Neutrophil Interactions with the Lymphatic System
Abstract
1. Introduction
2. The Lymphatic Vasculature
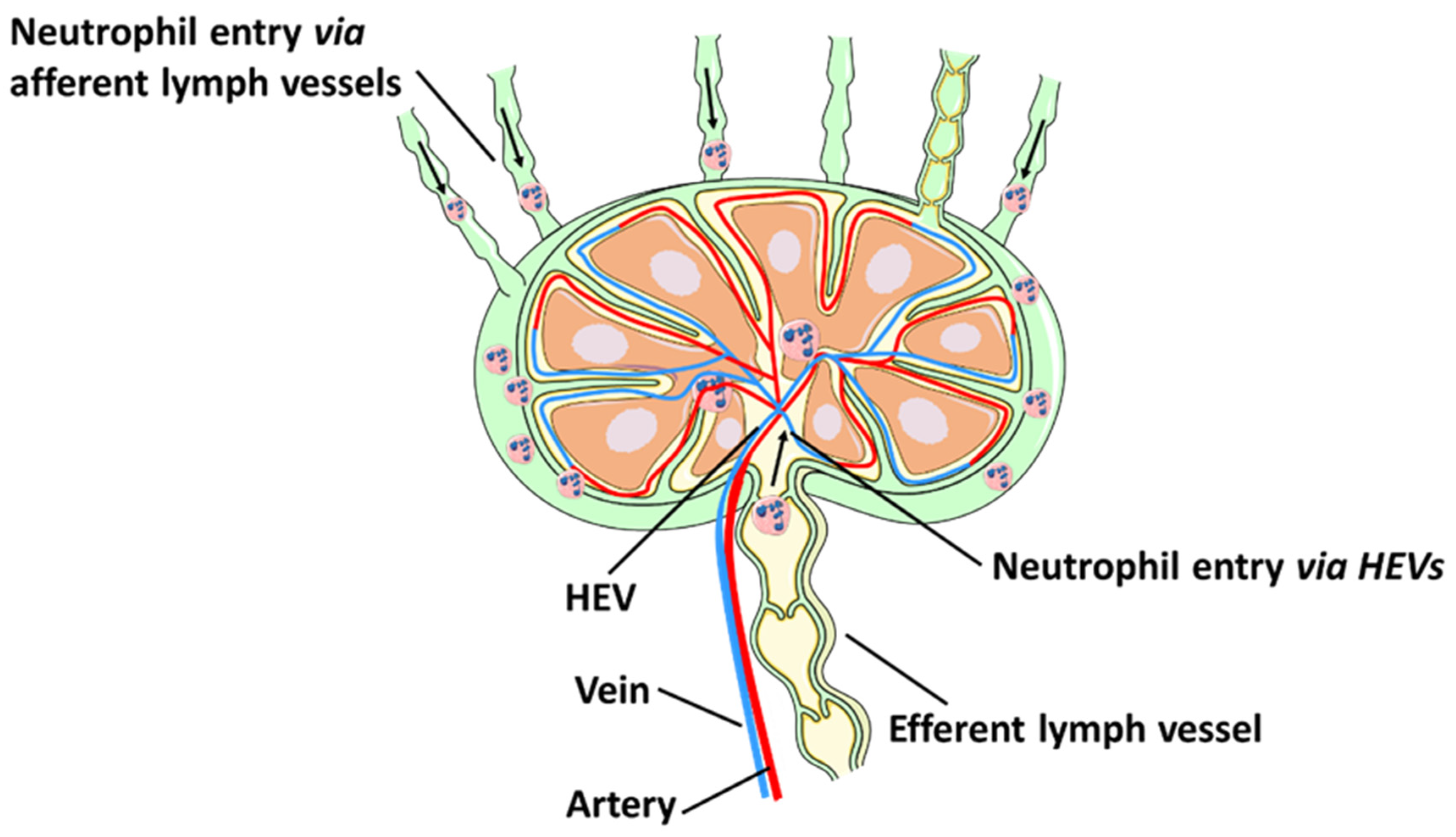
3. Neutrophil Migration to Lymphoid Organs
3.1. Neutrophil Migration via Lymphatic Vessels
3.2. Neutrophil Recruitment to Lymph Nodes via HEVs
4. Neutrophil Functions within the Lymphatic System
4.1. Pathogen Control versus Dissemination
4.2. Antigen Presentation
4.3. Neutrophil Modulation of T and B Lymphocytes
4.4. Neutrophil Contributions to Lymphangiogenesis
5. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Harvey, N.L. Lymphatic Vascular Development. Heart Dev. Regen. 2010, 543–565. [Google Scholar]
- Jackson, D.G. Leucocyte Trafficking via the Lymphatic Vasculature— Mechanisms and Consequences. Front. Immunol. 2019, 10, 471. [Google Scholar] [CrossRef]
- Liao, S.; von der Weid, P.-Y. Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis 2014, 17, 325–334. [Google Scholar] [CrossRef]
- Lok, L.S.C.; Dennison, T.W.; Mahbubani, K.M.; Saeb-Parsy, K.; Chilvers, E.R.; Clatworthy, M.R. Phenotypically distinct neutrophils patrol uninfected human and mouse lymph nodes. Proc. Natl. Acad. Sci. USA 2019, 116, 19083–19089. [Google Scholar] [CrossRef]
- Bogoslowski, A.; Wijeyesinghe, S.; Lee, W.-Y.; Chen, C.-S.; Alanani, S.; Jenne, C.; Steeber, D.A.; Scheiermann, C.; Butcher, E.C.; Masopust, D.; et al. Neutrophils Recirculate through Lymph Nodes to Survey Tissues for Pathogens. J. Immunol. 2020, 204, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.R.; Perrin, B.J.; Liu, T.X.; Kanki, J.; Look, A.T.; Huttenlocher, A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 2006, 80, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Hampton, H.R.; Bailey, J.; Tomura, M.; Brink, R.; Chtanova, T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat. Commun. 2015, 6, 7139. [Google Scholar] [CrossRef]
- Abadie, V.r.; Badell, E.; Douillard, P.; Ensergueix, D.; Leenen, P.J.M.; Tanguy, M.; Fiette, L.; Saeland, S.; Gicquel, B.; Winter, N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 2005, 106, 1843–1850. [Google Scholar] [CrossRef]
- Gorlino, C.V.; Ranocchia, R.P.; Harman, M.F.; García, I.A.; Crespo, M.I.; Morón, G.; Maletto, B.A.; Pistoresi-Palencia, M.C. Neutrophils Exhibit Differential Requirements for Homing Molecules in Their Lymphatic and Blood Trafficking into Draining Lymph Nodes. J. Immunol. 2014, 193, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Arokiasamy, S.; Zakian, C.; Dilliway, J.; Wang, W.; Nourshargh, S.; Voisin, M.-B. Endogenous TNFα orchestrates the trafficking of neutrophils into and within lymphatic vessels during acute inflammation. Sci. Rep. 2017, 7, 44189. [Google Scholar] [CrossRef]
- Kamenyeva, O.; Boularan, C.; Kabat, J.; Cheung, G.Y.C.; Cicala, C.; Yeh, A.J.; Chan, J.L.; Periasamy, S.; Otto, M.; Kehrl, J.H. Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to Staphylococcus aureus. PLoS Pathog. 2015, 11, e1004827. [Google Scholar] [CrossRef]
- Brackett, C.M.; Muhitch, J.B.; Evans, S.S.; Gollnick, S.O. IL-17 Promotes Neutrophil Entry into Tumor-Draining Lymph Nodes following Induction of Sterile Inflammation. J. Immunol. 2013, 191, 4348–4357. [Google Scholar] [CrossRef]
- Bogoslowski, A.; Butcher, E.C.; Kubes, P. Neutrophils recruited through high endothelial venules of the lymph nodes via PNAd intercept disseminating Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2018, 115, 2449–2454. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef]
- Baluk, P.; Tammela, T.; Ator, E.; Lyubynska, N.; Achen, M.G.; Hicklin, D.J.; Jeltsch, M.; Petrova, T.V.; Pytowski, B.; Stacker, S.A.; et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Investig. 2005, 115, 247–257. [Google Scholar] [CrossRef]
- Tan, K.W.; Chong, S.Z.; Wong, F.H.S.; Evrard, M.; Tan, S.M.-L.; Keeble, J.; Kemeny, D.M.; Ng, L.G.; Abastado, J.-P.; Angeli, V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood 2013, 122, 3666–3677. [Google Scholar] [CrossRef]
- Santambrogio, L. Lymph Formation and Composition. Lymphedema 2018, 139–152. [Google Scholar]
- Dzieciatkowska, M.; D’Alessandro, A.; Moore, E.E.; Wohlauer, M.; Banerjee, A.; Silliman, C.C.; Hansen, K.C. Lymph Is Not a Plasma Ultrafiltrate. Shock 2014, 42, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Veenstra, T.D. Proteomic analysis of serum, plasma, and lymph for the identification of biomarkers. Proteom. Clin. Appl. 2007, 1, 747–757. [Google Scholar] [CrossRef]
- Cueni, L.N.; Detmar, M. New Insights into the Molecular Control of the Lymphatic Vascular System and its Role in Disease. J. Investig. Dermatol. 2006, 126, 2167–2177. [Google Scholar] [CrossRef]
- Oliver, G. Lymphatic vasculature development. Nat. Rev. Immunol. 2004, 4, 35–45. [Google Scholar] [CrossRef]
- Kong, L.-L.; Yang, N.-Z.; Shi, L.-H.; Zhao, G.-H.; Zhou, W.; Ding, Q.; Wang, M.-H.; Zhang, Y.-S. The optimum marker for the detection of lymphatic vessels. Mol. Clin. Oncol. 2017, 7, 515–520. [Google Scholar] [CrossRef]
- Baluk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendini, C.; Dejana, E.; et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007, 204, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Schineis, P.; Runge, P.; Halin, C. Cellular traffic through afferent lymphatic vessels. Vasc. Pharm. 2019, 112, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bazigou, E.; Xie, S.; Chen, C.; Weston, A.; Miura, N.; Sorokin, L.; Adams, R.; Muro, A.F.; Sheppard, D.; Makinen, T. Integrin-α9 Is Required for Fibronectin Matrix Assembly during Lymphatic Valve Morphogenesis. Dev. Cell 2009, 17, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zarkada, G.; Yi, S.; Eichmann, A. Lymphatic Endothelial Cell Junctions: Molecular Regulation in Physiology and Diseases. Front. Physiol. 2020, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Hirosue, S.; Vokali, E.; Raghavan, V.R.; Rincon-Restrepo, M.; Lund, A.W.; Corthésy-Henrioud, P.; Capotosti, F.; Halin Winter, C.; Hugues, S.; Swartz, M.A. Steady-State Antigen Scavenging, Cross-Presentation, and CD8 + T Cell Priming: A New Role for Lymphatic Endothelial Cells. J. Immunol. 2014, 192, 5002–5011. [Google Scholar] [CrossRef] [PubMed]
- Gkountidi, A.O.; Garnier, L.; Dubrot, J.; Angelillo, J.; Harlé, G.; Brighouse, D.; Wrobel, L.J.; Pick, R.; Scheiermann, C.; Swartz, M.A.; et al. MHC Class II Antigen Presentation by Lymphatic Endothelial Cells in Tumors Promotes Intratumoral Regulatory T cell-Suppressive Functions. Cancer Immunol. Res. 2021, 9, 748–764. [Google Scholar] [CrossRef]
- Vokali, E.; Yu, S.S.; Hirosue, S.; Rinçon-Restrepo, M.; Duraes, F.V.; Scherer, S.; Corthésy-Henrioud, P.; Kilarski, W.W.; Mondino, A.; Zehn, D.; et al. Lymphatic endothelial cells prime naïve CD8+ T cells into memory cells under steady-state conditions. Nat. Commun. 2020, 11, 538. [Google Scholar] [CrossRef]
- Schulte-Merker, S.; Sabine, A.; Petrova, T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell. Biol. 2011, 193, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Vranova, M. Lymphatic Vessels in Inflammation. J. Clin. Cell Immunol 2014, 5, 1000250. [Google Scholar] [CrossRef]
- Tan, K.W.; Chong, S.Z.; Angeli, V. Inflammatory lymphangiogenesis: Cellular mediators and functional implications. Angiogenesis 2014, 17, 373–381. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.H.; Scallan, J.P. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef]
- Lynskey, N.N.; Banerji, S.; Johnson, L.A.; Holder, K.A.; Reglinski, M.; Wing, P.A.C.; Rigby, D.; Jackson, D.G.; Sriskandan, S. Rapid Lymphatic Dissemination of Encapsulated Group A Streptococci via Lymphatic Vessel Endothelial Receptor-1 Interaction. PLoS Pathog. 2015, 11, e1005137. [Google Scholar] [CrossRef]
- Hall, J.G.; Morris, B. The output of cells in lymph from the popliteal node of sheep. Q. J. Exp. Physiol. Cogn. Med. Sci. 1962, 47, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.B.; McIntosh, G.H.; Morris, B. The traffic of cells through tissues: A study of peripheral lymph in sheep. J. Anat. 1970, 107, 87–100. [Google Scholar]
- Olszewski, W. Immune cell traffic from blood through the normal human skin to lymphatics. Clin. Dermatol. 1995, 13, 473–483. [Google Scholar] [CrossRef]
- Egan, P.J.; Kimpton, W.; Seow, H.F.; Bowles, V.M.; Brandon, M.R.; Nash, A.D. Inflammation-induced changes in the phenotype and cytokine profile of cells migrating through skin and afferent lymph. Immunology 1996, 89, 539–546. [Google Scholar] [CrossRef]
- Haig, D.M.; Hopkins, J.; Miller, H.R. Local immune responses in afferent and efferent lymph. Immunology 1999, 96, 155–163. [Google Scholar] [CrossRef] [PubMed]
- de Veer, M.; Neeland, M.; Burke, M.; Pleasance, J.; Nathanielsz, J.; Elhay, M.; Meeusen, E. Cell recruitment and antigen trafficking in afferent lymph after injection of antigen and poly(I:C) containing liposomes, in aqueous or oil-based formulations. Vaccine 2013, 31, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Yam, A.O.; Chtanova, T. Imaging the neutrophil: Intravital microscopy provides a dynamic view of neutrophil functions in host immunity. Cell. Immunol. 2020, 350, 103898. [Google Scholar] [CrossRef] [PubMed]
- Rigby, D.A.; Ferguson, D.J.P.; Johnson, L.A.; Jackson, D.G. Neutrophils rapidly transit inflamed lymphatic vessel endothelium via integrin-dependent proteolysis and lipoxin-induced junctional retraction. J. Leukoc. Biol. 2015, 98, 897–912. [Google Scholar] [CrossRef]
- Teijeira, A.; Halin, C. Editorial: Breaching their way through: Neutrophils destroy intercellular junctions to transmigrate rapidly across lymphatic endothelium. J. Leukoc. Biol. 2015, 98, 880–882. [Google Scholar] [CrossRef]
- Beauvillain, C.; Cunin, P.; Doni, A.; Scotet, M.; Jaillon, S.; Loiry, M.-L.; Magistrelli, G.; Masternak, K.; Chevailler, A.; Delneste, Y.; et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 2011, 117, 1196–1204. [Google Scholar] [CrossRef]
- Solovjov, D.A.; Pluskota, E.; Plow, E.F. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J. Biol. Chem. 2005, 280, 1336–1345. [Google Scholar] [CrossRef]
- Hasenberg, A.; Hasenberg, M.; Männ, L.; Neumann, F.; Borkenstein, L.; Stecher, M.; Kraus, A.; Engel, D.R.; Klingberg, A.; Seddigh, P.; et al. Catchup: A mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat. Methods 2015, 12, 445–452. [Google Scholar] [CrossRef]
- Vigl, B.; Aebischer, D.; Nitschke, M.; Iolyeva, M.; Rothlin, T.; Antsiferova, O.; Halin, C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011, 118, 205–215. [Google Scholar] [CrossRef]
- Phillipson, M.; Heit, B.; Colarusso, P.; Liu, L.; Ballantyne, C.M.; Kubes, P. Intraluminal crawling of neutrophils to emigration sites: A molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 2006, 203, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, M.R.; Aronin, C.E.P.; Mathews, R.J.; Morgan, N.Y.; Smith, K.G.C.; Germain, R.N. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat. Med. 2014, 20, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Leirião, P.; del Fresno, C.; Ardavín, C. Monocytes as effector cells: Activated Ly-6Chigh mouse monocytes migrate to the lymph nodes through the lymph and cross-present antigens to CD8+T cells. Eur. J. Immunol. 2012, 42, 2042–2051. [Google Scholar] [CrossRef]
- Debes, G.F.; Arnold, C.N.; Young, A.J.; Krautwald, S.; Lipp, M.; Hay, J.B.; Butcher, E.C. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005, 6, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Gowans, J.L. The recirculation of lymphocytes from blood to lymph in the rat. J. Physiol. 1959, 146, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Voisin, M.B.; Nourshargh, S. Neutrophil trafficking to lymphoid tissues: Physiological and pathological implications. J. Pathol. 2019, 247, 662–671. [Google Scholar] [CrossRef]
- Miyasaka, M.; Tanaka, T. Lymphocyte trafficking across high endothelial venules: Dogmas and enigmas. Nat. Rev. Immunol 2004, 4, 360–370. [Google Scholar] [CrossRef]
- Berg, E.L.; Robinson, M.K.; Warnock, R.A.; Butcher, E.C. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J. Cell. Biol. 1991, 114, 343–349. [Google Scholar] [CrossRef]
- Rosen, S.D. Ligands for L-Selectin: Homing, Inflammation, and Beyond. Ann. Rev. Immunol. 2004, 22, 129–156. [Google Scholar] [CrossRef]
- Teng, T.-S.; Ji, A.-l.; Ji, X.-Y.; Li, Y.-Z. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Wang, X.; Hossain, M.; Bogoslowski, A.; Kubes, P.; Irimia, D. Chemotaxing neutrophils enter alternate branches at capillary bifurcations. Nat. Commun. 2020, 11, 2385. [Google Scholar] [CrossRef]
- de Castro Pinho, J.; Förster, R. Lymph-Derived Neutrophils Primarily Locate to the Subcapsular and Medullary Sinuses in Resting and Inflamed Lymph Nodes. Cells 2021, 10, 1486. [Google Scholar] [CrossRef]
- Kastenmüller, W.; Torabi-Parizi, P.; Subramanian, N.; Lämmermann, T.; Germain, R.N. A Spatially-Organized Multicellular Innate Immune Response in Lymph Nodes Limits Systemic Pathogen Spread. Cell 2012, 150, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Hollmén, M.; Dermadi, D.; Pan, J.; Brulois, K.F.; Kaukonen, R.; Lönnberg, T.; Boström, P.; Koskivuo, I.; Irjala, H.; et al. Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity 2019, 51, 561–572.e565. [Google Scholar] [CrossRef]
- Appelberg, R. Neutrophils and intracellular pathogens: Beyond phagocytosis and killing. Trends Microbiol. 2007, 15, 87–92. [Google Scholar] [CrossRef]
- Coombes, J.L.; Charsar, B.A.; Han, S.-J.; Halkias, J.; Chan, S.W.; Koshy, A.A.; Striepen, B.; Robey, E.A. Motile invaded neutrophils in the small intestine of Toxoplasma gondii-infected mice reveal a potential mechanism for parasite spread. Proc. Natl. Acad. Sci. USA 2013, 110, E1913–E1922. [Google Scholar] [CrossRef]
- Bonneau, M.; Epardaud, M.; Payot, F.; Niborski, V.; Thoulouze, M.-I.; Bernex, F.; Charley, B.; Riffault, S.; Guilloteau, L.A.; Schwartz-Cornil, I. Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J. Leukoc. Biol. 2006, 79, 268–276. [Google Scholar] [CrossRef]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In Vivo Imaging Reveals an Essential Role for Neutrophils in Leishmaniasis Transmitted by Sand Flies. Science 2008, 321, 970–974. [Google Scholar] [CrossRef]
- Gresham, H.D.; Lowrance, J.H.; Caver, T.E.; Wilson, B.S.; Cheung, A.L.; Lindberg, F.P. Survival of Staphylococcus aureus Inside Neutrophils Contributes to Infection. J. Immunol. 2000, 164, 3713–3722. [Google Scholar] [CrossRef]
- Denkers, E.K.; Tacchini-Cottier, F.; Van Zandbergen, G. Understanding Neutrophil Function During Toxoplasma gondii Infection. In Neutrophils in Infectious Diseases; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 59–66. [Google Scholar]
- Neeland, M.R.; Shi, W.; Collignon, C.; Taubenheim, N.; Meeusen, E.N.T.; Didierlaurent, A.M.; de Veer, M.J. The Lymphatic Immune Response Induced by the Adjuvant AS01: A Comparison of Intramuscular and Subcutaneous Immunization Routes. J. Immunol. 2016, 197, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Maletto, B.A.; Ropolo, A.S.; Alignani, D.O.; Liscovsky, M.V.; Ranocchia, R.P.; Moron, V.G.; Pistoresi-Palencia, M.a.C. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood 2006, 108, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- Chtanova, T.; Schaeffer, M.; Han, S.-J.; van Dooren, G.G.; Nollmann, M.; Herzmark, P.; Chan, S.W.; Satija, H.; Camfield, K.; Aaron, H.; et al. Dynamics of Neutrophil Migration in Lymph Nodes during Infection. Immunity 2008, 29, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Blomgran, R.; Ernst, J.D. Lung Neutrophils Facilitate Activation of Naive Antigen-Specific CD4 + T Cells during Mycobacterium tuberculosis Infection. J. Immunol. 2011, 186, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Fites, J.S.; Gui, M.; Kernien, J.F.; Negoro, P.; Dagher, Z.; Sykes, D.B.; Nett, J.E.; Mansour, M.K.; Klein, B.S. An unappreciated role for neutrophil-DC hybrids in immunity to invasive fungal infections. PLoS Pathog. 2018, 14, e1007073. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, H.; Geng, S.; Lu, R.; Okamoto, T.; Yao, Y.; Mayuzumi, N.; Kotol, P.F.; Chojnacki, B.J.; Miyazaki, T.; Gallo, R.L.; et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 2013, 121, 1677–1689. [Google Scholar] [CrossRef]
- Geng, S.; Matsushima, H.; Okamoto, T.; Yao, Y.; Lu, R.; Page, K.; Blumenthal, R.M.; Ward, N.L.; Miyazaki, T.; Takashima, A. Emergence, origin, and function of neutrophil–dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood 2013, 121, 1690–1700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Michaeli, J.; Shaul, M.E.; Mishalian, I.; Hovav, A.-H.; Levy, L.; Zolotriov, L.; Granot, Z.; Fridlender, Z.G. Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology 2017, 6, e1356965. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Ann. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Beauvillain, C.; Delneste, Y.; Scotet, M.; Peres, A.; Gascan, H.; Guermonprez, P.; Barnaba, V.; Jeannin, P. Neutrophils efficiently cross-prime naive T cells in vivo. Blood 2007, 110, 2965–2973. [Google Scholar] [CrossRef]
- Nadkarni, S.; Smith, J.; Sferruzzi-Perri, A.N.; Ledwozyw, A.; Kishore, M.; Haas, R.; Mauro, C.; Williams, D.J.; Farsky, S.H.P.; Marelli-Berg, F.M.; et al. Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc. Natl. Acad. Sci. USA 2016, 113, E8415–E8424. [Google Scholar] [CrossRef]
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cell. Mol. Life Sci. 2013, 70, 3813–3827. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, R.; Bertolotto, M.; Barisione, G.; Astigiano, S.; Mandruzzato, S.; Ottonello, L.; Dallegri, F.; Bronte, V.; Ferrini, S.; Barbieri, O. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J. Leukoc. Biol. 2011, 89, 721–727. [Google Scholar] [CrossRef]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.-W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Langereis, J.D.; Pickkers, P.; de Kleijn, S.; Gerretsen, J.; de Jonge, M.I.; Kox, M. Spleen-derived IFN-γ induces generation of PD-L1 + -suppressive neutrophils during endotoxemia. J. Leukoc. Biol. 2017, 102, 1401–1409. [Google Scholar] [CrossRef]
- de Kleijn, S.; Langereis, J.D.; Leentjens, J.; Kox, M.; Netea, M.G.; Koenderman, L.; Ferwerda, G.; Pickkers, P.; Hermans, P.W.M. IFN-γ-Stimulated Neutrophils Suppress Lymphocyte Proliferation through Expression of PD-L1. PLoS ONE 2013, 8, e72249. [Google Scholar] [CrossRef]
- Costa, S.; Bevilacqua, D.; Cassatella, M.A.; Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 2019, 156, 23–32. [Google Scholar] [CrossRef]
- Scapini, P.; Nardelli, B.; Nadali, G.; Calzetti, F.; Pizzolo, G.; Montecucco, C.; Cassatella, M.A. G-CSF–stimulated Neutrophils Are a Prominent Source of Functional BLyS. J. Exp. Med. 2003, 197, 297–302. [Google Scholar] [CrossRef]
- Huard, B.; McKee, T.; Bosshard, C.; Durual, S.; Matthes, T.; Myit, S.; Donze, O.; Frossard, C.; Chizzolini, C.; Favre, C.; et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Investig. 2008, 118, 2887–2895. [Google Scholar] [CrossRef]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell–helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2012, 13, 170–180. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun. Signal. 2019, 17, 147. [Google Scholar] [CrossRef]
- Gestermann, N.; Di Domizio, J.; Lande, R.; Demaria, O.; Frasca, L.; Feldmeyer, L.; Di Lucca, J.; Gilliet, M. Netting Neutrophils Activate Autoreactive B Cells in Lupus. J. Immunol. 2018, 200, 3364–3371. [Google Scholar] [CrossRef]
- Christiansen, A.; Detmar, M. Lymphangiogenesis and Cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef]
- Bálint, L.; Jakus, Z. Mechanosensation and Mechanotransduction by Lymphatic Endothelial Cells Act as Important Regulators of Lymphatic Development and Function. Int. J. Mol. Sci. 2021, 22, 3955. [Google Scholar] [CrossRef] [PubMed]
- Coso, S.; Bovay, E.; Petrova, T.V. Pressing the right buttons: Signaling in lymphangiogenesis. Blood 2014, 123, 2614–2624. [Google Scholar] [CrossRef]
- Secker, G.A.; Harvey, N.L. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev. Dyn. 2015, 244, 323–331. [Google Scholar] [CrossRef]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Ullmann, S.; Proulx, S.T.; Pytowski, B.; Alitalo, K.; Detmar, M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J. Exp. Med. 2010, 207, 2255–2269. [Google Scholar] [CrossRef]
- Kajiya, K.; Detmar, M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J. Investig. Dermatol. 2006, 126, 919–921. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, S.; Correale, C.; Tacconi, C.; Gandelli, A.; Pietrogrande, G.; Vetrano, S.; Genua, M.; Arena, V.; Spinelli, A.; Peyrin-Biroulet, L.; et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J. Clin. Investig. 2014, 124, 3863–3878. [Google Scholar] [CrossRef]
- Jurisic, G.; Sundberg, J.P.; Detmar, M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm. Bowel Dis. 2013, 19, 1983–1989. [Google Scholar] [CrossRef]
- Guo, R.; Zhou, Q.; Proulx, S.T.; Wood, R.; Ji, R.C.; Ritchlin, C.T.; Pytowski, B.; Zhu, Z.; Wang, Y.J.; Schwarz, E.M.; et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009, 60, 2666–2676. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and Tolerance in Corneal Transplantation. J. Immunol. 2016, 196, 3983–3991. [Google Scholar] [CrossRef]
- Hou, Y.; Bock, F.; Hos, D.; Cursiefen, C. Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival. Cells 2021, 10, 1661. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Immune cells and angiogenesis. J. Cell. Mol. Med. 2009, 13, 2822–2833. [Google Scholar] [CrossRef]
- Veikkola, T.; Jussila, L.; Makinen, T.; Karpanen, T.; Jeltsch, M.; Petrova, T.V.; Kubo, H.; Thurston, G.; McDonald, D.M.; Achen, M.G.; et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. Embo J. 2001, 20, 1223–1231. [Google Scholar] [CrossRef]
- Baldwin, M.E.; Halford, M.M.; Roufail, S.; Williams, R.A.; Hibbs, M.L.; Grail, D.; Kubo, H.; Stacker, S.A.; Achen, M.G. Vascular Endothelial Growth Factor D Is Dispensable for Development of the Lymphatic System. Mol. Cell. Biol. 2005, 25, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Houck, K.A.; Leung, D.W.; Rowland, A.M.; Winer, J.; Ferrara, N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 1992, 267, 26031–26037. [Google Scholar] [CrossRef]
- Poltorak, Z.; Cohen, T.; Sivan, R.; Kandelis, Y.; Spira, G.; Vlodavsky, I.; Keshet, E.; Neufeld, G. VEGF145, a Secreted Vascular Endothelial Growth Factor Isoform That Binds to Extracellular Matrix. J. Biol. Chem. 1997, 272, 7151–7158. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Okuda, K.S.; Misa, J.P.; Oehlers, S.H.; Hall, C.J.; Ellett, F.; Alasmari, S.; Lieschke, G.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. A zebrafish model of inflammatory lymphangiogenesis. Biol. Open 2015, 4, 1270–1280. [Google Scholar] [CrossRef]
- Sano, M.; Sasaki, T.; Hirakawa, S.; Sakabe, J.; Ogawa, M.; Baba, S.; Zaima, N.; Tanaka, H.; Inuzuka, K.; Yamamoto, N.; et al. Lymphangiogenesis and Angiogenesis in Abdominal Aortic Aneurysm. PLoS ONE 2014, 9, e89830. [Google Scholar] [CrossRef]
- Ji, R.C. Lymph node lymphangiogenesis: A new concept for modulating tumor metastasis and inflammatory process. Histol. Histopathol. 2009, 24, 377–384. [Google Scholar]
- Shrestha, B.; Hashiguchi, T.; Ito, T.; Miura, N.; Takenouchi, K.; Oyama, Y.; Kawahara, K.-i.; Tancharoen, S.; Ki-i, Y.; Arimura, N.; et al. B Cell-Derived Vascular Endothelial Growth Factor A Promotes Lymphangiogenesis and High Endothelial Venule Expansion in Lymph Nodes. J. Immunol. 2010, 184, 4819–4826. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef]
- Chyou, S.; Ekland, E.H.; Carpenter, A.C.; Tzeng, T.-C.J.; Tian, S.; Michaud, M.; Madri, J.A.; Lu, T.T. Fibroblast-Type Reticular Stromal Cells Regulate the Lymph Node Vasculature. J. Immunol. 2008, 181, 3887–3896. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Claesson-Welsh, L. Signal Transduction by Vascular Endothelial Growth Factor Receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef]
- Hampton, H.R.; Chtanova, T. The lymph node neutrophil. Semin. Immunol. 2016, 28, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hampton, H.R.; Chtanova, T. Lymphatic Migration of Immune Cells. Front. Immunol. 2019, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Bogoslowski, A.; Kubes, P. Lymph Nodes: The Unrecognized Barrier against Pathogens. ACS Infect. Dis. 2018, 4, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.; Perrin, H.; Abadie, V.; Benhabiles, N.; Boissonnas, A.; Liard, C.; Descours, B.; Reboulleau, D.; Bonduelle, O.; Verrier, B.; et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity 2012, 37, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.; Gonzalez, A. Depletion of Neutrophils Promotes the Resolution of Pulmonary Inflammation and Fibrosis in Mice Infected with Paracoccidioides brasiliensis. PLoS ONE 2016, 11, e0163985. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Arseneau, K.; Cominelli, F. Targeting Leukocyte Trafficking for the Treatment of Inflammatory Bowel Disease. Clin. Pharm. 2015, 97, 22–28. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Kröber, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006, 12, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of Hemangiogenesis and Lymphangiogenesis after Normal-Risk Corneal Transplantation by Neutralizing VEGF Promotes Graft Survival. Investig. Opthalmology Vis. Sci. 2004, 45, 2666. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, K.; Monzon-Medina, M.E.; Padera, R.F.; Wang, H.; George, G.; Toprak, D.; Abdelnour, E.; D’Agostino, E.; Goldberg, H.J.; et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J. Clin. Investig. 2015, 125, 4255–4268. [Google Scholar] [CrossRef]
- Kajiya, K.; Hirakawa, S.; Detmar, M. Vascular Endothelial Growth Factor-A Mediates Ultraviolet B-Induced Impairment of Lymphatic Vessel Function. Am. J. Pathol. 2006, 169, 1496–1503. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovija, A.; Chtanova, T. Neutrophil Interactions with the Lymphatic System. Cells 2021, 10, 2106. https://doi.org/10.3390/cells10082106
Jakovija A, Chtanova T. Neutrophil Interactions with the Lymphatic System. Cells. 2021; 10(8):2106. https://doi.org/10.3390/cells10082106
Chicago/Turabian StyleJakovija, Arnolda, and Tatyana Chtanova. 2021. "Neutrophil Interactions with the Lymphatic System" Cells 10, no. 8: 2106. https://doi.org/10.3390/cells10082106
APA StyleJakovija, A., & Chtanova, T. (2021). Neutrophil Interactions with the Lymphatic System. Cells, 10(8), 2106. https://doi.org/10.3390/cells10082106




