the progression of IVDD and delays matrix degradation | [50] |
| BMSCs | Cheng et al., 2018 | To evaluate the protective effect of MSC-Exos on NP cell apoptosis and IVDD, and the regulatory effect of miRNAs | Rat | Intradiscal injection of MSC-Exos alleviates NP cell apoptosis via miR-21 contained in exosomes | [134] |
| BMSCs | Liao et al., 2019 | To determine if the delivery of MSC-Exos could modulate endoplasmic reticulum (ER) stress in the IVD | Rat | Delivery of MSC-Exos modulates ER stress-related apoptosis in AGEs-associated IVDD | [135] |
| PLMSCs | Yuan et al., 2020 | To elucidate the potential therapeutic role of human placental MSC-derived exosomes carrying AntagomiR-4450 | Mouse | Inhibition of miR-4450 alleviates inflammation, apoptosis and damage to NP cells by upregulating ZNF121 | [136] |
| MSCs | Xie et al., 2020 | To characterize the effect and mechanism of MSC-derived exosomes and the inhibition of apoptosis and calcification in endplate chondrocytes (EPCs) | Rat | Sub-endplate injection of MSC-Exos reduces apoptosis and calcification in EPCs via regulation of miR-31-5p and ATF6-related ER stress | [137] |
| iMSCs | Sun et al., 2021 | To explore the therapeutic effect of exosomes derived from induced pluripotent stem cell (iPS)-derived MSCs (iMSCs) on IVDD | Rat | Intradiscal injection could rejuvenate senescent NP cells and restore IVD height 4 weeks after needle puncture | [138] |
| CESCs | Luo et al., 2021 | To compare the use of normal and degenerated cartilage endplate stem cell-derived exosomes to diminish apoptosis of NP cells | Rat | CEP inflammation aggravates IVDD and normal CESC-derived exosomes are better equipped to decrease the apoptotic rate of NP cells by activating the PI3K, AKT and autophagy pathways | [139] |
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, W.C.; Sze, K.L.; Samartzis, D.; Leung, V.Y.; Chan, D. Structure and biology of the intervertebral disk in health and disease. Orthop. Clin. N. Am. 2011, 42, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S. Pathophysiology of degenerative disc disease. Asian Spine J. 2009, 3, 39–44. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2014, 22, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef]
- Clouet, J.; Vinatier, C.; Merceron, C.; Pot-Vaucel, M.; Hamel, O.; Weiss, P.; Grimandi, G.; Guicheux, J. The intervertebral disc: From pathophysiology to tissue engineering. Joint Bone Spine 2009, 76, 614–618. [Google Scholar] [CrossRef]
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The pathophysiology of disc degeneration: A critical review. J. Bone Joint Surg. Br. 2008, 90, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef][Green Version]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Pennicooke, B.; Moriguchi, Y.; Hussain, I.; Bonssar, L.; Härtl, R. Biological Treatment Approaches for Degenerative Disc Disease: A Review of Clinical Trials and Future Directions. Cureus 2016, 8, e892. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur. Cells Mater. 2015, 30, 104–116; discussion 116–117. [Google Scholar] [CrossRef]
- Pritzker, K.P. Aging and degeneration in the lumbar intervertebral disc. Orthop. Clin. N. Am. 1977, 8, 66–77. [Google Scholar] [CrossRef]
- Nagae, M.; Ikeda, T.; Mikami, Y.; Hase, H.; Ozawa, H.; Matsuda, K.; Sakamoto, H.; Tabata, Y.; Kawata, M.; Kubo, T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007, 13, 147–158. [Google Scholar] [CrossRef]
- Chujo, T.; An, H.S.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine 2006, 31, 2909–2917. [Google Scholar] [CrossRef]
- Willems, N.; Bach, F.C.; Plomp, S.G.; van Rijen, M.H.; Wolfswinkel, J.; Grinwis, G.C.; Bos, C.; Strijkers, G.J.; Dhert, W.J.; Meij, B.P.; et al. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res. Ther. 2015, 17, 137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matta, A.; Erwin, W.M. Injectable Biologics for the Treatment of Degenerative Disc Disease. Curr. Rev. Musculoskelet. Med. 2020, 13, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Farhang, N.; Ginley-Hidinger, M.; Berrett, K.C.; Gertz, J.; Lawrence, B.; Bowles, R.D. Lentiviral CRISPR Epigenome Editing of Inflammatory Receptors as a Gene Therapy Strategy for Disc Degeneration. Hum. Gene Ther. 2019, 30, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Asazuma, T.; Ishihara, M.; Ishihara, M.; Kikuchi, T.; Kikuchi, M.; Fujikawa, K. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine 2003, 28, 548–553. [Google Scholar] [CrossRef]
- Hohaus, C.; Ganey, T.M.; Minkus, Y.; Meisel, H.J. Cell transplantation in lumbar spine disc degeneration disease. Eur. Spine J. 2008, 17 (Suppl. 4), 492–503. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Ueda, Y.; Miyazaki, K.; Koizumi, M.; Takakura, Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: A report of two case studies. Spine 2010, 35, E475–E480. [Google Scholar] [CrossRef]
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.Á.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; García, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Wang, W.; Guo, Z.; Yang, F.; Cai, X.; Xiong, L. Current Progress in the Endogenous Repair of Intervertebral Disk Degeneration Based on Progenitor Cells. Front. Bioeng. Biotechnol. 2021, 8, 1592. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, Y.; Liu, W.; Ni, W.; Huang, X.; Yuan, J.; Zhao, B.; Xiao, H.; Xue, F. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J. Cell Mol. Med. 2020, 24, 11742–11754. [Google Scholar] [CrossRef]
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.d.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Rahman, H.S.; Markov, A.; Endjun, J.J.; Zekiy, A.O.; Chartrand, M.S.; Beheshtkhoo, N.; Kouhbanani, M.A.J.; Marofi, F.; Nikoo, M.; et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res. Ther. 2021, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Marbán, E. The Secret Life of Exosomes: What Bees Can Teach Us About Next-Generation Therapeutics. J. Am. Coll. Cardiol. 2018, 71, 193–200. [Google Scholar] [CrossRef]
- Roberts, S.; Menage, J.; Urban, J.P. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine 1989, 14, 166–174. [Google Scholar] [CrossRef] [PubMed]
- De Geer, C.M. Intervertebral Disk Nutrients and Transport Mechanisms in Relation to Disk Degeneration: A Narrative Literature Review. J. Chiropr. Med. 2018, 17, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Newell, N.; Little, J.P.; Christou, A.; Adams, M.A.; Adam, C.J.; Masouros, S.D. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Nedresky, D.; Reddy, V.; Singh, G. Anatomy, Back, Nucleus Pulposus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120, 113–130. [Google Scholar]
- Trout, J.J.; Buckwalter, J.A.; Moore, K.C. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat. Rec. 1982, 204, 307–314. [Google Scholar] [CrossRef]
- Holm, S.; Maroudas, A.; Urban, J.P.; Selstam, G.; Nachemson, A. Nutrition of the intervertebral disc: Solute transport and metabolism. Connect. Tissue Res. 1981, 8, 101–119. [Google Scholar] [CrossRef]
- Bayliss, M.T.; Johnstone, B.; O’Brien, J.P. 1988 Volvo award in basic science. Proteoglycan synthesis in the human intervertebral disc. Variation with age, region and pathology. Spine 1988, 13, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Notochordal cells in the adult intervertebral disc: New perspective on an old question. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 29–41. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.; Moore, C.A.; Kim, K.; Karim, A.; Smith, K.; Barbosa, Z.; Van Eps, J.; Rameshwar, P.; Weiner, B. Novel therapeutic strategies for degenerative disc disease: Review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018, 6, 2050312118761674. [Google Scholar] [CrossRef]
- Marchand, F.; Ahmed, A.M. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990, 15, 402–410. [Google Scholar] [CrossRef]
- Hastreiter, D.; Ozuna, R.M.; Spector, M. Regional variations in certain cellular characteristics in human lumbar intervertebral discs, including the presence of alpha-smooth muscle actin. J. Orthop. Res. 2001, 19, 597–604. [Google Scholar] [CrossRef]
- Feng, G.; Yang, X.; Shang, H.; Marks, I.W.; Shen, F.H.; Katz, A.; Arlet, V.; Laurencin, C.T.; Li, X. Multipotential differentiation of human anulus fibrosus cells: An in vitro study. J. Bone Joint Surg. Am. 2010, 92, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Sztrolovics, R.; Alini, M.; Roughley, P.J.; Mort, J.S. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem. J. 1997, 326 (Pt 1), 235–241. [Google Scholar] [CrossRef]
- Eyre, D.R.; Muir, H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim. Biophys. Acta. 1977, 492, 29–42. [Google Scholar] [CrossRef]
- Donohue, P.J.; Jahnke, M.R.; Blaha, J.D.; Caterson, B. Characterization of link protein(s) from human intervertebral-disc tissues. Biochem. J. 1988, 251, 739–747. [Google Scholar] [CrossRef]
- Sivan, S.S.; Hayes, A.J.; Wachtel, E.; Caterson, B.; Merkher, Y.; Maroudas, A.; Brown, S.; Roberts, S. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur. Spine J. 2014, 23, 344–353. [Google Scholar] [CrossRef]
- Roberts, S.; Caterson, B.; Menage, J.; Evans, E.H.; Jaffray, D.C.; Eisenstein, S.M. Matrix metalloproteinases and aggrecanase: Their role in disorders of the human intervertebral disc. Spine 2000, 25, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine 2013, 13, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zeng, Z.; Fang, B.; Tao, M.; Gu, C.; Zheng, L.; Wang, Y.; Shi, Y.; Fang, C.; Mei, S.; et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic. Biol. Med. 2019, 143, 1–15. [Google Scholar] [CrossRef]
- Cazzanelli, P.; Wuertz-Kozak, K. MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology. Int. J. Mol. Sci. 2020, 21, 3601. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Haglund, L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine J. 2013, 3, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; An, R.; Xiang, Q.; Li, G.; Wang, K.; Song, Y.; Liao, Z.; Li, S.; Hua, W.; Feng, X.; et al. Acid-sensing ion channels regulate nucleus pulposus cell inflammation and pyroptosis via the NLRP3 inflammasome in intervertebral disc degeneration. Cell Prolif. 2021, 54, e12941. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Zhang, Y.; Geng, W.; Liu, W.; Gao, Y.; Li, S.; Wang, K.; Wu, X.; Kang, L.; et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. J. Cell Mol. Med. 2017, 21, 1373–1387. [Google Scholar] [CrossRef]
- Wang, J.; Nisar, M.; Huang, C.; Pan, X.; Lin, D.; Zheng, G.; Jin, H.; Chen, D.; Tian, N.; Huang, Q.; et al. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Sudo, H.; Minami, A. Regulation of apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2 overexpression. J. Orthop. Res. 2010, 28, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Zotti, M.G.; Osti, O.L. Operative Management of Lumbar Degenerative Disc Disease. Asian Spine J. 2016, 10, 801–819. [Google Scholar] [CrossRef]
- Fritzell, P.; Hägg, O.; Wessberg, P.; Nordwall, A. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine 2001, 26, 2521–2532; discussion 2532–2534. [Google Scholar] [CrossRef]
- Li, J.; Yoon, S.T.; Hutton, W.C. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J. Spinal Disord. Tech. 2004, 17, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.P.; Oegema, T.R., Jr.; Bradford, D.S. Stimulation of mature canine intervertebral disc by growth factors. Spine 1991, 16, 253–260. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Alimi, M.; Khair, T.; Manolarakis, G.; Berlin, C.; Bonassar, L.J.; Härtl, R. Biological Treatment Approaches for Degenerative Disk Disease: A Literature Review of In Vivo Animal and Clinical Data. Global Spine J. 2016, 6, 497–518. [Google Scholar] [CrossRef]
- Walsh, A.J.; Bradford, D.S.; Lotz, J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine 2004, 29, 156–163. [Google Scholar] [CrossRef]
- Guterl, C.C.; See, E.Y.; Blanquer, S.B.; Pandit, A.; Ferguson, S.J.; Benneker, L.M.; Grijpma, D.W.; Sakai, D.; Eglin, D.; Alini, M.; et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur. Cell Mater. 2013, 25, 1–21. [Google Scholar] [CrossRef]
- Dowdell, J.; Erwin, M.; Choma, T.; Vaccaro, A.; Iatridis, J.; Cho, S.K. Intervertebral Disk Degeneration and Repair. Neurosurgery 2017, 80, S46–S54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, C.; Wang, W.; Peng, J.; Cheng, X.; Shangguan, Y.; Xu, M.; Li, J.; Qu, R.; Chen, X.; et al. Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation. Theranostics 2020, 10, 7015–7033. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Gu, J.M.; Xie, Z.A.; Gu, Y.; Jie, Z.W.; Huang, K.M.; Wang, J.Y.; Fan, S.W.; Jiang, X.S.; Hu, Z.J. Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radic. Biol. Med. 2018, 120, 368–379. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhou, M.; Bai, Z.; Wen, Y.; Shen, J.; Hu, Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway. Biochem. Biophys. Res. Commun. 2020, 526, 772–779. [Google Scholar] [CrossRef]
- Takeoka, Y.; Yurube, T.; Nishida, K. Gene Therapy Approach for Intervertebral Disc Degeneration: An Update. Neurospine 2020, 17, 3–14. [Google Scholar] [CrossRef]
- Sawamura, K.; Ikeda, T.; Nagae, M.; Okamoto, S.; Mikami, Y.; Hase, H.; Ikoma, K.; Yamada, T.; Sakamoto, H.; Matsuda, K.; et al. Characterization of in vivo effects of platelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng. Part A 2009, 15, 3719–3727. [Google Scholar] [CrossRef]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM&R 2016, 8, 1–10; quiz 10. [Google Scholar] [CrossRef]
- Gullung, G.B.; Woodall, J.W.; Tucci, M.A.; James, J.; Black, D.A.; McGuire, R.A. Platelet-rich plasma effects on degenerative disc disease: Analysis of histology and imaging in an animal model. Evid. Based Spine Care J. 2011, 2, 13–18. [Google Scholar] [CrossRef]
- Monfett, M.; Harrison, J.; Boachie-Adjei, K.; Lutz, G. Intradiscal platelet-rich plasma (PRP) injections for discogenic low back pain: An update. Int. Orthop. 2016, 40, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, Y.; Xu, G.; Wang, H.; Wang, L. The role of miRNA, lncRNA and circRNA in the development of intervertebral disk degeneration (Review). Exp. Ther. Med. 2021, 21, 555. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; He, Q.; Ding, Y.; Hou, L.; Li, J.; Luk, K.D. Intervertebral disc transplantation in the treatment of degenerative spine disease: A preliminary study. Lancet 2007, 369, 993–999. [Google Scholar] [CrossRef]
- Berlemann, U.; Schwarzenbach, O. An injectable nucleus replacement as an adjunct to microdiscectomy: 2 year follow-up in a pilot clinical study. Eur. Spine J. 2009, 18, 1706–1712. [Google Scholar] [CrossRef]
- Vadalà, G.; Russo, F.; Ambrosio, L.; Loppini, M.; Denaro, V. Stem cells sources for intervertebral disc regeneration. World J. Stem Cells 2016, 8, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Zhang, C.; Hwang, Y.S.; Varghese, S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 97–106. [Google Scholar] [CrossRef]
- Le Blanc, K.; Ringdén, O. Mesenchymal stem cells: Properties and role in clinical bone marrow transplantation. Curr. Opin. Immunol. 2006, 18, 586–591. [Google Scholar] [CrossRef]
- Guyer, R.D.; McAfee, P.C.; Banco, R.J.; Bitan, F.D.; Cappuccino, A.; Geisler, F.H.; Hochschuler, S.H.; Holt, R.T.; Jenis, L.G.; Majd, M.E.; et al. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: Five-year follow-up. Spine 2009, 9, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.F.; Villarón, E.M.; Pescador, D.; da Casa, C.; Gómez, V.; Redondo, A.M.; López-Villar, O.; López-Parra, M.; Muntión, S.; Sánchez-Guijo, F. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: Results of a prospective phase I/II clinical trial with long-term follow-up. Stem Cell Res. Ther. 2019, 10, 63. [Google Scholar] [CrossRef]
- Haufe, S.M.; Mork, A.R. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006, 15, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Marfia, G.; Campanella, R.; Navone, S.E.; Zucca, I.; Scotti, A.; Figini, M.; Di Vito, C.; Alessandri, G.; Riboni, L.; Parati, E. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: A preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res. Ther. 2014, 16, 457. [Google Scholar] [CrossRef]
- Chun, H.J.; Kim, Y.S.; Kim, B.K.; Kim, E.H.; Kim, J.H.; Do, B.R.; Hwang, S.J.; Hwang, J.Y.; Lee, Y.K. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg. 2012, 78, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Comella, K.; Silbert, R.; Parlo, M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J. Transl. Med. 2017, 15, 12. [Google Scholar] [CrossRef]
- Meisel, H.J.; Siodla, V.; Ganey, T.; Minkus, Y.; Hutton, W.C.; Alasevic, O.J. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol. Eng. 2007, 24, 5–21. [Google Scholar] [CrossRef]
- Ju, D.G.; Kanim, L.E.; Bae, H.W. Intervertebral Disc Repair: Current Concepts. Global Spine J. 2020, 10, 130s–136s. [Google Scholar] [CrossRef]
- Pettine, K.A.; Murphy, M.B.; Suzuki, R.K.; Sand, T.T. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 2015, 33, 146–156. [Google Scholar] [CrossRef]
- Hingert, D.; Nawilaijaroen, P.; Ekström, K.; Baranto, A.; Brisby, H. Human Levels of MMP-1 in Degenerated Disks Can Be Mitigated by Signaling Peptides from Mesenchymal Stem Cells. Cells Tissues Organs 2020, 209, 144–154. [Google Scholar] [CrossRef]
- Silverman, L.I.; Dulatova, G.; Tandeski, T.; Erickson, I.E.; Lundell, B.; Toplon, D.; Wolff, T.; Howard, A.; Chintalacharuvu, S.; Foley, K.T. In vitro and in vivo evaluation of discogenic cells, an investigational cell therapy for disc degeneration. Spine J. 2020, 20, 138–149. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, T.; Sun, X.; Han, C.; Zhang, K.; Zhao, C.; Li, X.; Tian, H.; Yang, X.; Zhou, Y.; et al. Autologous fibroblasts induce fibrosis of the nucleus pulposus to maintain the stability of degenerative intervertebral discs. Bone Res. 2020, 8, 7. [Google Scholar] [CrossRef]
- Ouyang, A.; Cerchiari, A.E.; Tang, X.; Liebenberg, E.; Alliston, T.; Gartner, Z.J.; Lotz, J.C. Effects of cell type and configuration on anabolic and catabolic activity in 3D co-culture of mesenchymal stem cells and nucleus pulposus cells. J. Orthop. Res. 2017, 35, 61–73. [Google Scholar] [CrossRef]
- Ma, K.; Chen, S.; Li, Z.; Deng, X.; Huang, D.; Xiong, L.; Shao, Z. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthr. Cartil. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Xia, H.; Li, X.; Gao, W.; Fu, X.; Fang, R.H.; Zhang, L.; Zhang, K. Tissue repair and regeneration with endogenous stem cells. Nat. Rev. Mater. 2018, 3, 174–193. [Google Scholar] [CrossRef]
- Frapin, L.; Clouet, J.; Chédeville, C.; Moraru, C.; Samarut, E.; Henry, N.; André, M.; Bord, E.; Halgand, B.; Lesoeur, J.; et al. Controlled release of biological factors for endogenous progenitor cell migration and intervertebral disc extracellular matrix remodelling. Biomaterials 2020, 253, 120107. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peroglio, M.; Alini, M.; Grad, S. Potential and limitations of intervertebral disc endogenous repair. Curr. Stem Cell Res. Ther. 2015, 10, 329–338. [Google Scholar] [CrossRef]
- Liu, L.T.; Huang, B.; Li, C.Q.; Zhuang, Y.; Wang, J.; Zhou, Y. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS ONE 2011, 6, e26285. [Google Scholar] [CrossRef]
- Liang, H.; Chen, S.; Huang, D.; Deng, X.; Ma, K.; Shao, Z. Effect of Compression Loading on Human Nucleus Pulposus-Derived Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 1481243. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; Vendrig, T.; Hopmans, E.S.; van Eijndhoven, M.; Middeldorp, J.M.; Pegtel, D.M. Exosomes: Fit to deliver small RNA. Commun. Integr. Biol. 2010, 3, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Yang, I.; Hua, W.; Mao, Y.; Carter, B.S.; Chen, C.C. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 2016, 17, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969. [Google Scholar] [CrossRef]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes—The enigmatic regulators of bone homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef]
- Welton, J.L.; Khanna, S.; Giles, P.J.; Brennan, P.; Brewis, I.A.; Staffurth, J.; Mason, M.D.; Clayton, A. Proteomics analysis of bladder cancer exosomes. Mol. Cell Proteomics 2010, 9, 1324–1338. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Eldh, M.; Ekström, K.; Valadi, H.; Sjöstrand, M.; Olsson, B.; Jernås, M.; Lötvall, J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Eirin, A.; Riester, S.M.; Zhu, X.Y.; Tang, H.; Evans, J.M.; O’Brien, D.; van Wijnen, A.J.; Lerman, L.O. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 2014, 551, 55–64. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Nazari-Shafti, T.Z.; Neuber, S.; Falk, V.; Emmert, M.Y. Toward next-generation advanced therapies: Extracellular vesicles and cell therapy—Partners or competitors? Regen. Med. 2021, 16, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Kang, M.; Wu, X.; Chen, J.; Teng, L.; Qiu, L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci. 2020, 256, 118002. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- D’Alimonte, I.; Lannutti, A.; Pipino, C.; Di Tomo, P.; Pierdomenico, L.; Cianci, E.; Antonucci, I.; Marchisio, M.; Romano, M.; Stuppia, L.; et al. Wnt signaling behaves as a "master regulator" in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. Rep. 2013, 9, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S., Jr.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Liao, F.L.; Tan, L.; Liu, H.; Wang, J.J.; Ma, X.T.; Zhao, B.; Chen, Y.; Bihl, J.; Yang, Y.; Chen, R.L. Hematopoietic stem cell-derived exosomes promote hematopoietic differentiation of mouse embryonic stem cells in vitro via inhibiting the miR126/Notch1 pathway. Acta Pharmacol. Sin. 2018, 39, 552–560. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, K.; Zhang, X.; Zheng, Z.; Liu, K. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res. Ther. 2018, 9, 318. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Y.; Liu, L.; Wang, H.; Shen, P.; Yang, H. Mesenchymal stem cells-derived exosomes ameliorate nucleus pulposus cells apoptosis via delivering miR-142-3p: Therapeutic potential for intervertebral disc degenerative diseases. Cell Cycle 2020, 19, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yang, X.; Peng, C.; Yu, L.; Hao, Y. Exosomal miR-532–5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Exp. Cell Res. 2020, 393, 112109. [Google Scholar] [CrossRef]
- Hingert, D.; Ekström, K.; Aldridge, J.; Crescitelli, R.; Brisby, H. Extracellular vesicles from human mesenchymal stem cells expedite chondrogenesis in 3D human degenerative disc cell cultures. Stem Cell Res. Ther. 2020, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.R.; Pan, S.; Li, H.Y.; Sun, C.; Chang, X.; Lu, K.; Jiang, C.Q.; Zuo, R.; Zhou, Y.; Li, C.Q. Inhibition of the Notch1 Pathway Promotes the Effects of Nucleus Pulposus Cell-Derived Exosomes on the Differentiation of Mesenchymal Stem Cells into Nucleus Pulposus-Like Cells in Rats. Stem Cells Int. 2019, 2019, 8404168. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Li, H.Y.; Yang, K.; Wu, J.L.; Cai, X.W.; Zhou, Y.; Li, C.Q. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: In-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Moen, A.; Jacobsen, D.; Phuyal, S.; Legfeldt, A.; Haugen, F.; Røe, C.; Gjerstad, J. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J. Transl. Med. 2017, 15, 89. [Google Scholar] [CrossRef]
- Poon, K.-S.; Palanisamy, K.; Chang, S.-S.; Sun, K.-T.; Chen, K.-B.; Li, P.-C.; Lin, T.-C.; Li, C.-Y. Plasma exosomal miR-223 expression regulates inflammatory responses during cardiac surgery with cardiopulmonary bypass. Sci. Rep. 2017, 7, 10807. [Google Scholar] [CrossRef]
- Wang, H.; Hao, P.; Zhang, H.; Xu, C.; Zhao, J. MicroRNA-223 inhibits lipopolysaccharide-induced inflammatory response by directly targeting Irak1 in the nucleus pulposus cells of intervertebral disc. IUBMB Life 2018, 70, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Libregts, S.; Creemers, L.; Meij, B.; Ito, K.; Wauben, M.; Tryfonidou, M. Notochordal-cell derived extracellular vesicles exert regenerative effects on canine and human nucleus pulposus cells. Oncotarget 2017, 8, 88845–88856. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, R.; Richardson, S.M.; Hoyland, J.A. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur. Spine J. 2014, 23, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D.; Xiao, Z. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr. Stem Cell Res. Ther. 2018, 13, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, G.; Zhang, L.; Hu, Y.; Zhang, K.; Sun, X.; Zhao, C.; Li, H.; Li, Y.M.; Zhao, J. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell Mol. Med. 2018, 22, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Luo, R.; Li, G.; Song, Y.; Zhan, S.; Zhao, K.; Hua, W.; Zhang, Y.; Wu, X.; Yang, C. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019, 9, 4084–4100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, X.; Liu, L.; Cai, Y.; Zhao, X.; Ma, H.; Zhang, Y. Exosomes Derived from Human Placental Mesenchymal Stromal Cells Carrying AntagomiR-4450 Alleviate Intervertebral Disc Degeneration Through Upregulation of ZNF121. Stem Cells Dev. 2020, 29, 1038–1058. [Google Scholar] [CrossRef]
- Xie, L.; Chen, Z.; Liu, M.; Huang, W.; Zou, F.; Ma, X.; Tao, J.; Guo, J.; Xia, X.; Lyu, F.; et al. MSC-Derived Exosomes Protect Vertebral Endplate Chondrocytes against Apoptosis and Calcification via the miR-31-5p/ATF6 Axis. Mol. Ther. Nucleic Acids 2020, 22, 601–614. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Li, X. Induced pluripotent stem cell-derived mesenchymal stem cells deliver exogenous miR-105-5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration. Stem Cell Res. Ther. 2021, 12, 286. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jian, X.; Sun, H.; Qin, J.; Wang, Y.; Zhang, J.; Shen, Z.; Yang, D.; Li, C.; Zhao, P.; et al. Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate Akt/autophagy. Stem Cells 2021, 39, 467–481. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Shahabipour, F.; Banach, M.; Sahebkar, A. Exosomes as nanocarriers for siRNA delivery: Paradigms and challenges. Arch. Med. Sci. 2016, 12, 1324–1326. [Google Scholar] [CrossRef]
- Malekpour, K.; Hazrati, A.; Zahar, M.; Markov, A.; Zekiy, A.O.; Navashenaq, J.G.; Roshangar, L.; Ahmadi, M. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes for Orthopedic Diseases Treatment. Stem Cell Rev. Rep. 2021, 1–19. [Google Scholar] [CrossRef]
- Riau, A.K.; Ong, H.S.; Yam, G.H.F.; Mehta, J.S. Sustained Delivery System for Stem Cell-Derived Exosomes. Front. Pharmacol. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Piazza, N.; Dehghani, M.; Gaborski, T.R.; Wuertz-Kozak, K. Therapeutic Potential of Extracellular Vesicles in Degenerative Diseases of the Intervertebral Disc. Front. Bioeng. Biotechnol. 2020, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.B.; Gray, K.M.; Santharam, Y.; Lamichhane, T.N.; Stroka, K.M.; Jay, S.M. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2017, 2, 170–179. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Jin, Y.-Q.; Liu, W.; Zhang, W.J.; Hong, T.-H.; Zhou, G.; Baggett, L.S.; Mikos, A.G.; Cao, Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008, 9, 60. [Google Scholar] [CrossRef]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekström, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021, 11, 1954. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Ayers, L.; Pink, R.; Carter, D.R.F.; Nieuwland, R. Clinical requirements for extracellular vesicle assays. J. Extracell. Vesicles 2019, 8, 1593755. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, M.H.; Kink, J.A.; Hematti, P.; Capitini, C.M. Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front. Cell Dev. Biol. 2020, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Loibl, M.; Wuertz-Kozak, K.; Vadala, G.; Lang, S.; Fairbank, J.; Urban, J.P. Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine 2019, 2, e1043. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krut, Z.; Pelled, G.; Gazit, D.; Gazit, Z. Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration. Cells 2021, 10, 2241. https://doi.org/10.3390/cells10092241
Krut Z, Pelled G, Gazit D, Gazit Z. Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration. Cells. 2021; 10(9):2241. https://doi.org/10.3390/cells10092241
Chicago/Turabian StyleKrut, Zoe, Gadi Pelled, Dan Gazit, and Zulma Gazit. 2021. "Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration" Cells 10, no. 9: 2241. https://doi.org/10.3390/cells10092241
APA StyleKrut, Z., Pelled, G., Gazit, D., & Gazit, Z. (2021). Stem Cells and Exosomes: New Therapies for Intervertebral Disc Degeneration. Cells, 10(9), 2241. https://doi.org/10.3390/cells10092241






