1. Introduction
Membrane intrinsic protein (MIP), also known as aquaporin 0 (AQP0), is the most abundant lens membrane protein. Absence or mutation thereof leads to cataract formation [
1]. It has been shown to have a number of functions in the lens including water transport, adhesion, and regulation of functions of other proteins, such as connexin 50 [
2] and filensin [
3,
4]. Historically, water transport has been the most studied function of AQP0, largely in expression systems, but in recent years, adhesive properties of AQP0 have begun to receive more attention. Since lens optics depend on the precise architecture achieved by compact cell-to-cell adhesion, understanding how proper cell-to-cell adhesion is achieved would shed light on normal lens physiology and pathophysiology.
A structural role for AQP0 was first proposed based on observations of square array junctions [
5] and later confirmed by others [
6,
7]. Self-adhesive properties of human AQP0 were first demonstrated in vitro by expressing AQP0 in adhesion-deficient mouse fibroblast L cells [
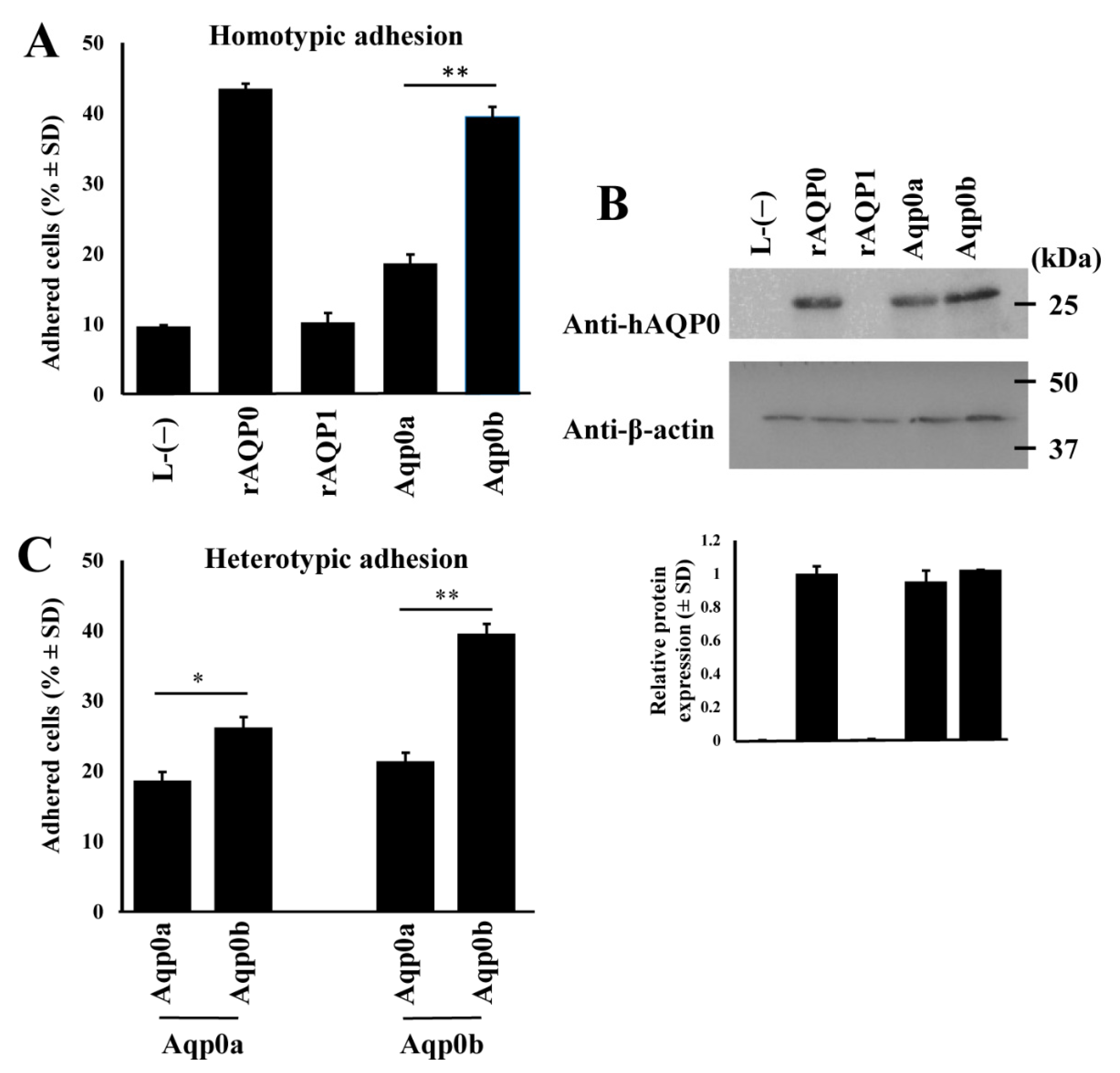
8]. This assay utilized the L cells stably expressing AQP0, dyed red and seeded in a dish, on the top of which AQP0-expressing cells dyed blue were loaded. The percentage of adherent cells was then quantified by flow cytometry. A human AQP0 mutation, R33C, had reduced adhesive properties, while water permeability was unaffected [
9]. Bovine AQP0 reconstituted into large unilamellar liposomes increased adhesion via electrostatic interactions with the negatively charged membrane [
10]. Similarly, mouse AQP0 was shown to adhere to untransfected adhesion-deficient mouse fibroblast L cells, negatively charged L-α-phosphatidylserine large unilamellar vesicles, and lens vesicles derived from the WT or AQP0-KO mouse lens cortex [
11]. Electron crystallography of the sheep lens suggested that AQP0 connects through
109Pro,
110Pro,
113Arg, and
123Pro in the C-loop between two AQP0 molecules on adjoining membranes [
12]. By mutating these residues in rat AQP0 and transfecting them in vitro, we confirmed their importance utilizing the aforementioned adhesion assay [
13]. P109A/P110A significantly reduced adhesion between homotypic pairs of cells, while AQP0 V107I and R113G mutations did not alter adhesion [
13].
In mammalian systems, however, it has been impossible to study adhesion separately from other functions of AQP0. For this reason, it is useful to study other models with duplicated AQP0 orthologs, including teleost fishes such as zebrafish, medaka, and Atlantic salmon. Tetraploid Atlantic salmon (
Salmo salar) AQP0 channels (
Aqp0a1, 0a2, 0b1, 0b2) are all highly expressed in the lens and all permeate water in the
Xenopus laevis oocyte expression system with differing pH sensitivities [
14]. All four Aqp0s have strong auto-adhesive properties and lower heterotypic adhesive affinities to other salmon Aqp0 paralogs as well as human AQP0 [
15]. This suggests that the adhesive properties of Aqp0s in teleosts are likely conserved with their mammalian orthologs, making them useful for studying the underlying mechanisms. Here, we utilized the zebrafish, which we had shown partitions at least some of the functions of AQP0 among the two duplicated genes,
aqp0a and
aqp0b [
16,
17,
18], but whose adhesive properties had not been tested.
Aqp0b-mediated water permeability appears to be unnecessary for adult lens transparency [
18], but Aqp0b is likely to have an additional function, possibly adhesion. While both Aqp0a and Aqp0b permeate water in the
Xenopus laevis oocyte expression system [
14], Aqp0b lacking the water transport function can rescue the cataract formed in 3-day-old zebrafish lenses after Aqp0b knock-down [
16]. An adhesive function for Aqp0b may also help explain why a non-water-permeable variant of Aqp0b (S19 compared to G19) has also been identified in some strains of zebrafish [
14,
16,
17,
19]. In this study, we tested the adhesive properties of zebrafish Aqp0a and Aqp0b in homotypic and heterotypic stably expressed L-type mouse fibroblast cell lines. In addition, we performed a mutational screen to identify key residues required for these functions.
2. Materials and Methods
2.1. Cell Culture
Mouse fibroblast L cells (ATCC cell bank, Manassas, VA, USA) were cultured under standard culture conditions of 5% CO2 at 37 °C in Dulbecco’s modified Eagle’s minimum essential medium (DMEM; Nacalai Tesque, Kyoto, Japan) with a penicillin–streptomycin antibiotic mixture (100 U/mL and 100 mg/mL, respectively) containing 10% fetal calf serum (FBS; Life Technologies, Waltham, CA, USA).
2.2. Cell-to-Cell Adhesion Assay
Cell-to-cell adhesion assays were carried out as previously described [
13]. Briefly, stable cell lines were established expressing pcDNA-Aqp0a, pcDNA-Aqp0b, or pcDNA-MIPfun wildtype\ or mutant forms. WT pcDNA-Aqp0a and WT pcDNA-Aqp0b were previously generated in the Hall lab. MIPfun was cloned into pcDNA3.1 (+) using XbaI and HindIII restriction sites with CTGGCTAGCGTTTAAACTTAATGTGGGAGTTCAGGTCC (forward) and AGCGGGTTTAAACGGGCCCTTTATAGGGCCTGCGTCTTC (reverse) primers using a Gibson Assembly Cloning Kit (E5510S New England Biolabs Inc, Ipswich, MA, USA). Cells transfected with empty pcDNA3 were used as negative controls. Two groups with equal numbers of cells in each cell line were generated. The first group was loaded with CellTracker Red CMPTX dye (Life Technologies) and cultured for 18 h until confluent. The second group of cells was loaded with CellTracker Blue CMAC dye (Life Technologies) and seeded over the first layer for 18 h. After washing non-adherent cells off with PBS three times, the percentage of red or blue positive cells was measured by flow cytometry (Aria III, high-speed multicolor digital analyzer, BD Bioscience, Franklin Lakes, NJ, USA).
2.3. Statistical Analysis
One-way ANOVA with post-hoc Tukey’s multiple comparisons was used to test for statistically significant differences in adhesion between groups (SPSS version 24; IBM Corporation, Armonk, New York, NY, USA). Each experiment was performed at least six times.
2.4. Western Blot Analysis
Western blot analysis was carried out as previously described [
13] with minor modifications. Briefly, cell lysates were prepared in the EBC lysis buffer (120 mM NaCl, 0.5% NP-40, 50 mM Tris HCl (pH 8.0)) with a protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). Protein concentrations were measured using a Bradford assay. Equal amounts of protein samples were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Burlington, MA, USA). To quantify protein expression levels, we tested a number of anti-mammalian AQP0 antibodies against zebrafish lens homogenates, and the polyclonal rabbit anti-human AQP0 C-terminus antibodies (H44, Santa Cruz sc-99059) gave the strongest and cleanest signal in detecting both, Aqp0a and Aqp0b, by Western blotting (data not shown). This antibody does, however, have a stronger affinity for rat AQP0 (total score of 81.3 and 84.09% identity) compared to zebrafish Aqp0s, which are similar—Aqp0a (total score of 62.4 and 61.35% identity) and Aqp0b (total score of 58.9 and 54.55% identity;
Supplementary Table S1). Polyclonal antisera against zebrafish Aqp0a C-terminus and zebrafish Aqp0b C-terminus [
17] shown to be ortholog-specific [
18] were purified (Econo-Pac Serum IgG; Bio-Rad Laboratories, Hercules, CA, USA). Anti-β-actin antibody (C-4, Santa Cruz Biotechnologies, Santa Cruz, CA, USA) was used as a loading control. Anti-rabbit HRP-conjugated secondary antibodies were used (Cell Signaling Technology, Danvers, MA, USA). Image J was used to quantify protein loading in at least three different Western blots.
2.5. Mutagenesis
Protein sequence alignments were carried out using an NCBI Constraint-based Multiple Alignment Tool (COBALT) and viewed using the NCBI Multiple Sequence Alignment Viewer, version 1.18.1. Protein sequence IDs for Homo sapiens AQP0—NP_036196.1; for Bos taurus AQP0—NP_776362.1; for Mus musculus—NP_032626.2; for Rattus norvegicus—NP_001099189.1; for Fundulus heteroclitus MIPfun—AAF04146.1; for Danio rerio Aqp0a and Aqp0b— NP_001003534.1 and NP_001018356.1; for Salmo salar Aqp0a1, Aqp0a2, Aqp0b1, and Aqp0b2—AJD87691.1, AJD87688.1, AJD87689.1, and AJD87690.1, respectively. Mutations were generated using a QuikChange II XL site-directed mutagenesis kit (200521 Agilent, Santa Clara, CA, USA) and confirmed by sequencing (Genewiz, South Plainfield, NJ, USA). Primers (forward, reverse) used to generate Aqp0a T110N were 5′-cagatttcctctcatattgtttggcgtgaccccataaag-3′ and 5′-ctttatggggtcacgccaaacaatatgagaggaaatctg-3′); Aqp0a Y195T—5′-agtggttaatgaagttcctagtgagcacagcaggggcaaaa-3′ and 5′-ttttgcccctgctgtgctcactaggaacttcattaaccact-3′; Aqp0b N110T or MIPfun N110T—5′-gtgcccctcatgttggtgggtgtaactccgt-3′ and 5′-acggagttacacccaccaacatgaggggcac-3′; Aqp0b T195Y—5′-cagtggttgatgaaattcctatagataacagcaggggcgaaagac-3′ and 5′-gtctttcgcccctgctgttatctataggaatttcatcaaccactg-3′; MIPfun N110P—5′-aggttccccctcatgttgggaggtgtgactccatacag-3′ and 5′-ctgtatggagtcacacctcccaacatgagggggaacct-3′.
4. Discussion
We showed a functional difference in adhesive properties between zebrafish Aqp0a and Aqp0b in an in vitro adhesion assay. Aqp0b has strong auto-adhesive properties while Aqp0a does not. The difference appears to be largely due to an amino acid difference at residue 110 in the extracellular C-loop. Aqp0a has a T at position 110 while Aqp0b has an N. This difference highlights the conservation of residue 110 and its importance in adhesion between zebrafish Aqp0b and mammalian AQP0, for which P110 (as well as P109) is a key residue required for adhesion [
13]. It also suggests that zebrafish Aqp0a has largely lost adhesive activity and that its contribution to lens transparency is largely due to its water permeability. These results clarify genetic studies in zebrafish showing that Aqp0a is essential for lens development and transparency while Aqp0b is not, at least under homeostatic conditions, providing some of the first evidence separating requirements for AQP0′s functions in water permeability versus adhesion [
18].
We show that Aqp0b promotes adhesion when stably expressed in L-type mouse fibroblast cells while Aqp0a does not. While at face value rat AQP0 and zebrafish Aqp0b appear to have similar levels of adhesion in homotypic cell lines (
Figure 1), we cannot make quantitative comparisons between absolute protein expression levels between species. We also cannot compare absolute adhesive properties between species either since the anti-human AQP0 antibody has a higher predicted affinity to rat AQP0. However, differences in the relative adhesive properties between Aqp0a and Aqp0b can be reliably compared since the predicted affinity of the antibody is similar for these two proteins.
Interestingly, the adhesion of Aqp0b plated on top of Aqp0a is slightly higher than of Aqp0a plated on Aqp0a, suggesting that Aqp0b can weakly adhere to Aqp0a or directly to the membrane. Previous studies using electron crystallography and adhesion assays have indicated the importance of extracellular loops for AQP0–AQP0 adhesion [
12]. Protein alignments of the A-, C-, and E-loops reveal that residues T110 and Y195 differ between zebrafish Aqp0a and Aqp0b, as well as between Aqp0a and Aqp0s in other species with known adhesive properties (
Figure 2). Swapping residues between Aqp0b and Aqp0a at sites 110 and 195 results in reduced adhesion in Aqp0b N110T and increased adhesion in Aqp0a T110N, while mutating residue 195 has no effect (
Figure 3). Interestingly, these two mutations alone do not increase adhesion mediated by Aqp0a to similar levels as does WT Aqp0b, and similarly adhesion mediated by Aqp0b does not decrease to the same level as does Aqp0a with both mutations. These results confirm the importance of N110 and the extracellular C-loop in the adhesive properties of Aqp0b. Furthermore, it is interesting that adhesion mediated by zebrafish Aqp0a is so weak, while all the Atlantic salmon Aqp0s show strong adhesive properties.
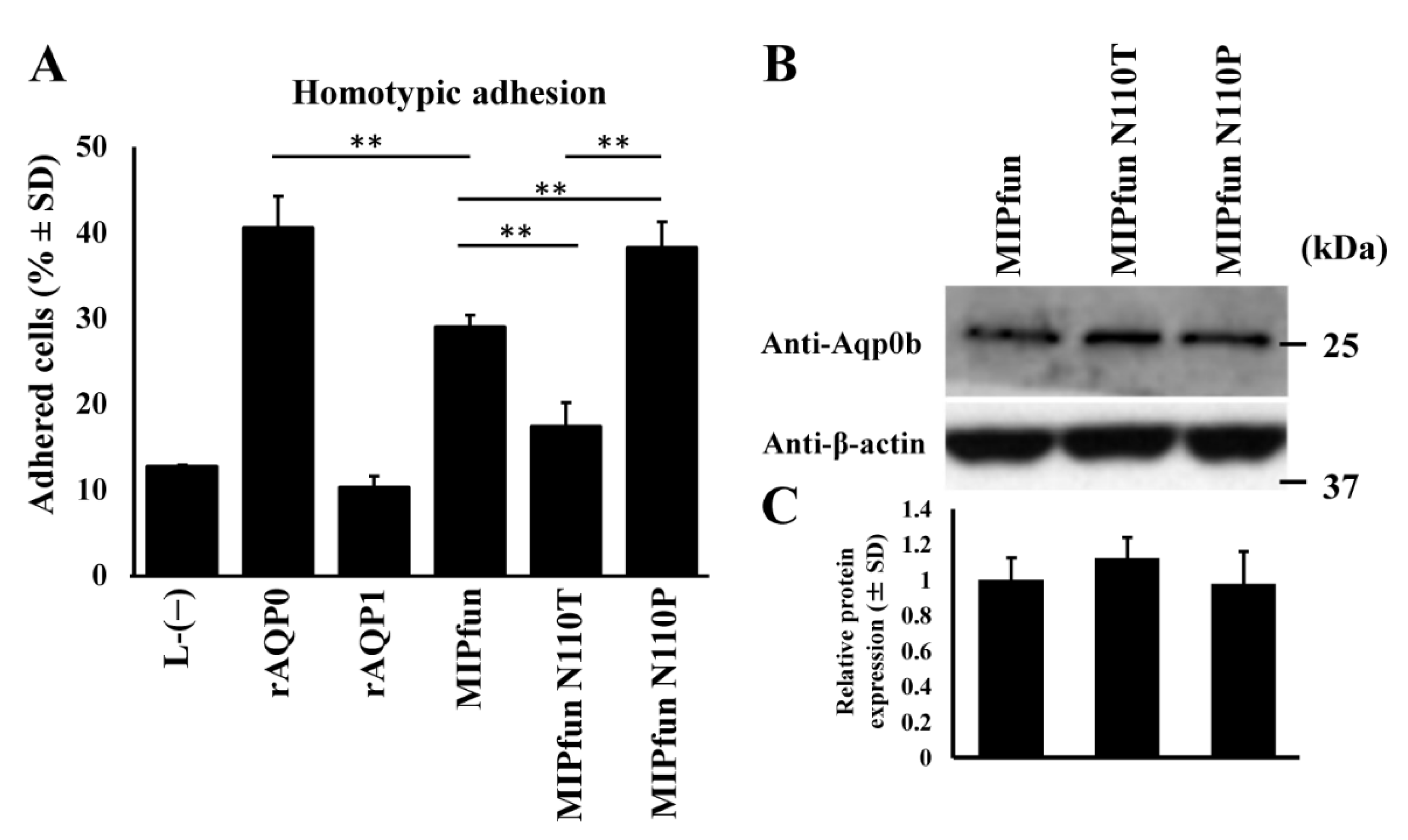
In addition, we show that a similar N110T mutation in the Aqp0 of
Fundulus heteroclitus, MIPfun, also reduces adhesion (
Figure 4). This result is consistent with evidence that MIPfun has both water transport [
17] and adhesive functions. Knockdown or loss-of-function mutants in zebrafish
aqp0a or
aqp0b increases the frequency of cataract at 2 and 3 days post-fertilization, which recovers by 4 dpf [
16,
17,
18], and both can be rescued by transient overexpression of WT MIPfun. In contrast, a MIPfun N68Q mutant lacking water permeability rescues loss of Aqp0b but not of Aqp0a [
16]. Thus, Aqp0a requires water transport function for embryonic lens transparency while Aqp0b does not. In the future, we will test the ability of MIPfun N110T with reduced adhesive properties to rescue an embryonic cataract in non-functional
aqp0b−/− mutant zebrafish. Although they form cataracts initially,
aqp0b−/− mutant lenses are indistinguishable from WT in terms of transparency, size, gross morphology, and optics after 4 dpf [
18,
20]. This suggests that the adhesion function of Aqp0b is dispensable once the lens matures, at least under homeostatic conditions, and can be compensated by other mechanisms. Other cell-to-cell adhesion mechanisms could potentially compensate for loss of adhesion by Aqp0b such as N-cadherin junctions [
12] or connexin 50 [
21]. Furthermore,
aqp0b−/− mutant lenses may also be sensitive to stress, e.g., osmotic, or mechanical perturbation. Future studies are needed to test the mechanical integrity and stiffness of
aqp0b−/− mutant lenses under load [
22].
While there is high conservation between species in the extracellular loop residues of Aqp0s, there are variations between mammalian and piscine species in specific regions (
Table 1). Notably, while P109 is conserved in all the species tested, P110 in mammals is mainly N110 in piscine species, as well as piscine N111 and M112, instead of mammalian A111 and V112. In the E-loop, site 195 varies from T195 in mammals to V195 and F195 in Atlantic salmon and killifish, while zebrafish Aqp0b has T195 and Aqp0a has Y195. It also appears that mammalian P110 yields a higher level of auto-adhesion compared to N110, as seen in MIPfun N110P (
Figure 4). This suggests that mammalian AQP0 may have stronger cell-to-cell adhesion than piscine species, possibly to support mechanical integrity of the larger mammalian lens. Since sheep AQP0 is thought to connect through
109Pro,
110Pro,
113Arg, and
123Pro in the C-loop [
12], it is likely that zebrafish Aqp0b may also connect to other Aqp0b through
109Pro,
110Asn,
113Arg, and
123Pro.
It is also interesting to speculate that having a proline with a nonpolar aliphatic residue that results in stronger auto-adhesion compared to an asparagine with polar neutral side chains leads to stronger adhesion as we showed for MIPfun. Molecular dynamics simulations could test this by quantifying the absolute adhesive force exerted per protein molecule to test how changes in N110P and other residues in the extracellular loops affect strength of AQP0–AQP0 interactions. If indeed mammalian AQP0 can exert stronger adhesive forces compared to zebrafish or killifish, this might suggest that the larger size of mammalian lenses requires a stronger mechanical integrity, for example, to withstand the mechanical stress due to accommodation by the ciliary body compared to piscine species that either move the whole lens to accommodate or, in the case of zebrafish, do not accommodate [
23].