Physiological Shear Stress Enhances Differentiation, Mucus-Formation and Structural 3D Organization of Intestinal Epithelial Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
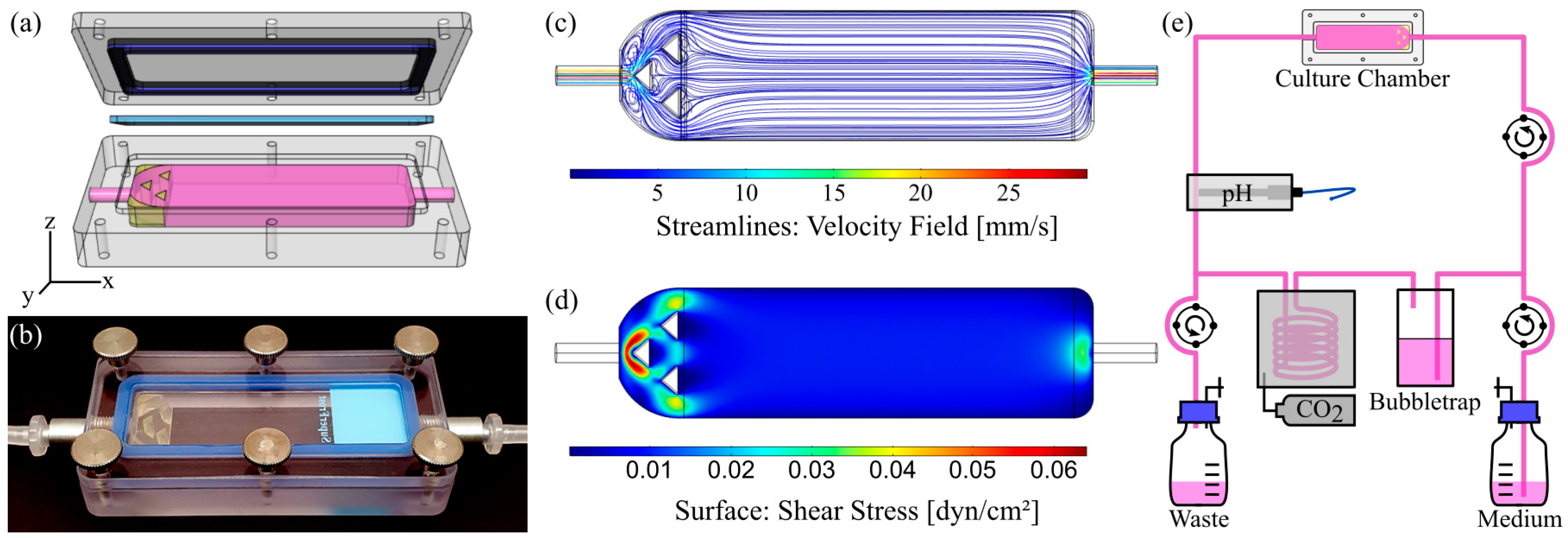
2.2. Chamber Design and Fabrication
2.2.1. Slide Chamber for Dynamic Culture on Solid Substrates
2.2.2. Insert Chamber for Dynamic Culture on Membranes
2.2.3. Computational Fluid Dynamics (CFD)
2.3. Cell Culture
2.3.1. Solid Substrates
2.3.2. Membranes
2.4. Dynamic Cell Culture
2.4.1. Bioreactor Setup for Dynamic Culture on Solid Substrates
2.4.2. Bioreactor Setup for Dynamic Culture on Membranes
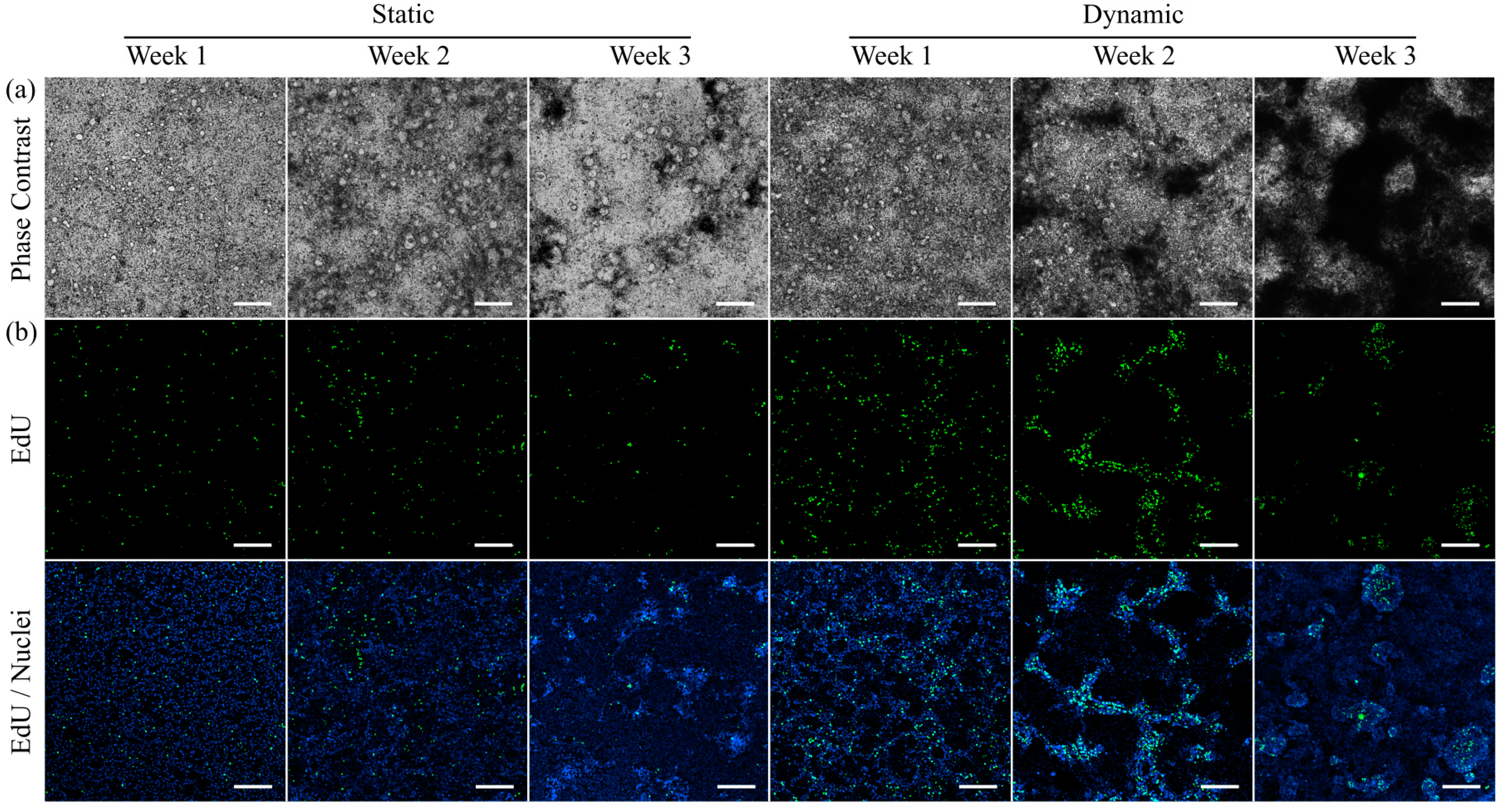
2.5. Cell Proliferation Assay
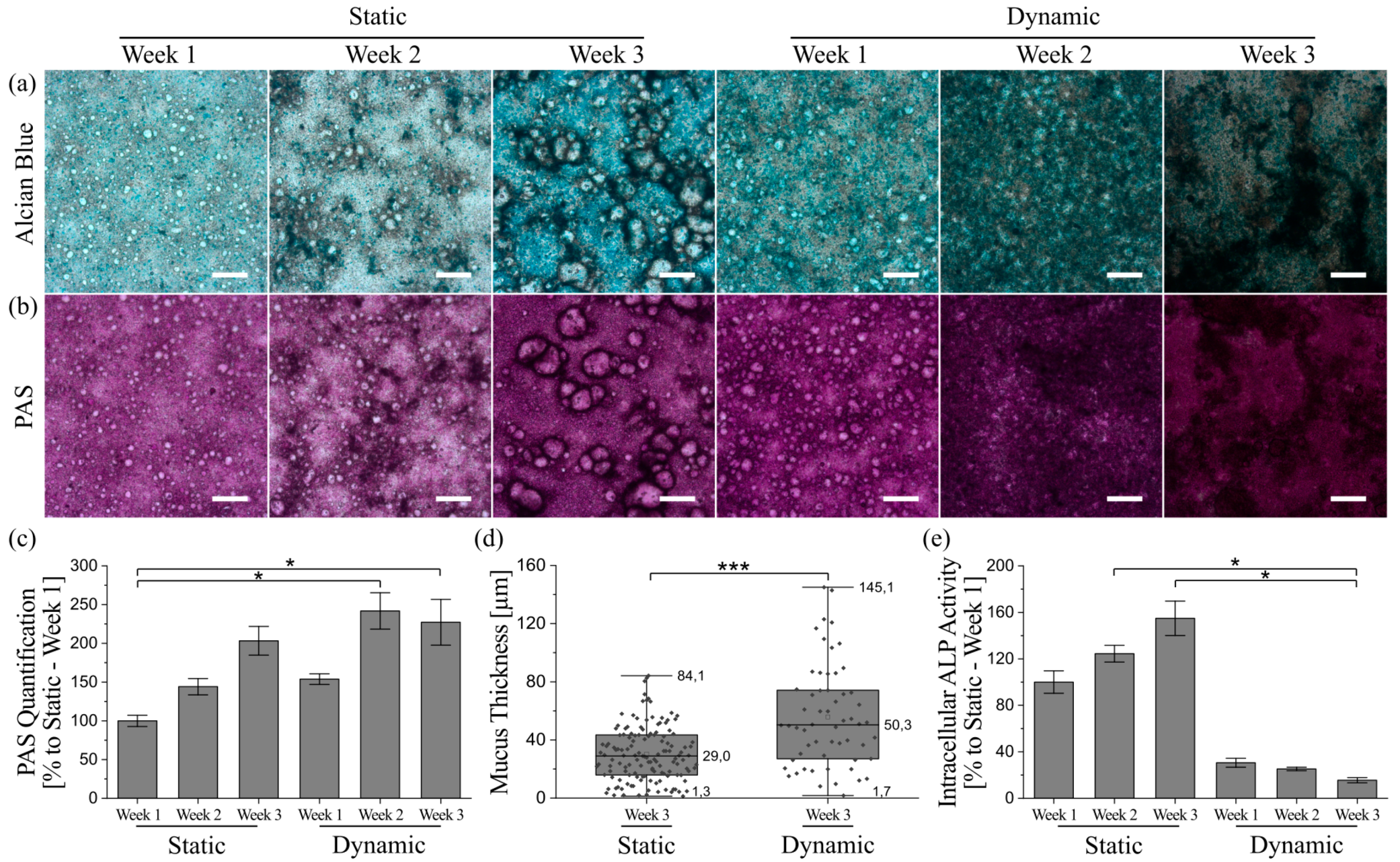
2.6. Mucus Staining and Quantification
2.7. Thickness-Determination of the Adherent Mucus Layer
2.8. Alkaline Phosphatase (ALP) Activity Assay
2.9. Immunostaining and Direct Labelling
2.10. Transepithelial Electrical Resistance (TEER)
2.11. Statistics
3. Results and Discussion
3.1. Characterization of Flow Chamber for Solid Substrates
3.2. Design of Experiments
3.3. Morphology and Proliferation of HT29-MTX Cells under Static and Dynamic Conditions
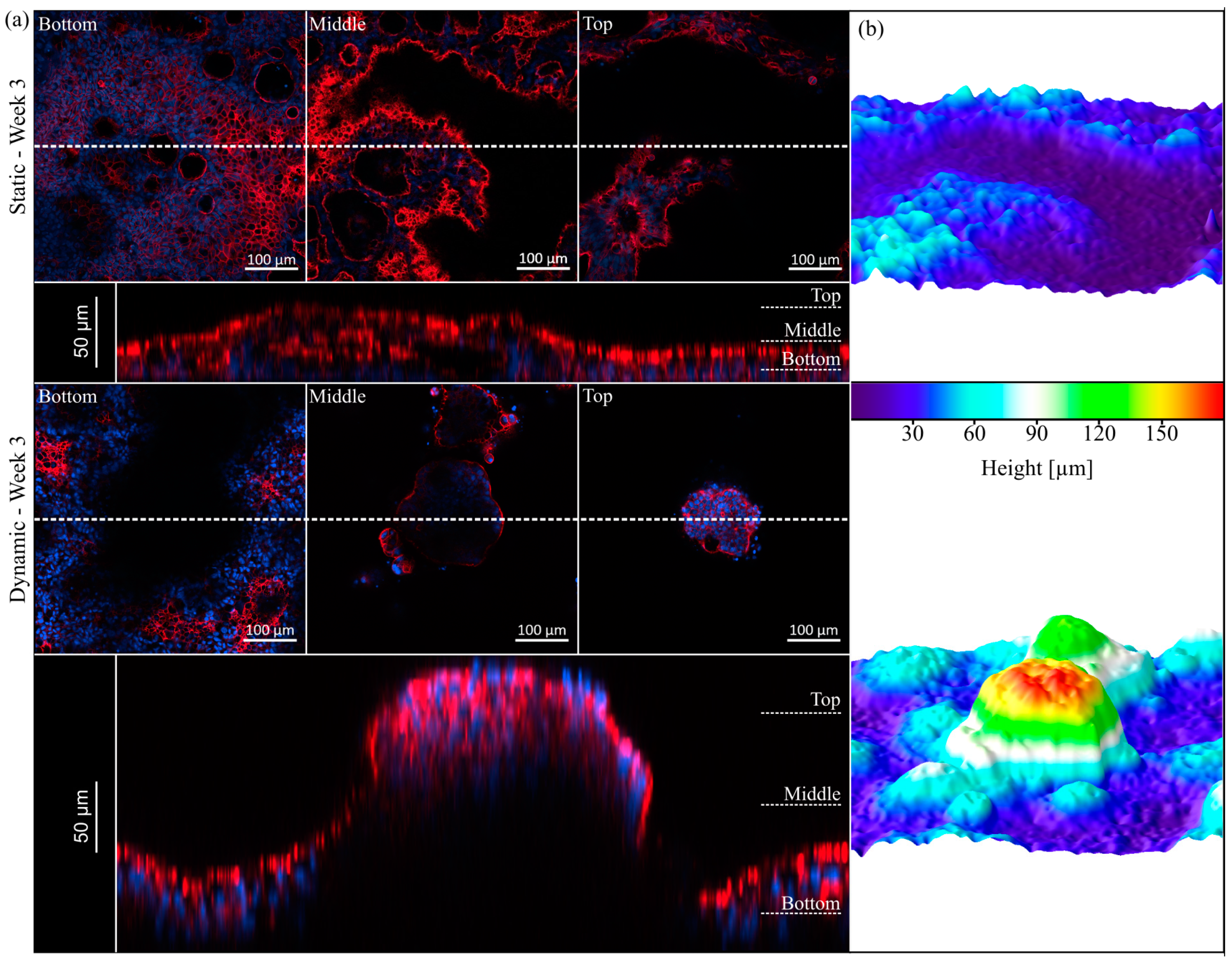
3.4. Villi Formation
3.5. Mucus Characterization
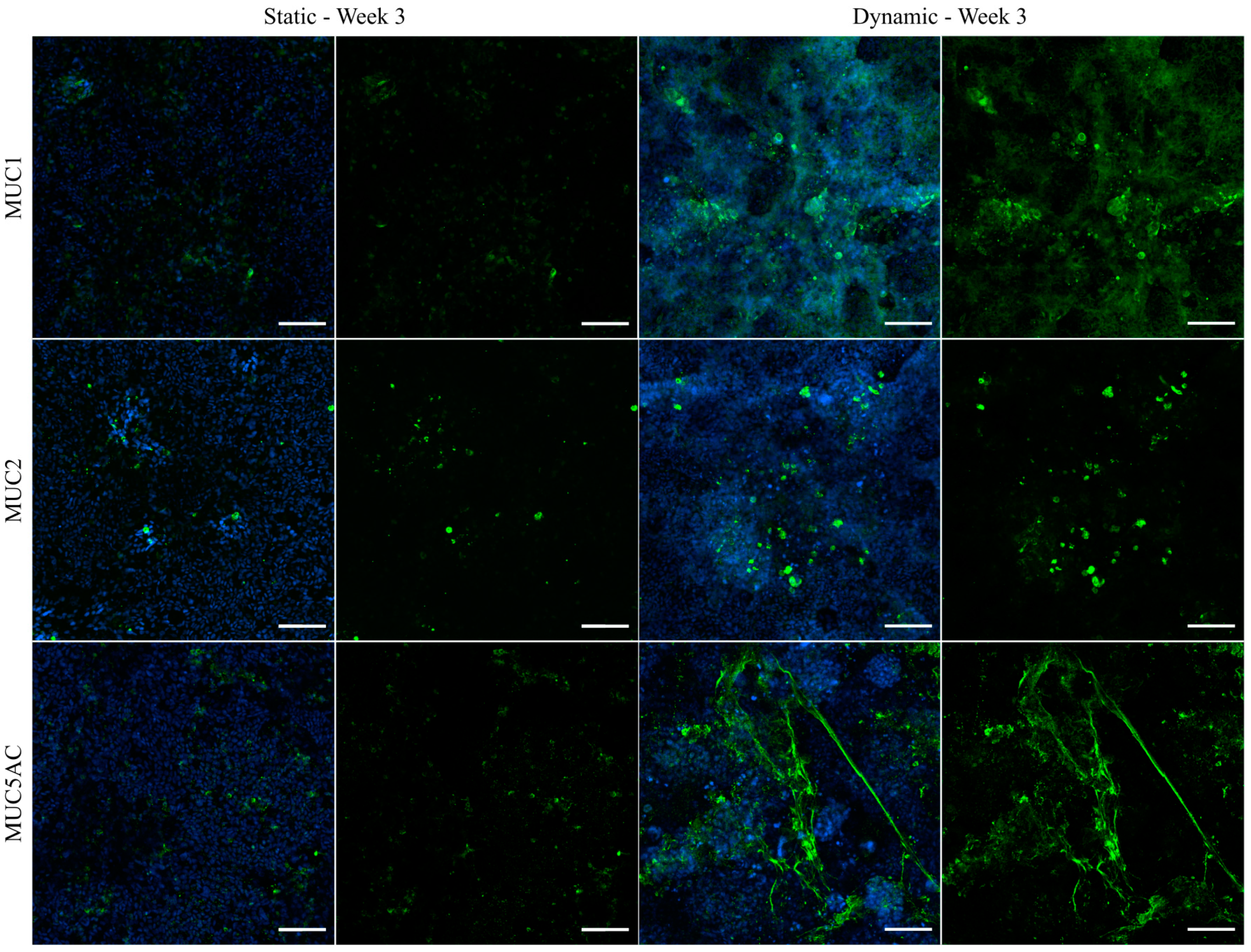
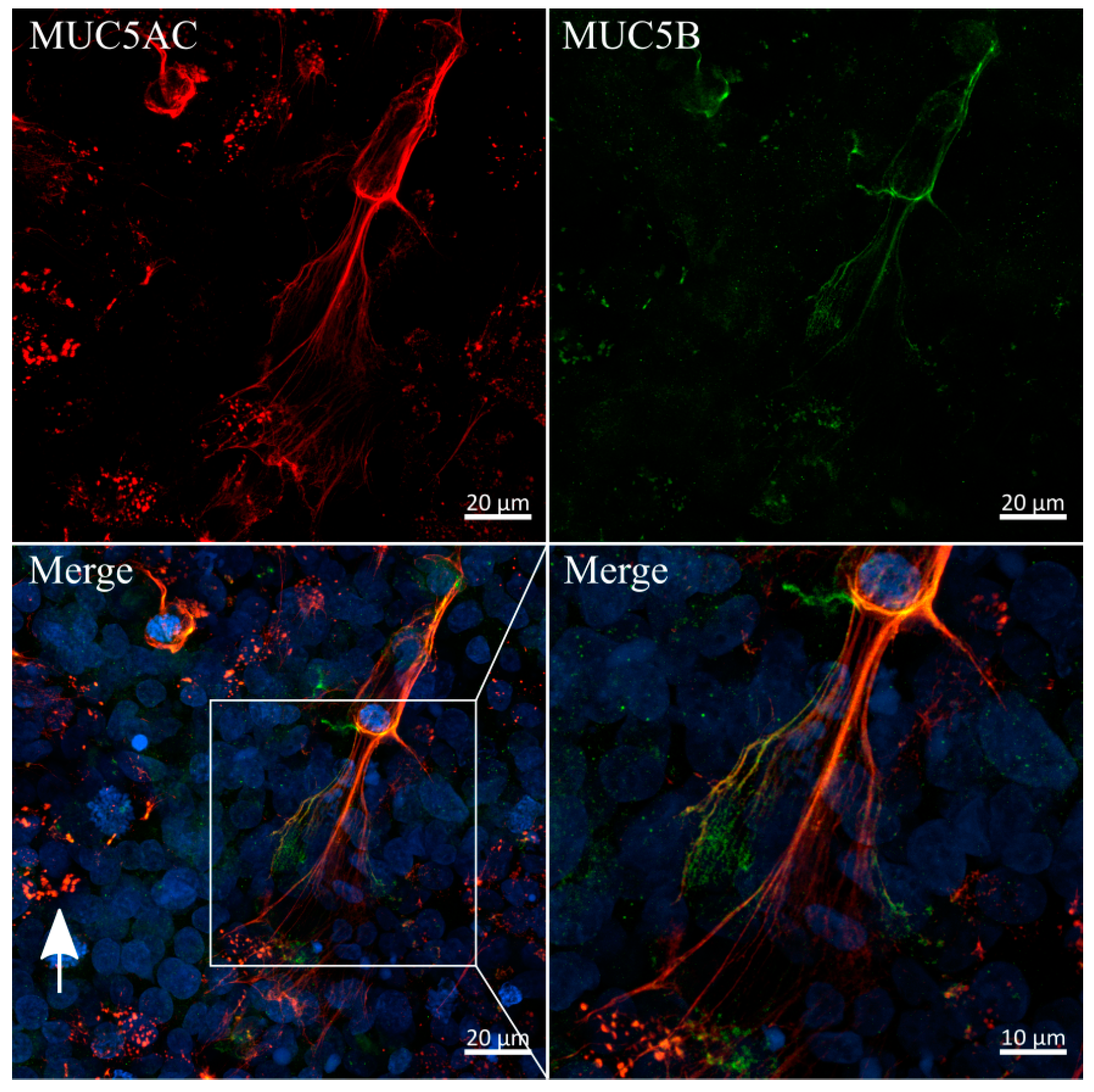
3.6. Immunofluorescent Staining of Selected Mucins
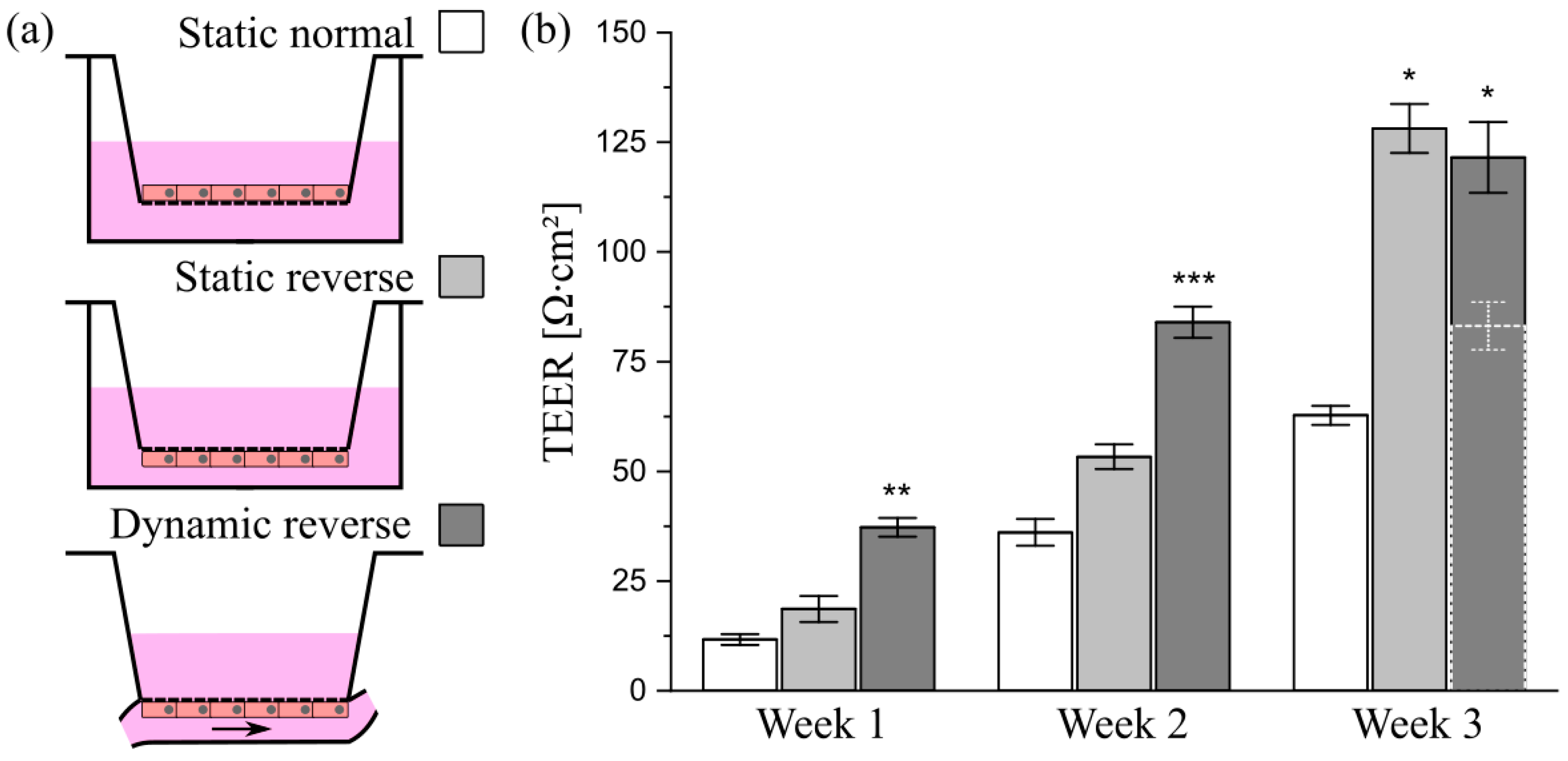
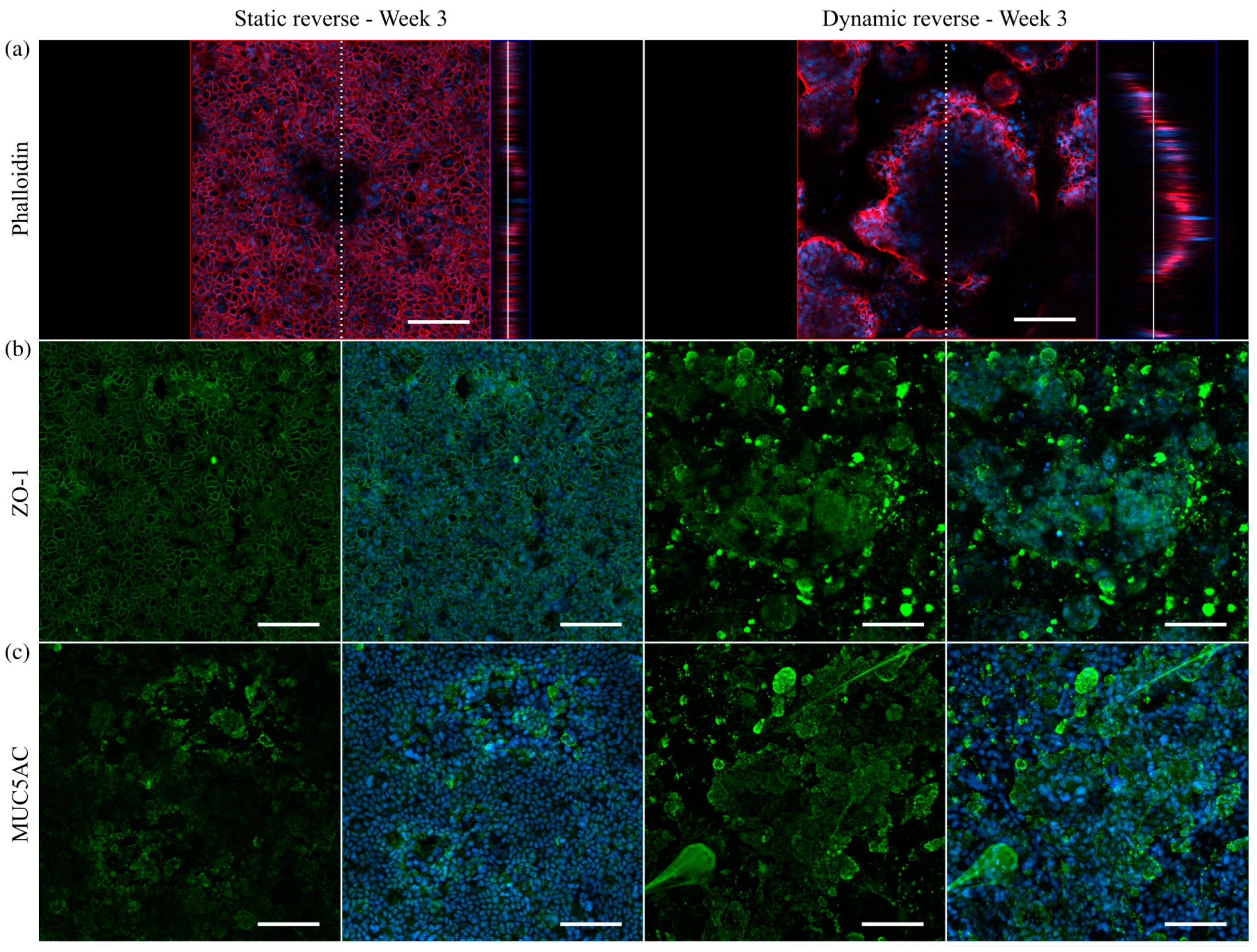
3.7. Transfer of the Model System from Solid Substrates to Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, B.O. Fight them or feed them: How the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, S.; Rodes, L.; Coussa-Charley, M.; Tomaro-Duchesneau, C. Gut microbiota: Next frontier in understanding human health and development of biotherapeutics. Biologics 2011, 5, 71–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, M.; Gresnigt, M.S.; Last, A.; Wollny, T.; Berlinghof, F.; Pospich, R.; Cseresnyes, Z.; Medyukhina, A.; Graf, K.; Groger, M.; et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 2019, 220, 119396. [Google Scholar] [CrossRef] [PubMed]
- Schultsz, C.; Van Den Berg, F.M.; Ten Kate, F.W.; Tytgat, G.N.; Dankert, J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology 1999, 117, 1089–1097. [Google Scholar] [CrossRef]
- Corfield, A.P.; Carroll, D.; Myerscough, N.; Probert, C.S. Mucins in the gastrointestinal tract in health and disease. Front. Biosci. 2001, 6, D1321–D1357. [Google Scholar] [CrossRef]
- Yildiz, H.M.; Speciner, L.; Ozdemir, C.; Cohen, D.E.; Carrier, R.L. Food-associated stimuli enhance barrier properties of gastrointestinal mucus. Biomaterials 2015, 54, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Macierzanka, A.; Mackie, A.R.; Bajka, B.H.; Rigby, N.M.; Nau, F.; Dupont, D. Transport of particles in intestinal mucus under simulated infant and adult physiological conditions: Impact of mucus structure and extracellular DNA. PLoS ONE 2014, 9, e95274. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Leithner, K.; Hauptstein, S.; Hintzen, F.; Salvenmoser, W.; Bernkop-Schnürch, A. Preparation and characterization of mucus-penetrating papain/poly(acrylic acid) nanoparticles for oral drug delivery applications. J. Nanopart. Res. 2012, 15, 1353. [Google Scholar] [CrossRef]
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef]
- Harding, S.E. Trends in muco-adhesive analysis. Trends Food Sci. Technol. 2006, 17, 255–262. [Google Scholar] [CrossRef]
- Groo, A.C.; Saulnier, P.; Gimel, J.C.; Gravier, J.; Ailhas, C.; Benoit, J.P.; Lagarce, F. Fate of paclitaxel lipid nanocapsules in intestinal mucus in view of their oral delivery. Int. J. Nanomed. 2013, 8, 4291–4302. [Google Scholar] [CrossRef] [Green Version]
- Boegh, M.; Baldursdottir, S.G.; Mullertz, A.; Nielsen, H.M. Property profiling of biosimilar mucus in a novel mucus-containing in vitro model for assessment of intestinal drug absorption. Eur. J. Pharm. Biopharm. 2014, 87, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Youhanna, S.; Lauschke, V.M. The Past, Present and Future of Intestinal In Vitro Cell Systems for Drug Absorption Studies. J. Pharm. Sci. 2021, 110, 50–65. [Google Scholar] [CrossRef]
- Darling, N.J.; Mobbs, C.L.; Gonzalez-Hau, A.L.; Freer, M.; Przyborski, S. Bioengineering Novel in vitro Co-culture Models That Represent the Human Intestinal Mucosa With Improved Caco-2 Structure and Barrier Function. Front. Bioeng. Biotechnol. 2020, 8, 992. [Google Scholar] [CrossRef]
- Dosh, R.H.; Jordan-Mahy, N.; Sammon, C.; Le Maitre, C.L. Tissue Engineering Laboratory Models of the Small Intestine. Tissue Eng. Part B Rev. 2018, 24, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Beduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef]
- van Klinken, B.J.; Oussoren, E.; Weenink, J.J.; Strous, G.J.; Buller, H.A.; Dekker, J.; Einerhand, A.W. The human intestinal cell lines Caco-2 and LS174T as models to study cell-type specific mucin expression. Glycoconj. J. 1996, 13, 757–768. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Barbat, A.; Dussaulx, E.; Zweibaum, A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990, 50, 6334–6343. [Google Scholar] [PubMed]
- Schoultz, I.; Keita, A.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Walter, E.; Janich, S.; Roessler, B.J.; Hilfinger, J.M.; Amidon, G.L. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: In vitro-in vivo correlation with permeability data from rats and humans. J. Pharm. Sci. 1996, 85, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Stenberg, P.; Artursson, P.; Kissel, T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm. Res. 2001, 18, 1138–1145. [Google Scholar] [CrossRef]
- Navabi, N.; McGuckin, M.A.; Linden, S.K. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS ONE 2013, 8, e68761. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kim, R.; Sims, C.E.; Allbritton, N.L. Building a Thick Mucus Hydrogel Layer to Improve the Physiological Relevance of In Vitro Primary Colonic Epithelial Models. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 653–655.e655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sontheimer-Phelps, A.; Chou, D.B.; Tovaglieri, A.; Ferrante, T.C.; Duckworth, T.; Fadel, C.; Frismantas, V.; Sutherland, A.D.; Jalili-Firoozinezhad, S.; Kasendra, M.; et al. Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 507–526. [Google Scholar] [CrossRef] [Green Version]
- Reuter, C.; Alzheimer, M.; Walles, H.; Oelschlaeger, T.A. An adherent mucus layer attenuates the genotoxic effect of colibactin. Cell. Microbiol. 2018, 20, e12812. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Mahler, G.J.; Esch, M.B.; Glahn, R.P.; Shuler, M.L. Characterization of a gastrointestinal tract microscale cell culture analog used to predict drug toxicity. Biotechnol. Bioeng. 2009, 104, 193–205. [Google Scholar] [CrossRef]
- Chi, M.; Yi, B.; Oh, S.; Park, D.J.; Sung, J.H.; Park, S. A microfluidic cell culture device (muFCCD) to culture epithelial cells with physiological and morphological properties that mimic those of the human intestine. Biomed. Microdevices 2015, 17, 9966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cole, T.; Zhang, Y.; Tang, S.Y. Mechanical Strain-Enabled Reconstitution of Dynamic Environment in Organ-on-a-Chip Platforms: A Review. Micromachines 2021, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Nasiri, R.; Barros, N.R.; Tebon, P.; Thakor, J.; Goudie, M.; Shamloo, A.; Martin, M.G.; Khademhosseini, A. Gut-on-a-chip: Current progress and future opportunities. Biomaterials 2020, 255, 120196. [Google Scholar] [CrossRef]
- Poon, C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. bioRxiv 2020. [Google Scholar] [CrossRef]
- Furter, M.; Sellin, M.E.; Hansson, G.C.; Hardt, W.D. Mucus Architecture and Near-Surface Swimming Affect Distinct Salmonella Typhimurium Infection Patterns along the Murine Intestinal Tract. Cell Rep. 2019, 27, 2665–2678.e2663. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Gustafsson, J.K.; Holmen-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjovall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, J.; Jalilian, I.; Shi, A.; Heng Yeoh, G.; Knothe Tate, M.L.; Ebrahimi Warkiani, M. Flow-induced stress on adherent cells in microfluidic devices. Lab Chip. 2015, 15, 4114–4127. [Google Scholar] [CrossRef]
- Wong, T.Y.; Chang, S.N.; Jhong, R.C.; Tseng, C.J.; Sun, G.C.; Cheng, P.W. Closer to Nature Through Dynamic Culture Systems. Cells 2019, 8, 942. [Google Scholar] [CrossRef] [Green Version]
- Lesuffleur, T.; Porchet, N.; Aubert, J.P.; Swallow, D.; Gum, J.R.; Kim, Y.S.; Real, F.X.; Zweibaum, A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 1993, 106 Pt 3, 771–783. [Google Scholar] [CrossRef]
- Reuter, C.; Oelschlaeger, T.A. Enhancement of Mucus Production in Eukaryotic Cells and Quantification of Adherent Mucus by ELISA. Bio-Protocol 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Berger, E.; Nassra, M.; Atgie, C.; Plaisancie, P.; Geloen, A. Oleic Acid Uptake Reveals the Rescued Enterocyte Phenotype of Colon Cancer Caco-2 by HT29-MTX Cells in Co-Culture Mode. Int. J. Mol. Sci. 2017, 18, 1573. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.M.; Phillipsen, M.B.; Hartmanis, L.M.; Kwasnica, M.A.; Chen, V.; Hackam, D.; Chang, M.W.; Bentley, W.E.; March, J.C. Microscale Bioreactors for in situ characterization of GI epithelial cell physiology. Sci. Rep. 2017, 7, 12515. [Google Scholar] [CrossRef]
- Helander, H.F.; Fandriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Lindner, M.; Laporte, A.; Block, S.; Elomaa, L.; Weinhart, M. Physiological shear stress enhances differentiation and mucus-formation of intestinal epithelial cells in vitro. Authorea Prepr. 2020. [Google Scholar] [CrossRef]
- Rohe, I.; Huttner, F.J.; Plendl, J.; Drewes, B.; Zentek, J. Comparison of different histological protocols for the preservation and quantification of the intestinal mucus layer in pigs. Eur. J. Histochem. 2018, 62, 2874. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.F.; Austen, W.G., Jr.; Zhang, X.; Munene, G.; Mostafa, G.; Biswas, S.; McCormack, M.; Eberlin, K.R.; Nguyen, J.T.; Tatlidede, H.S.; et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc. Natl. Acad. Sci. USA 2008, 105, 3551–3556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciak-Stothard, B.; Ridley, A.J. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J. Cell Biol. 2003, 161, 429–439. [Google Scholar] [CrossRef]
- Kim, H.J.; Ingber, D.E. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 2013, 5, 1130–1140. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Sheehan, J.K.; Kirkham, S.; Howard, M.; Woodman, P.; Kutay, S.; Brazeau, C.; Buckley, J.; Thornton, D.J. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J. Biol. Chem. 2004, 279, 15698–15705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehan, J.K.; Brazeau, C.; Kutay, S.; Pigeon, H.; Kirkham, S.; Howard, M.; Thornton, D.J. Physical characterization of the MUC5AC mucin: A highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem. J. 2000, 347 Pt 1, 37–44. [Google Scholar] [CrossRef]
- Fischer, A.J.; Pino-Argumedo, M.I.; Hilkin, B.M.; Shanrock, C.R.; Gansemer, N.D.; Chaly, A.L.; Zarei, K.; Allen, P.D.; Ostedgaard, L.S.; Hoffman, E.A.; et al. Mucus strands from submucosal glands initiate mucociliary transport of large particles. JCI Insight 2019, 4, e124863. [Google Scholar] [CrossRef] [Green Version]
- IVTech Srl Home Page of LiveBox2 Bioreactor. Available online: https://www.ivtech.it/Products/LiveBox2-(LB2) (accessed on 5 August 2021).
- Cacopardo, L.; Costa, J.; Giusti, S.; Buoncompagni, L.; Meucci, S.; Corti, A.; Mattei, G.; Ahluwalia, A. Real-time cellular impedance monitoring and imaging of biological barriers in a dual-flow membrane bioreactor. Biosens. Bioelectron. 2019, 140, 111340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaderer, V.; Hermann, M.; Lass-Florl, C.; Posch, W.; Wilflingseder, D. Turning the World Upside-Down in Cellulose for Improved Culturing and Imaging of Respiratory Challenges within a Human 3D Model. Cells 2019, 8, 1292. [Google Scholar] [CrossRef] [Green Version]
- Schimpel, C.; Teubl, B.; Absenger, M.; Meindl, C.; Frohlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles. Mol. Pharm. 2014, 11, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Meng, Q.; Zhang, G. Design of 3D printed insert for hanging culture of Caco-2 cells. Biofabrication 2014, 7, 015003. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindner, M.; Laporte, A.; Block, S.; Elomaa, L.; Weinhart, M. Physiological Shear Stress Enhances Differentiation, Mucus-Formation and Structural 3D Organization of Intestinal Epithelial Cells In Vitro. Cells 2021, 10, 2062. https://doi.org/10.3390/cells10082062
Lindner M, Laporte A, Block S, Elomaa L, Weinhart M. Physiological Shear Stress Enhances Differentiation, Mucus-Formation and Structural 3D Organization of Intestinal Epithelial Cells In Vitro. Cells. 2021; 10(8):2062. https://doi.org/10.3390/cells10082062
Chicago/Turabian StyleLindner, Marcus, Anna Laporte, Stephan Block, Laura Elomaa, and Marie Weinhart. 2021. "Physiological Shear Stress Enhances Differentiation, Mucus-Formation and Structural 3D Organization of Intestinal Epithelial Cells In Vitro" Cells 10, no. 8: 2062. https://doi.org/10.3390/cells10082062
APA StyleLindner, M., Laporte, A., Block, S., Elomaa, L., & Weinhart, M. (2021). Physiological Shear Stress Enhances Differentiation, Mucus-Formation and Structural 3D Organization of Intestinal Epithelial Cells In Vitro. Cells, 10(8), 2062. https://doi.org/10.3390/cells10082062





