Human Platelet Mitochondrial Function Reflects Systemic Mitochondrial Alterations: A Protocol for Application in Field Studies
Abstract
:1. Introduction
2. Materials and Methods: A Guideline for HRR in PLTs
2.1. Sampling and Cell Purification
2.2. High-Resolution Respirometry
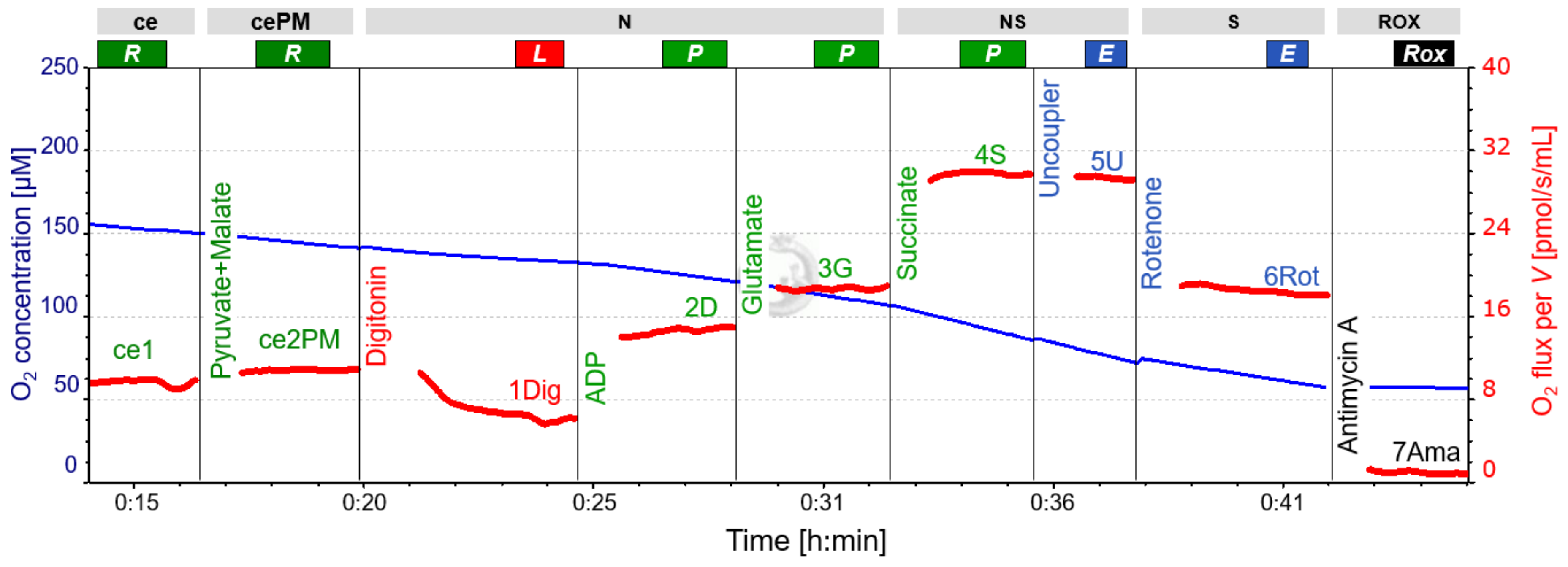
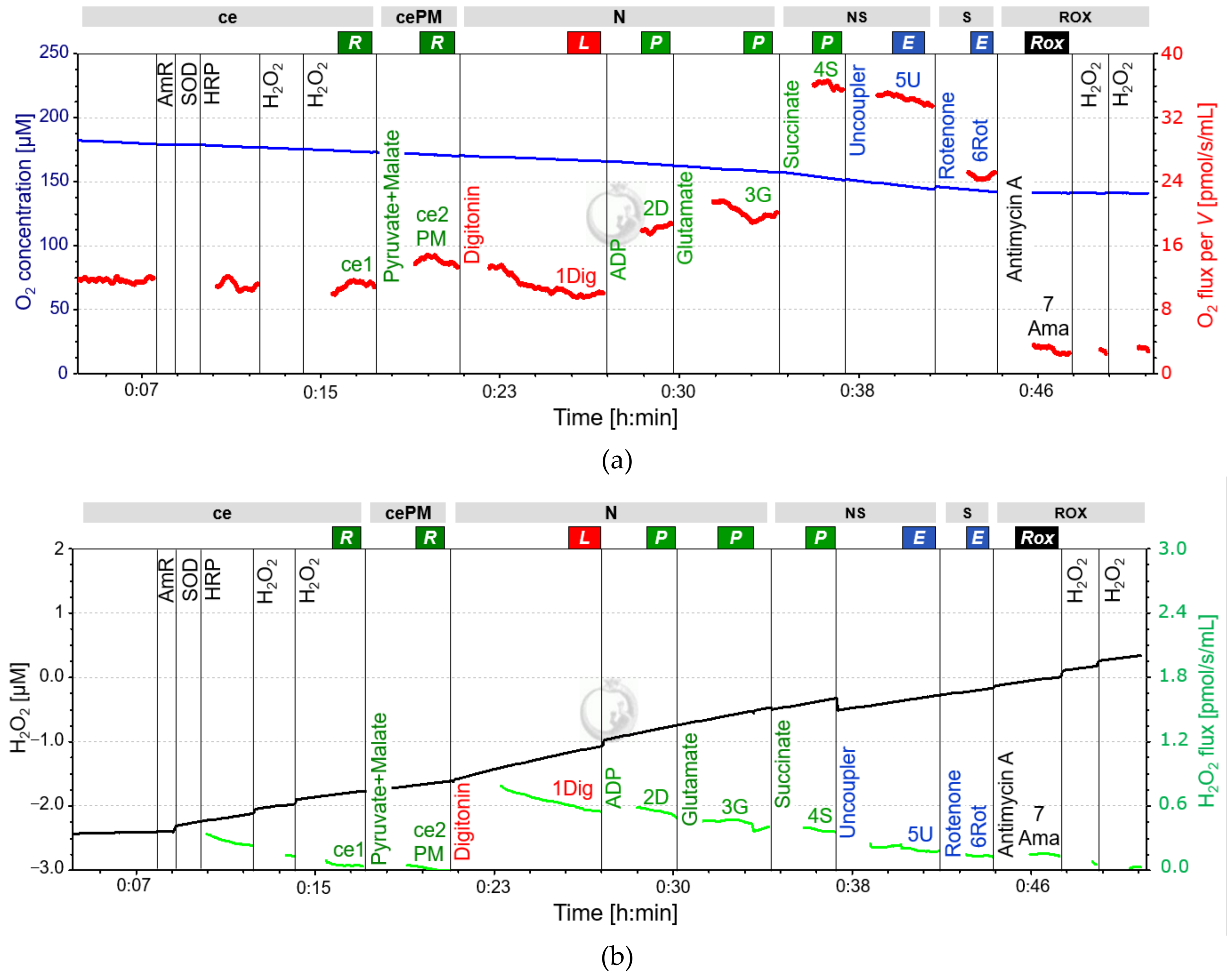
2.3. SUIT-Protocol for Platelets
3. Experimental Reproducibility: From Lab to Field
3.1. Methods and Study Design
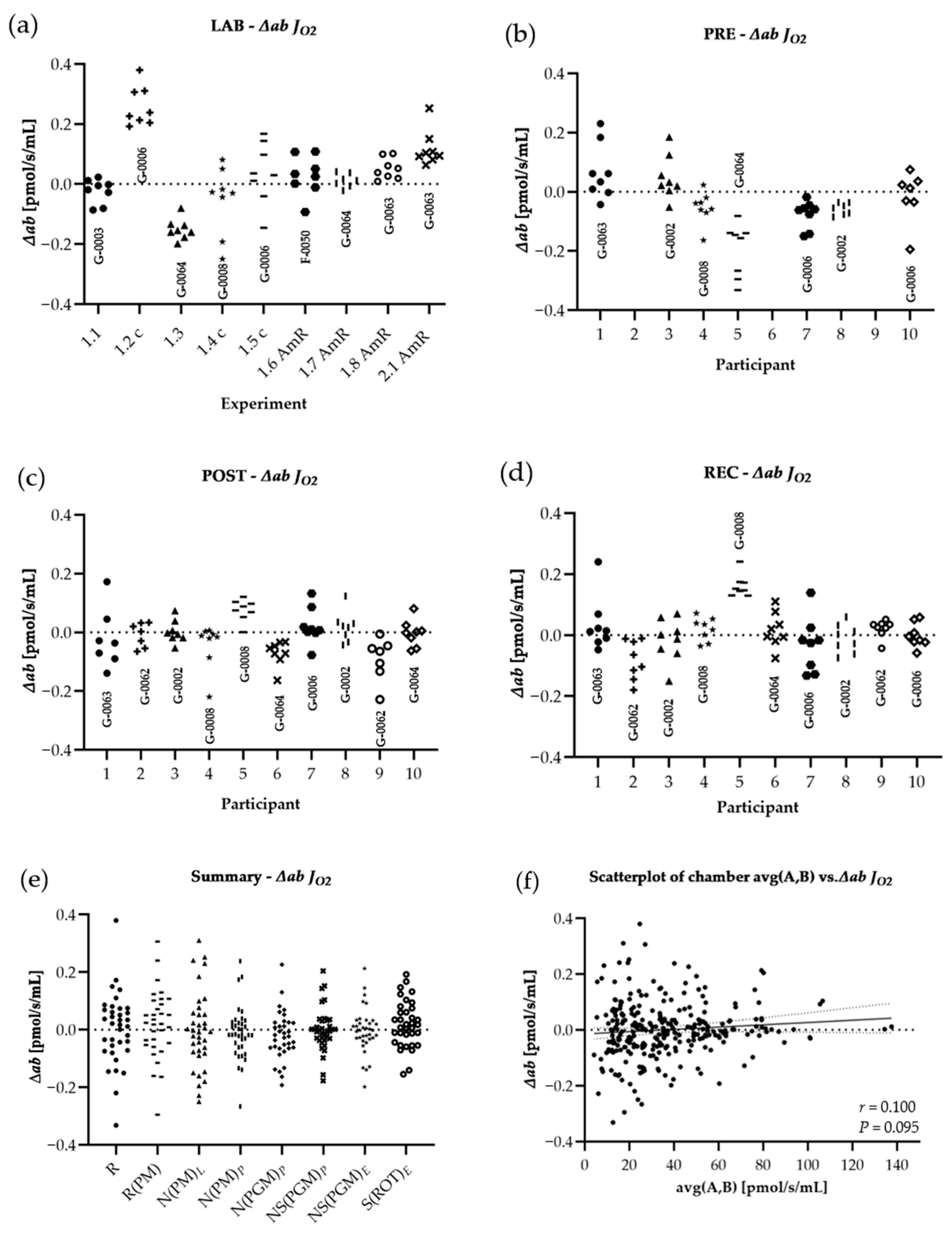
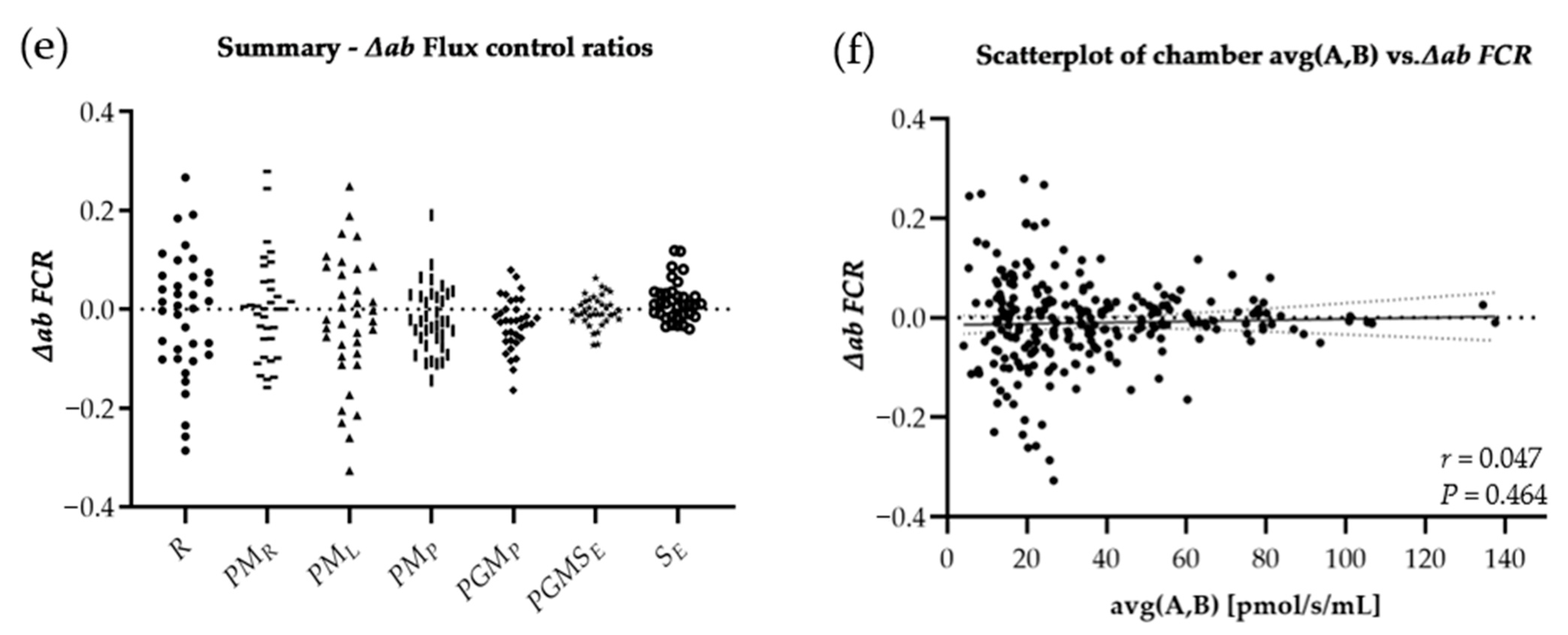
3.2. Statistics
3.3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| O2 Background a° | O2 Background b° | |||
|---|---|---|---|---|
| O2k Serial Number | A | B | A | B |
| LAB | ||||
| G-0003 | −2.8046 | −2.0394 | 0.0287 | 0.0275 |
| G-0006 | −2.2446 | −2.9382 | 0.0295 | 0.0311 |
| G-0064 | −2.0428 | −1.8178 | 0.0249 | 0.0273 |
| G-0008 | −2.3598 | −2.7908 | 0.0301 | 0.0334 |
| G-0006 | −2.2446 | −2.9382 | 0.0295 | 0.0311 |
| F-0050 * | −2.0000 | −2.0000 | 0.0250 | 0.0250 |
| G-0064 | −2.4825 | −2.0258 | 0.0305 | 0.0275 |
| G-0063 | −1.6941 | −2.2292 | 0.0273 | 0.0296 |
| G-0063 | −2.4168 | −2.4971 | 0.0327 | 0.0317 |
| PRE, POST, REC | ||||
| G-0002 | −2.5226 | −3.0570 | 0.0289 | 0.0313 |
| G-0006 | −2.0208 | −1.7848 | 0.0318 | 0.0309 |
| G-0008 | −2.7778 | −2.1008 | 0.0263 | 0.0263 |
| G-0062 | −2.0303 | −1.5078 | 0.0267 | 0.0250 |
| G-0063 | −1.6941 | −2.2292 | 0.0273 | 0.0296 |
| G-0064 | −2.4825 | −2.0258 | 0.0305 | 0.0275 |
| R1 | J°O2 POS | Experiment | |||
|---|---|---|---|---|---|
| O2k Serial Number | A | B | A | B | |
| LAB | |||||
| G-0003 | 2.0851 | 2.2999 | 2.70 | 2.95 | Exp. 1.1 |
| G-0006 | 1.9956 | 2.0786 | 2.57 | 2.67 | Exp. 1.2 |
| G-0064 | 1.8829 | 1.8695 | 2.45 | 2.41 | Exp. 1.3 |
| G-0008 | 2.0124 | 2.1300 | 2.57 | 2.74 | Exp. 1.4 |
| G-0006 | 1.9900 | 2.0883 | 2.56 | 2.68 | Exp. 1.5 |
| F-0050 | 2.0575 | 1.9936 | 2.65 | 2.57 | Exp. 1.6 |
| G-0064 | 1.9910 | 2.0127 | 2.58 | 2.59 | Exp. 1.7 |
| G-0063 | 2.1835 | 1.8911 | 2.81 | 2.43 | Exp. 1.8 |
| G-0063 | 2.1778 | 1.8760 | 2.79 | 2.40 | Exp. 2.1 |
| PRE | |||||
| G-0002 | 1.9937 | 1.9171 | 2.57 | 2.47 | Part. 3, 8 |
| G-0006 | 2.0233 | 2.0927 | 2.60 | 2.69 | Part. 7, 10 |
| G-0008 | 2.0502 | 1.9348 | 2.64 | 2.49 | Part. 4 |
| G-0062 | 2.0845 | 2.3000 | 2.70 | 2.95 | Part. 2, 9 |
| G-0063 | 2.1757 | 1.9804 | 2.80 | 2.54 | Part. 1 |
| G-0064 | 2.0203 | 2.0680 | 2.61 | 2.67 | Part. 5,6 |
| POST | |||||
| G-0002 | 1.9926 | 1.9679 | 2.57 | 2.53 | Part. 3, 8 |
| G-0006 | 2.0280 | 2.0724 | 2.61 | 2.66 | Part. 7 |
| G-0008 | 2.0410 | 1.9473 | 2.62 | 2.51 | Part. 4, 5 |
| G-0062 | 2.0961 | 2.3069 | 2.71 | 2.96 | Part. 2, 9 |
| G-0063 | 2.2393 | 1.9716 | 2.88 | 2.53 | Part. 1 |
| G-0064 | 2.0203 | 2.0680 | 2.61 | 2.67 | Part. 6, 10 |
| REC | |||||
| G-0002 | 1.9755 | 1.9808 | 2.55 | 2.55 | Part. 3, 8 |
| G-0006 | 2.0087 | 2.0628 | 2.58 | 2.65 | Part. 7, 10 |
| G-0008 | 2.0339 | 1.9403 | 2.61 | 2.50 | Part. 4, 5 |
| G-0062 | 2.0826 | 2.2991 | 2.69 | 2.95 | Part. 2, 9 |
| G-0063 | 2.2352 | 1.9420 | 2.87 | 2.49 | Part. 1 |
| G-0064 | 2.0312 | 2.0677 | 2.63 | 2.67 | Part. 6 |
References
- Doerrier, C.; Garcia-Souza, L.; Krumschnabel, G.; Wohlfarter, Y.; Mészáros, A.; Gnaiger, E. High-Resolution FluoRespirometry and OXPHOS Protocols for Human Cells, Permeabilized Fibers from Small Biopsies of Muscle, and Isolated Mitochondria. Methods Mol. Biol. 2018, 1782, 31–70. [Google Scholar] [CrossRef]
- Gnaiger, E.; Aasander Frostner, E.; Abdul Karim, N.; Abumrad, N.; Acuna-Castroviejo, D.; Adiele, R.C.; Ahn, B.; Ali, S.S.; Alton, L.; Alves, M.G.; et al. Mitochondrial respiratory states and rates. MitoFit Prepr. Arch. 2019, 1–40. [Google Scholar] [CrossRef]
- Distefano, G.; Standley, R.; Zhang, X.; Carnero, E.; Yi, F.; Cornnell, H.; Coen, P. Physical activityunveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J. Cachexia Sarcopenia Muscle 2018, 9, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Bakkman, L.; Fernström, M.; Loogna, P.; Rooyackers, O.; Brandt, L.; Lagerros, Y. Reduced respiratory capacity in muscle mitochondria of obese subjects. Obes. Facts 2010, 3, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P.; Jacobs, R.; Bonne, T.; Flück, D.; Bangsbo, J.; Lundby, C. A short period of high-intensity interval training improves skeletal muscle mitochondrial function and pulmonary oxygen uptake kinetics. J. Appl. Physiol. 2016, 120, 1319–1327. [Google Scholar] [CrossRef] [Green Version]
- Porter, C.; Reidy, P.; Bhattarai, N.; Sidossis, L.; Rasmussen, B. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef] [Green Version]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [CrossRef]
- Pesta, D.; Hoppel, F.; Macek, C.; Messner, H.; Faulhaber, M.; Kobel, C.; Parson, W.; Burtscher, M.; Schocke, M.; Gnaiger, E. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 1078–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boushel, R.; Lundby, C.; Qvortrup, R.; Sahlin, K. Mitochondrial Plasticity with Exercise Training and Extreme Environments. Exerc. Sport Sci. Rev. 2014, 42, 169–174. [Google Scholar] [CrossRef]
- Larsen, S.; Hey-Mogensen, M.; Rabol, R.; Stride, N.; Helge, J.; Dela, F. The influence of age and aerobic fitness: Effects of mitochondrial respiration on skeletal muscle. Acta Physiol. 2012, 205, 423–432. [Google Scholar] [CrossRef]
- Sumbalova, Z.; Droescher, S.; Hiller, E.; Chang, S.; Garcia-Souza, L.; Calabria, E.; Volani, C.; Krumschnabel, G.; Gnaiger, E. O2k-Protocols: Isolation of peripheral blood mononuclear cells and platelets from human blood for HRR. MiPNet 2018, 21, 1–16. [Google Scholar]
- Tyrrell, D.J.; Bharadwaj, M.S.; Jorgensen, M.J.; Register, T.C.; Molina, A.J. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: Implications for a minimally invasive biomarker of mitochondrial health. Redox Biol. 2016, 10, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Braganza, A.; Corey, C.G.; Santanasto, A.J.; Distefano, G.; Coen, P.M.; Glynn, N.W.; Nouraie, S.M.; Goodpaster, B.H.; Newman, A.B.; Shiva, S. Platelet bioenergetics correlate with muscle energetics and are altered in older adults. JCI Insight 2019, 5, e128248. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, D.J.; Bharadwaj, M.S.; Jorgensen, M.J.; Register, T.C.; Shively, C.; Andrews, R.N.; Neth, B.; Keene, C.D.; Mintz, A.; Craft, S.; et al. Blood-Based Bioenergetic Profiling Reflects Differences in Brain Bioenergetics and Metabolism. Oxid. Med. Cell. Longev. 2017, 2017, 7317251. [Google Scholar] [CrossRef] [Green Version]
- Braganza, A.; Annarapu, G.K.; Shiva, S. Blood-based bioenergetics: An emerging translational and clinical tool. Mol. Aspects Med. 2020, 71, 100835. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. Preparation of permeabilized muscle fibres for diagnosis of mitochondrial respiratory function. MiPNet 2015, 14, 1–5. [Google Scholar]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Physiological and Pathophysiological Responses to Ultramarathon Running in Non-elite Runners. Front. Physiol. 2019, 10, 1300. [Google Scholar] [CrossRef] [Green Version]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Effects of Ultramarathon Running on Mitochondrial Function of Platelets and Oxidative Stress Parameters: A Pilot Study. Front. Physiol. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.A.; Ravi, S.; Chacko, B.; Johnson, M.S.; Darley-Usmar, V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014, 2, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchinger, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of Platelet Mitochondria: Life in a Nucleus-Free Zone. Front. Cardiovasc. Med. 2019, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer, K.; Haass, C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta 2010, 1802, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, Y.; Luo, L.; Lu, Y.; Yew, D.T.; Xu, J.; Guo, K. Platelet mitochondrial dysfunction of DM rats and DM patients. Int. J. Clin. Exp. Med. 2015, 15, 6937–6946. [Google Scholar]
- Valla, J.; Schneider, L.; Niedzielko, T.; Coon, K.; Caselli, R.; Sabbagh, M.; Ahern, G.L.; Baxter, L.; Alexander, G.; Walker, D.G.; et al. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion 2006, 6, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Carvalho, E.; Diaz, E.C.; Cotter, M.; Bennuri, S.C.; Azhar, G.; Frye, R.E.; Adams, S.H.; Børsheim, E. A comparative study of mitochondrial respiration in circulating blood cells and skeletal muscle fibers in women. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E503–E512. [Google Scholar] [CrossRef]
- Gvozdjakova, A.; Sumbalova, Z.; Kucharska, J.; Chladekova, A.; Rausova, Z.; Vancova, O.; Komlosi, M.; Ulicna, O.; Mojto, V. Platelet mitochondrial bioenergetic analysis in patients with nephropathies and non-communicable diseases: A new method. Bratisl. Lek. Listy 2019, 120, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Gvozdjáková, A.; Sumbalová, Z.; Kucharská, J.; Komlósi, M.; Rausová, Z.; Vančová, O.; Számošová, M.; Mojto, V. Platelet Mitochondrial Respiration, Endogenous Coenzyme Q10 and Oxidative Stress in Patients with Chronic Kidney Disease. Diagnostics 2020, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Winnica, D.; Corey, C.; Mullett, S.; Reynolds, M.; Hill, G.; Wendell, S.; Que, L.; Holguin, F.; Shiva, S. Bioenergetic Differences in the Airway Epithelium of Lean Versus Obese Asthmatics Are Driven by Nitric Oxide and Reflected in Circulating Platelets. Antioxid. Redox Signal. 2019, 31, 673–686. [Google Scholar] [CrossRef]
- Nguyen, Q.L.; Corey, C.; White, P.; Watson, A.; Gladwin, M.T.; Simon, M.A.; Shiva, S. Platelets from pulmonary hypertension patients show increased mitochondrial reserve capacity. JCI Insight 2017, 2, e91415. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.C.; Tsai, H.H.; Fu, T.C.; Wang, J.S. Exercise Training Enhances Platelet Mitochondrial Bioenergetics in Stroke Patients: A Randomized Controlled Trial. J. Clin. Med. 2019, 812, 2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lucas, R.; Caputo, F.; Mendes de Souza, K.; Sigwalt, A.; Ghisoni, K.; Lock Silveira, P.; Remor, A.P.; da Luz Scheffer, D.; Guglielmo, L.G.; Latini, A. Increased platelet oxidative metabolism, blood oxidative stress and neopterin levels after ultra-endurance exercise. J. Sports Sci. 2014, 32, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gatterer, H.; Menz, V.; Salazar-Martinez, E.; Sumbalova, Z.; Garcia-Souza, L.F.; Velika, B.; Gnaiger, E.; Burtscher, M. Exercise Performance, Muscle Oxygen Extraction and Blood Cell Mitochondrial Respiration after Repeated-Sprint and Sprint Interval Training in Hypoxia: A Pilot Study. J. Sports Sci. Med. 2018, 17, 339–347. [Google Scholar] [PubMed]
- Lin, M.L.; Fu, T.C.; Hsu, C.C.; Huang, S.C.; Lin, Y.T.; Wang, J.S. Cycling Exercise Training Enhances Platelet Mitochondrial Bioenergetics in Patients with Peripheral Arterial Disease: A Randomized Controlled Trial. Thromb. Haemost. 2021, 121, 900–912. [Google Scholar] [CrossRef]
- Wu, L.H.; Chang, S.C.; Fu, T.C.; Huang, C.H.; Wang, J.S. High-intensity Interval Training Improves Mitochondrial Function and Suppresses Thrombin Generation in Platelets undergoing Hypoxic Stress. Sci. Rep. 2017, 7, 4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjövall, F.; Ehlinger, J.; Mareisson, S.; Morota, S.; Frostner, E.; Uchino, H.; Lundgren, J.; Arnbjörnsson, E.; Hansson, M.J.; Fellman, V.; et al. Mitochondrial respiration in human viable platelets—Methodology and influence of gender, age and storage. Mitochondrion 2013, 13, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Fasching, M.; Gnaiger, E. O2k Quality Control 2: Instrumental oxygen background correction and accuracy of oxygen flux. MiPNet 2018, 14, 1–14. [Google Scholar]
- Lemieux, H.; Blier, P.; Gnaiger, E. Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: Electron flow through the Q-junction in permeabilized fibers. Sci. Rep. 2017, 7, 2840. [Google Scholar] [CrossRef] [Green Version]
- Fasching, M.; Fontana-Ayoub, M.; Gnaiger, E. Mitochondrial Respiration Medium–MiR06. MiPNet 2016, 14, 1–4. [Google Scholar]
- Gnaiger, E. Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. MiPNet 2014, 19, 12. [Google Scholar]
- Sahlin, K.; Shabalina, I.; Mattsson, C.; Bakkman, L.; Fernström, M.; Rozhdestvenskaya, Z.; Engyist, J.K.; Nedergaard, J.; Ekblom, B.; Tonkonogi, M. Ultraendurance exercise increases the production of reactive oxygen species in isolated mitochondria from human skeletal muscle. J. Appl. Physiol. 2010, 108, 780–787. [Google Scholar] [CrossRef] [Green Version]
- Krumschnabel, G.; Fontana-Ayoub, M.; Sumbalova, Z.; Heidler, J.; Gauper, K.; Fasching, M.; Gnaiger, E. Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol. Biol. 2015, 1264, 245–261. [Google Scholar] [CrossRef]
- Kratz, A.; Wood, M.; Siegel, A.; Hiers, J.; Van Cott, E. Effects of marathon running on platelet activation markers: Direct evidence for in vivo platelet activation. Am. J. Clin. Pathol. 2006, 125, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.P.; Pichaud, N.; Ballard, J.W. Review: Quantifying mitochondrial dysfunction in complex diseases of aging. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 1022–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heber, S.; Volf, I. Effects of Physical (In) activity on Platelet Function. BioMed Res. Int. 2015, 2015, 165078. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Materials for Blood Sampling | Provider | |
|---|---|---|
| 1 | 9 mL VACUETTE® K3EDTA tubes and 21-gauge butterfly needles (2 per participant) | Greiner Bio-One, Kremsmünster, Austria |
| 2 | Set of automatic pipettes and tips (1 × 2.5 mL, 1 × 1 mL, 1 × 2 µL) | |
| 3 | Respiratory medium (MiR05) 1 (minimum 4.5 mL for each experiment; SUIT, background exp., O2 calib.) | Oroboros Instruments, Innsbruck, Austria |
| 4 | Centrifuge with a swinging bucket rotor and slots for 50 mL and 15 mL tubes and 9 mL vials; min. 1000× g available | Hettich Rotina 35, Tuttlingen, Germany |
| 5 | Eppendorf centrifuge with slots for 1.5 mL tubes; min. 1000× g available | Eppendorf AG, Hamburg, Germany |
| 6 | Eppendorf tubes, 1.5 mL (2 tubes for each SUIT experiment) | Eppendorf AG, Hamburg, Germany |
| 7 | Thermometer | |
| 8 | 50 mL Falcon tubes (2 for each sampling/experiment) | BD Becton Dickinson GmbH, Heidelberg, Germany |
| 9 | Polystyrene box with dry ice, protected from light | |
| 10 | PBS (1 mL for each experiment) | BE 17-516F, Lonza |
| 11 | Automated Cell counter | See discussion |
| Materials for Respirometry | Provider | |
|---|---|---|
| 1 | O2k for high-resolution Fluo-respirometry including Titration-Injection micropump (TIP), ISS-Integrated Suction System (ISS) (6 instruments) | Oroboros Instruments, Innsbruck, Austria |
| 2 | Aqua distilled; 70% Ethanol; 100% Ethanol | |
| 3 | Respiratory medium (MiR05) (4.5 mL for both each O2 calibration and instrumental O2 background exp.) | Oroboros Instruments, Innsbruck, Austria |
| 4 | Chemicals for O2k assay (see Table 4) | |
| 5 | O2k titration sets (6 × 10 µL, 6 × 25 µL, 1 × 50 µL, 1 × 100 µL, 1 × 500 µL; Hamilton Syringes) (number according to number of simultaneous working researchers) | Oroboros Instruments, Innsbruck, Austria |
| 6 | O2k-Fluo LED2-Module (6 modules) 1 | Oroboros Instruments, Innsbruck, Austria |
| 7 | O2k titration set (3 × 10 µL; Hamilton syringes) (number according to number of simultaneous working researchers) | Oroboros Instruments, Innsbruck, Austria |
| 8 | Chemicals for fluorometry (see Table 4) |
| Step | Chemical | Stock Concentration | Titration Volume | Final Concentration |
|---|---|---|---|---|
| SUIT protocol for HRR | ||||
| ce1 | Sample | |||
| ce1P | Pyruvate | 2 M | 5 µL | 5 mM |
| ce2M | Malate | 400 mM | 10 µL | 2 mM |
| 1Dig | Digitonin | 50 mg/mL | 8 µL | 200 µg/mL |
| 2D | Adenosine diphosphate | 500 mM | 4 µL | 1 mM |
| 3G | Glutamate | 2 M | 5 µL | 5 mM |
| 4S | Succinate | 1 M | 20 µL | 10 mM |
| 5U 1 | Carbonyl cyanide m-chloro phenyl hydrazine | 1 nM | 1 µL/step | 0.5 µM steps |
| 6Rot | Rotenone | 1 mM | 4 µL | 2 µM |
| 7Ama | Antimycin A | 5 mM | 0.5 µL | 1.25 µM |
| Optional SUIT protocol for Fluorometry | ||||
| ce1AmR | Amplex UltraRed | 10 mM | 1 µL | 5 µM |
| ce2HRP | Horseradish Peroxidase | 500 U/mL | 4 µL | 1 U/mL |
| ce3SOD | Superoxide Dismutase | 5000 U/mL | 1 µL | 2.5 U/mL |
| perform SUIT protocol for respirometry 2 | ||||
| ce4H2O2 | Hydrogen Peroxide | 40 µM | 5 µL | 0.1 µM |
| ce5H2O2 | Hydrogen Peroxide | 40 µM | 5 µL | 0.1 µM |
| Contents of Every Single Step of the Procedure | |
|---|---|
| 1. | Pre-heat MiR05 before use in a prepared water bath at 37 °C in a Styrofoam box shielded from light. |
| 2. | Run air calibration of the O2k with MiR05 at 37 °C including a stirrer test according to Fasching & Gnaiger, 2018 [36]; keep them running in MiR05 until cells are added. |
| 3. | Collect 2 × 9 mL blood in VACUETTE® K3EDTA vials from each participant |
| 5. | Centrifuge both 9 mL K3EDTA vials at 400× g at RT for 10 min; set no brake to avoid perturbation of cell layers. |
| 6. | Pipette PRP gently until ~1 cm above cell pellet remains, transfer PRP into a 50 mL Falcon tube, store regularly for up to 10 min at 37 °C protected from light. |
| 7. | Centrifuge PRP to collect PLTs and PPP (1000× g, RT, 10 min, acceleration 9, brake 6). |
| 9. | Resuspend cell pellet (step 7) by gentle pipetting in 4.5 mL pre-heated MiR05; use 1 mL tips. |
| 10. | Determine cell concentration 1 and dilute to 200 × 106 cells per mL cells with preheated MiR05. |
| 11. | Siphon off all MiR05 from the O2k and add 2.25 mL sample to each O2k chamber, close the chambers avoiding air bubbles, and siphon off excess medium from the stopper receptacle. |
| 12. | Start data recording with DatLab. |
| 13. | After terminating the respirometric experiment, transfer subsamples of the cells suspension (1 mL) from the O2k chamber while the stirrer is running, pellet the cells in Eppendorf tubes (1000× g, RT, 10 min, brake), resuspend in 300 µL PBS, and store on dry ice in Eppendorf tubes. |
| State | Flux | After Event | FCR | JZ-Y |
|---|---|---|---|---|
| ROUTINE | R | ce1 | R | |
| ROUTINE (PM) | R(PM) | ce2PM | PMR | |
| LEAK (L) | N(PM)L | 1Dig | PML | |
| OXPHOS (P) | N(PM)P | 2D | PMP | 1-N(PM)L/N(PM)P |
| OXPHOS (P) | N(PGM)P | 3G | PGMP | 1-N(PM)P/N(PGM)P |
| OXPHOS (P) | NS(PGM)P1 | 4S | 1-N(PGM)P/NS(PGM)P | |
| ET (E) | NS(PGM)E | 5U | PGMSE | 1-NS(PGM)E/N(PGMS)p |
| ET S (E) | S(Rot)E | 6Rot | SE | 1-S(Rot)E/NS(PGM)P2 |
| Field | |||||
|---|---|---|---|---|---|
| LAB | PRE | POST | REC | Adjusted p | |
| │∆ab│ Volume specific flux | |||||
| R | 0.099 ± 0.112 | 0.066 ± 0.103 | 0.075 ± 0.058 | 0.095 ± 0.057 | 0.983 |
| R(PM) | 0.097 ± 0.095 | 0.064 ± 0.096 | 0.059 ± 0.058 | 0.080 ± 0.067 | 0.983 |
| N(PM)L | 0.149 ± 0.106 * | 0.090 ± 0.091 $ | 0.057 ± 0.069 | 0.081 ± 0.072 | 0.102 |
| N(PM)P | 0.076 ± 0.073 | 0.068 ± 0.089 | 0.041 ± 0.043 | 0.052 ± 0.053 | 0.204 |
| N(PGM)P | 0.093 ± 0.077 | 0.045 ± 0.044 | 0.045 ± 0.049 | 0.054 ± 0.046 | 0.135 |
| NS(PGM)P | 0.079 ± 0.079 | 0.034 ± 0.048 | 0.036 ± 0.033 | 0.040 ± 0.048 | 0.905 |
| NS(PGM)E | 0.080 ± 0.078 | 0.038 ± 0.044 $ | 0.040 ± 0.047 | 0.043 ± 0.051 | 0.066 |
| S(ROT)E | 0.083 ± 0.071 # | 0.053 ± 0.052 | 0.059 ± 0.040 | 0.032 ± 0.034 | 0.513 |
| ROX | 0.245 ± 0.174 | 0.311 ± 0.303 | 0.401 ± 0.429 | 0.277 ± 0.204 | 0.565 |
| │∆ab│ Flux control ratio | |||||
| R | 0.095 ± 0.084 | 0.049 ± 0.060 | 0.079 ± 0.076 | 0.136 ± 0.078 | 0.298 |
| PMR | 0.066 ± 0.053 | 0.038 ± 0.059 | 0.062 ± 0.075 | 0.094 ± 0.081 | 0.479 |
| PML | 0.124 ± 0.101 | 0.096 ± 0.104 | 0.066 ± 0.061 | 0.086 ± 0.068 | 0.368 |
| PMP | 0.056 ± 0.043 | 0.050 ± 0.061 | 0.060 ± 0.034 | 0.045 ± 0.031 | 0.565 |
| PGMP | 0.057 ± 0.052 | 0.021 ± 0.026 | 0.043 ± 0.039 | 0.049 ± 0.026 | 0.576 |
| PGMSE | 0.020 ± 0.015 | 0.013 ± 0.013 | 0.026 ± 0.024 | 0.029 ± 0.022 | 0.060 |
| SE | 0.019 ± 0.015 | 0.030 ± 0.038 | 0.035 ± 0.036 | 0.029 ± 0.024 | 0.607 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppel, F.; Garcia-Souza, L.F.; Kantner-Rumplmair, W.; Burtscher, M.; Gnaiger, E.; Pesta, D.; Calabria, E. Human Platelet Mitochondrial Function Reflects Systemic Mitochondrial Alterations: A Protocol for Application in Field Studies. Cells 2021, 10, 2088. https://doi.org/10.3390/cells10082088
Hoppel F, Garcia-Souza LF, Kantner-Rumplmair W, Burtscher M, Gnaiger E, Pesta D, Calabria E. Human Platelet Mitochondrial Function Reflects Systemic Mitochondrial Alterations: A Protocol for Application in Field Studies. Cells. 2021; 10(8):2088. https://doi.org/10.3390/cells10082088
Chicago/Turabian StyleHoppel, Florian, Luiz Felipe Garcia-Souza, Wilhelm Kantner-Rumplmair, Martin Burtscher, Erich Gnaiger, Dominik Pesta, and Elisa Calabria. 2021. "Human Platelet Mitochondrial Function Reflects Systemic Mitochondrial Alterations: A Protocol for Application in Field Studies" Cells 10, no. 8: 2088. https://doi.org/10.3390/cells10082088
APA StyleHoppel, F., Garcia-Souza, L. F., Kantner-Rumplmair, W., Burtscher, M., Gnaiger, E., Pesta, D., & Calabria, E. (2021). Human Platelet Mitochondrial Function Reflects Systemic Mitochondrial Alterations: A Protocol for Application in Field Studies. Cells, 10(8), 2088. https://doi.org/10.3390/cells10082088







