Review of Biological Effects of Acute and Chronic Radiation Exposure on Caenorhabditis elegans
Abstract
1. Introduction
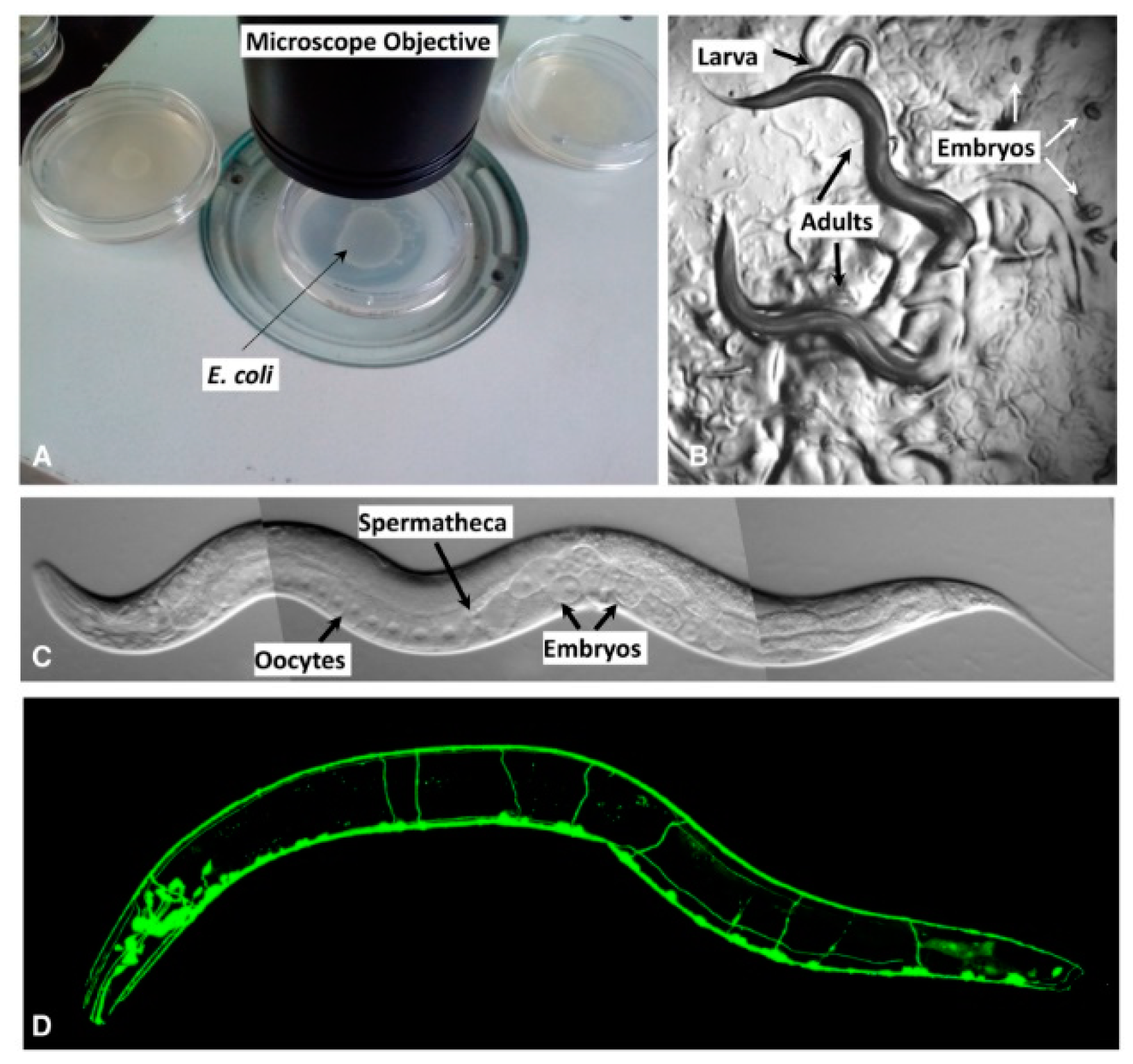
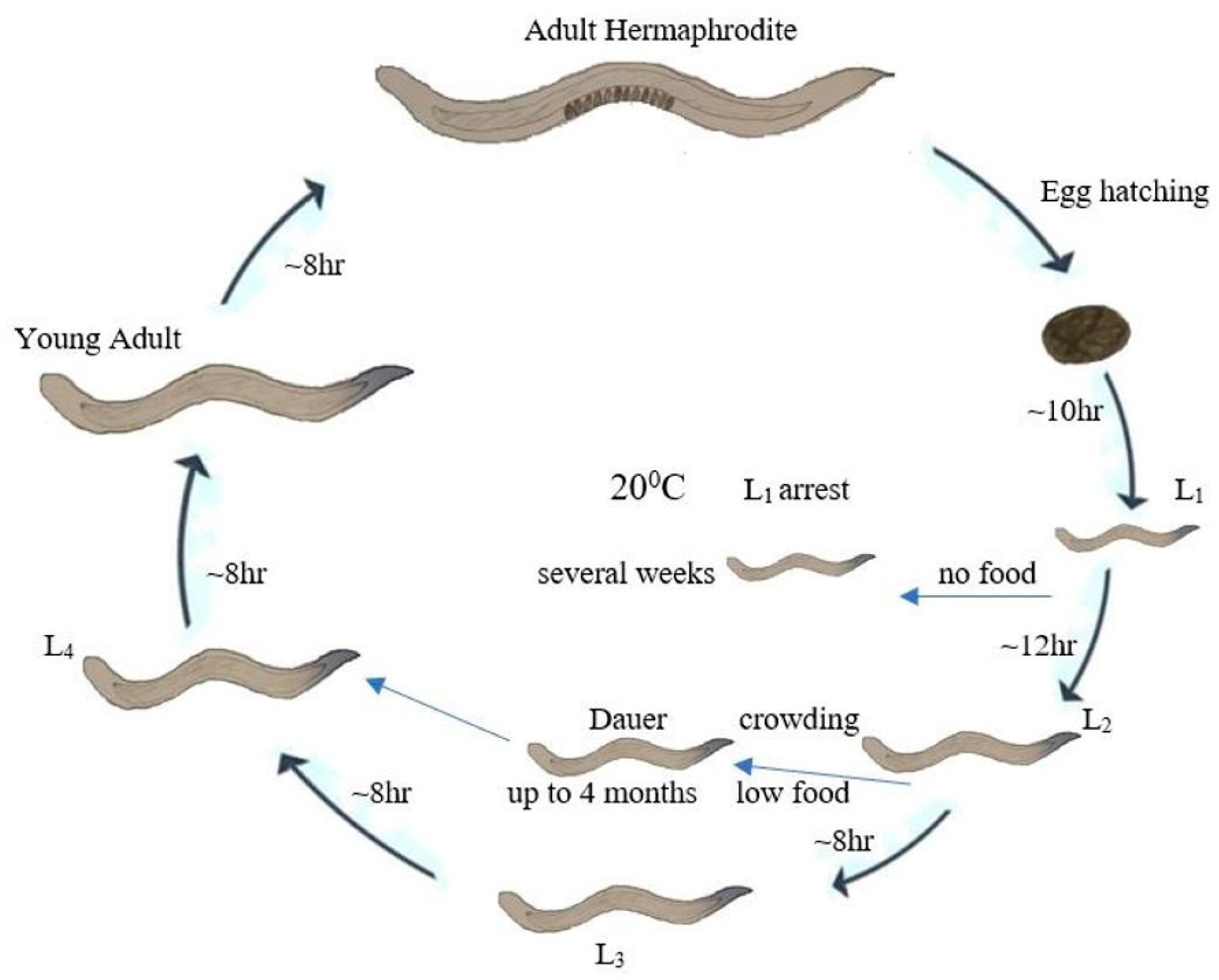
2. Life Cycle
2.1. Factors Affecting the Life Span of C. elegans
2.1.1. Temperature
2.1.2. Diet
2.1.3. Reproduction
2.1.4. Oxygen Level
3. Acute and Chronic Irradiation in C. elegans
3.1. Space vs. Ground Experiments
3.2. Effect on Aging
3.3. Effect on Reproduction
3.4. Effect on DNA Response
| Type of Radiation and Exposure Time | Radiation Dose/Dose Rate | Findings | Year/ Studies |
|---|---|---|---|
| Gamma Radiation for about an hour | 0.027 Gy/min | ≥0.1 Gy is needed to reduce the mean life of C. elegans. Dauers are the most sensitive and 8-day-old adults are the most resistant to ionizing radiation. | Johnson et al., 1984 [36] |
| Targeted micro bream (12C ion particles) | 20, 40, 60, and 100 Gy | Reproduction in C. elegans eggs arrested for both whole body and tip irradiation. In spot irradiation, the neighboring cells around the targeted point did not arrest the reproduction of germ cells nor did apoptosis happen. | Sugimoto et al., 2006 [76] |
| Gamma radiation | 100 Gy (32 Gy/min) | Chemotaxis reaction of C. elegans toward NaCl decreases. The role of ionizing irradiation in associative learning of C. elegans toward NaCl is elaborated. | Sakashita et al., 2008 [69] |
| Gamma radiation for 18 s | 6 × 10−3 to 2.8 × 10−2 Gy (for 1 month) 36 × 10–3 to 16.8 × 10–2 Gy (for 6 months) 144 × 10–3 to 67.2 × 10–2 Gy (for 2 years) | C. elegans would experience some damage from irradiation during long-term space flight, there are changes in genes related to DNA damage response, oxidative stress, and cell death, and the gamma rays induce apoptosis. | S. Yi et al., 2013 [64] |
| Accelerated proton for 18 s | 33.6 × 10–3 to 16.8 × 10–2 Gy (for 6 months) 144 × 10–3 Gy (for 2 years) | DNA repair mechanism was reduced due to proton exposure. Accelerated protons induce the expression of genes that are related to the DNA damage response and anti-apoptosis. | S. Yi et al., 2013 [64] |
| Gamma radiation | 25, 50, 60, 90, 120 Gy | 53BP1 homolog, HSR-9 increases the cell death in muted C. elegans exposed to acute irradiation. HSR-9 does not involve in C. elegans cells’ response to DNA damage due to ionizing irradiation. | Ryu et al., 2013 [82] |
| Proton particles microbeam | 1.65, 6.6, 16.5, 33 and 66 Gy (0.033 Gy per proton particle) | Proton irradiation increases the germ cell apoptosis in neighboring cells around the radiation spot. Ionized irradiation causes bystander effects in C. elegans cells | Guo et al., 2013 [66] |
| Proton microbeam and gamma rays (137Cs) (Time not specified) | Proton bream: 3.2 MeV with linear energy transfer rate Gamma rays: 75 and 100 Gy | DNA damage in worms was unique and in somatic cells, which include vulval cells the DNA damage checkpoint was not active. Radio-adaptive responses of the whole C. elegans organisms were improved by bystander effect, which was induced by radiation. | Tang et al., 2016 [67] |
| Targeted micro bream (12C ion particles) | 500 Gy | Development of a method to irradiate active C. elegans. Whole-body irradiation decreased the movement rate of C. elegans significantly. Regional irradiation on the head, middle, and tail of C. elegans did not have a significant effect on the movement rate. | Suzuki et al., 2017 [72] |
| Gamma radiation (137Cs) | 0, 60, 90, and 120 Gy | Ionized irradiation caused significant damage in C. elegans DNA but did not reduce the reproduction cells. Sensitivity to ionized irradiation increased in C. elegans mutants compared to the wild-type strain. BUB has a role in the response of C. elegans to DNA damage. | Bertolini et al., 2017 [83] |
| Gamma radiation (137Cs) | 2.5, 6.5, 14.4, 50, 100, and 200 Gy | Dependency of hatchability on irradiation dose was shown by the result of the decrease in significant number of progenies per individual after irradiation from and above 50 Gy, until 200 Gy. | Dubois et al., 2018 [88] |
| Gamma radiation (137Cs) | 1 Gy·min−1 0.5 Gy, 1 Gy, and 3.3 Gy | 369 proteins were found with significant differences. The molecular mechanisms induced by chronic irradiation differ from those induced by acute irradiation. | Dubois et al., 2019 [71] |
| X-ray (600C/D linear accelerator) | 0 gray [64], 200 Gy, and 400 Gy (not specified) | Genes related to several biological processes, such as behavior, regulation growth and locomotion, positive regulation of growth, calcium ion transport, and di- and trivalent inorganic cation transport, are differentially expressed | Xu et al., 2019 [68] |
| Gamma Radiation | Dose Rate: 1445 mGy·h−1 Total dose up to 6 Gy | Acute irradiation does not induce a significant change in reproduction. | Maremonti et al., 2019 [89] |
| Targeted micro bream (12C ion particles) | 0, 500, 100, and 1500 Gy | The decrease in mortality depends on the dose due to central nervous system (CNS) targeted irradiation and may partly be due to body-wall muscle cells around the CNS. | Suzuki et al., 2020 [73] |
| Targeted micro bream (12C ion particles) | 500 Gy | Targeted heavy ion microbeam smaller than 10 µm. The preparation and irradiation method for the device is provided. Targeted irradiation on the specific spots did not have an impact on the movement of C. elegans. | Funayama et al., 2020 [74] |
| Gamma Radiation | 0, 5, 10, 25, and 50 Gy (3.37 Gy/min) | Germ cell apoptosis decreases when C. elegans are treated with Ceramide. Ceramide influences C. elegans’s response to DNA damage. Ceramide involves in the functioning of mitochondria in C. elegans under ionizing irradiation. | Yang et al., 2020 [85] |
| X-ray | 0, 25, 37.5, 50, and 75 Gy | NHEJ factor in C. elegans is reported. NHJ-1 causes ionized radiation sensitivity in N2 wild-type C. elegans. | Vujin et al., 2020 [84] |
| Type of Radiation & Exposure Time | Radiation Dose | Findings | Year/Studies |
|---|---|---|---|
| Low dose gamma-ray radiation for 219.5 h | 0.268 to 0.306 cGy | The mutation frequency increased significantly due to exposure to space radiation. The charged iron particles are the major mutagenic component and the increased mutation frequencies caused significant cancer risk inherent in extended space travel. | Hartman et al., 2001 [61] |
| Low dose gamma-ray radiation for 11 days | Not specified | No significant increase in the mutation rate Introduction of eT1 balancer system for longer-term measurement of biological damage in space. | Zhao et al., 2006 [62] |
| Gamma radiation for 4 h | 100 Gy (0.42 Gy/min) | The avoidance response of C. elegans toward NaCl decreased significantly. | Sakashita et al., 2008 [69] |
| Accelerated proton for 18 s | 33.6 × 10–3 to 16.8 × 10–2 Gy (for 6 months) 144 × 10–3 Gy (for 2 years) | DNA repair mechanism was reduced due to proton exposure. Accelerated protons induce the expression of genes that are related to the DNA damage response and anti-apoptosis. | Yi et al., 2013 [64] |
| Gamma Radiation (137Cs source) for 64 h | Dose rate 7 and 52 mGy/h Dose: 0.5 and 3.3 Gy | Life span is significantly shortened in irradiated C. elegans. There was a significant difference between different absorbed doses for the same dose rate. | Kuzmic et al., 2019 [70] |
| Gamma Radiation (137Cs source) for 19 days | Dose rate 7 and 52 mGy/h Dose: 3.3 and 24 Gy | Life span is significantly shortened in irradiated C. elegans. There was a significant difference in absorbed doses in the treatments between 3.3 Gy cumulative irradiation (with 7 mGy/h) and 24 Gy cumulative irradiation (with 52 mGy/h) | Kuzmic et al., 2019 [70] |
| Gamma Radiation (137Cs source) for 65 h | Six dose rates 7, 14, 45, 50, 75, and 100 mGy/h Dose: 0.5, 1, 3.3 Gy | There are no effects from irradiation on the percentage of the hatch after chronic irradiation compared to control C. elegans. | Dubois et al., 2018 [88] |
| Gamma radiation (137Cs source) for 65 h | Dose rate: 7, 14, 50 mGy·h−1 corresponding to cumulated doses (0.5, 1, and 3.3 Gy) | 168 proteins were found with significant differences. The molecular mechanisms induced by chronic irradiation differ from those that were induced by acute irradiation. | Dubois et al., 2019 [71] |
| Gamma Radiation for 62 h | Dose rate: 0.9 to 227 mGy·h−1 Total dose up to 228 Gy | The number of larvae hatched was significantly decreased (by 43 and 61%, when chronically exposed from egg to young adult stage to a total dose of 6.7 Gy and 14 Gy, respectively) with increased germ cell apoptosis, impaired sperm meiosis, and adverse effects on sperm production. | Maremonti et al., 2019 [90] |
| Gamma Radiation for 72 h | Dose rate: 0.4 to 100 mGy Dose: 0.03 to 72 | Significant increase in mtDNA copy number (approx. 1.6-fold). | Maremonti et al., 2019 [90] |
| Gamma Radiation for 96 h | Dose rate: 40 and 100 mGy·h−1 Total dose: ~3.9 and 9.6 Gy | Toxic effect in reproduction. No of offspring reduced by 20 and 40%. | Maremonti et al., 2020 [91] |
4. Dietary or Pharmacological Interventions to Reduce Effects of Radiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ryan, J.L. Ionizing Radiation: The Good, the Bad, and the Ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef]
- Wong, J.Y.; Filippi, A.R.; Dabaja, B.S.; Yahalom, J.; Specht, L.K. Total Body Irradiation: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. 2018, 101, 521–529. [Google Scholar] [CrossRef]
- Wills, C.; Cherian, S.; Yousef, J.; Wang, K.; Mackley, H.B. Total body irradiation: A practical review. Appl. Rad. Oncol. 2016, 5, 11–17. [Google Scholar]
- Yasunari, T.J.; Stohl, A.; Hayano, R.; Burkhart, J.; Eckhardt, S. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. USA 2011, 108, 19530–19534. [Google Scholar] [CrossRef] [PubMed]
- National Geographic Partners. Early Manned Spaceflight. 2019. Available online: https://www.nationalgeographic.com/science/space/space-exploration/early-manned-spaceflight/ (accessed on 14 October 2019).
- Astronaut/Cosmonaut Statistics. Available online: http://www.worldspaceflight.com (accessed on 6 December 2019).
- Mettler, F.A. Medical effects and risks of exposure to ionizing radiation. J. Radiol. Prot. 2012, 32, N9. [Google Scholar] [CrossRef] [PubMed]
- Tzortzis, M.; Svoukis, E.; Tsertos, H. A comprehensive study of natural gamma radioactivity levels and associated dose rates from surface soils in Cyprus. Radiat. Prot. Dosim. 2004, 109, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.C.; Chao, M.Y. From odors to behaviors in Caenorhabditis elegans. In The Neurobiology of Olfaction; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Wood, W. The Nematode Caenorhabditis Elegans; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1998; p. 1. [Google Scholar]
- Zhao, Y.; Johnsen, R.; Baillie, D.; Rose, A. Worms in space? A model biological dosimeter. Gravit. Space Biol. Bull. Publ. Am. Soc. Gravit. Space Biol. 2005, 18, 11–16. [Google Scholar]
- Sakashita, T.; Takanami, T.; Yanase, S.; Hamada, N.; Suzuki, M.; Kimura, T.; Kobayashi, Y.; Ishii, N.; Higashitani, A. Radiation Biology of Caenorhabditis elegans: Germ Cell Response, Aging and Behavior. J. Radiat. Res. 2010, 51, 107–121. [Google Scholar] [CrossRef]
- McKay, S.; Johnsen, R.; Khattra, J.; Asano, J.; Baillie, D.; Chan, S.; Dube, N.; Fang, L.; Goszczynski, B.; Ha, E.; et al. Gene Expression Profiling of Cells, Tissues, and Developmental Stages of the Nematode C. elegans. Cold Spring Harb. Symp. Quant. Biol. 2003, 68, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Prasad, R.A.; Nfonsam, V.; Bernstein, H. DNA damage, DNA repair, and cancer, in New Research Directions in DNA Repair. IntechOpen 2013. [Google Scholar] [CrossRef]
- Bouyanfif, A.; Jayarathne, S.; Koboziev, I.; Moustaid-Moussa, N. The nematode Caenorhabditis elegans as a model organism to study metabolic effects of ω-3 polyunsaturated fatty acids in obesity. Adv. Nutr. 2019, 10, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Chin-Sang, I.; George, S.; Ding, M.; Moseley, S.L.; Lynch, A.S.; Chisholm, A.D. The Ephrin VAB-2/EFN-1 Functions in Neuronal Signaling to Regulate Epidermal Morphogenesis in C. elegans. Cell 1999, 99, 781–790. [Google Scholar] [CrossRef]
- Herman, R.K.; Albertson, D.G.; Brenner, S. Chromosome rearrangements in Caenorhabditis elegans. Genetics 1976, 83, 91–105. [Google Scholar] [CrossRef]
- Hartman, P.S. Epistatic interactions of radiation-sensitive (rad) mutants of Caenorhabditis elegans. Genetics 1985, 109, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.S.; Simpson, V.J.; Johnson, T.; Mitchell, D. Radiation sensitivity and DNA repair in Caenorhabditis elegans strains with different mean life spans. Mutat. Res. Lett. 1988, 208, 77–82. [Google Scholar] [CrossRef]
- Hartman, P.; Childress, E.; Beyer, T. Nematode development is inhibited by methyl viologen and high oxygen concentrations at a rate inversely proportional to life span. J. Gerontol. Ser. A Biol. Sci. Med Sci. 1995, 50, B322–B326. [Google Scholar] [CrossRef] [PubMed]
- Corsi, K.A.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Corsi, A.; Wightman, B.; Chalfie, M. A transparent window into biology: A primer on Caenorhabditis elegans. In WormBook: The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2015. [Google Scholar] [CrossRef]
- Riddle, L.D.; Blumenthal, T.; Meyer, J.B.; Priess, J.R. Developmental Genetics of the Germ Line—C. elegans II; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Hosono, R.; Mitsui, Y.; Sato, Y.; Aizawa, S.; Miwa, J. Life span of the wild and mutant nematode Caenorhabditis elegans: Effects of sex, sterilization, and temperature. Exp. Gerontol. 1982, 17, 163–172. [Google Scholar] [CrossRef]
- Klass, M.R. Aging in the nematode Caenorhabditis elegans: Major biological and environmental factors influencing life span. Mech. Ageing Dev. 1977, 6, 413–429. [Google Scholar] [CrossRef]
- Herndon, L.A. Introduction to C. elegans Anatomy. 2012. Available online: https://www.wormatlas.org/hermaphrodite/introduction/mainframe.htm (accessed on 14 June 2020).
- Cassada, R.C.; Russell, R.L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975, 46, 326–342. [Google Scholar] [CrossRef]
- Albert, P.S.; Riddle, D.L. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev. Biol. 1988, 126, 270–293. [Google Scholar] [CrossRef]
- Riddle, D.L. The genetics of development and behavior in Caenorhabditis elegans. J. Nematol. 1978, 10, 1. [Google Scholar] [PubMed]
- Reis, R.J.S.; Bharill, P.; Tazearslan, C.; Ayyadevara, S. Extreme-longevity mutations orchestrate silencing of multiple signaling pathways. Biochim. Et Biophys. Acta BBA Gen. Subj. 2009, 1790, 1075–1083. [Google Scholar] [CrossRef][Green Version]
- Anderson, J.L.; Morran, L.T.; Phillips, P.C. Outcrossing and the Maintenance of Males within C. elegans Populations. J. Hered. 2010, 101, S62–S74. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-E.H.; Jung, Y.; Lee, A.S.-J.V. Survival assays using Caenorhabditis elegans. Mol. Cells 2017, 40, 90–99. [Google Scholar] [CrossRef]
- Gómez-Orte, E.; Cornes, E.; Zheleva, A.; Sáenz-Narciso, B.; DE Toro, M.; Iñiguez, M.; López, R.; San-Juan, J.-F.; Ezcurra, B.; Sacristán, B.; et al. Effect of the diet type and temperature on the C. elegans transcriptome. Oncotarget 2017, 9, 9556–9571. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, R.; Ronan, E.A.; He, Y.; Hsu, A.-L.; Liu, J.; Xu, X. Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep. 2015, 11, 1414–1424. [Google Scholar] [CrossRef]
- Wong, A.; Boutis, P.; Hekimi, S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics 1995, 139, 1247–1259. [Google Scholar] [CrossRef]
- Johnson, T.E.; Mitchell, D.H.; Kline, S.; Kemal, R.; Foy, J. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech. Ageing Dev. 1984, 28, 23–40. [Google Scholar] [CrossRef]
- Lee, G.; Wilson, M.A.; Zhu, M.; Wolkow, C.A.; de Cabo, R.; Ingram, D.K.; Zou, S. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 2006, 5, 515–524. [Google Scholar] [CrossRef]
- Kaeberlein, T.L.; Smith, E.D.; Tsuchiya, M.; Welton, K.L.; Thomas, J.H.; Fields, S.; Kennedy, B.K.; Kaeberlein, M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 2006, 5, 487–494. [Google Scholar] [CrossRef]
- Shtonda, B.B.; Avery, L. Dietary choice behavior in Caenorhabditis elegans. J. Exp. Biol. 2006, 209, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin Gallate from Green Tea (Camellia sinensis) Increases Lifespan and Stress Resistance in Caenorhabditis elegans. Planta Med. 2008, 75, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Wiegant, F.A.C.; Surinova, S.; Ytsma, E.; Langelaar-Makkinje, M.; Wikman, G.; Post, J.A. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 2008, 10, 27–42. [Google Scholar] [CrossRef]
- Jayarathne, S.; Ramalingam, L.; Edwards, H.; Vanapalli, S.A.; Moustaid-Moussa, N. Tart cherry increases lifespan in caenorhabditis elegans by altering metabolic signaling pathways. Nutrients 2020, 12, 1482. [Google Scholar] [CrossRef] [PubMed]
- Hsin, H.; Kenyon, C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 1999, 399, 362–366. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Tissenbaum, H.A. Reproduction and longevity: Secrets revealed by C. elegans. Trends Cell Biol. 2007, 17, 65–71. [Google Scholar] [CrossRef]
- McCormick, M.; Chen, K.; Ramaswamy, P.; Kenyon, C. New genes that extend Caenorhabditis elegans’ lifespan in response to reproductive signals. Aging Cell 2011, 11, 192–202. [Google Scholar] [CrossRef]
- Berman, J.R.; Kenyon, C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 2006, 124, 1055–1068. [Google Scholar] [CrossRef]
- Yamawaki, M.T.; Arantes-Oliveira, N.; Berman, R.J.; Zhang, P.; Kanyon, C. Distinct activities of the germline and somatic reproductive tissues in the regulation of Caenorhabditis elegans’ longevity. Genetics 2008, 178, 513–526. [Google Scholar] [CrossRef]
- Van Voorhies, W.A. Production of sperm reduces nematode lifespan. Nature 1992, 360, 456. [Google Scholar] [CrossRef]
- Gems, D.; Riddle, D.L. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nat. Cell Biol. 1996, 379, 723–725. [Google Scholar] [CrossRef]
- Honda, S.; Ishii, N.; Suzuki, K.; Matsuo, M. Oxygen-Dependent Perturbation of Life Span and Aging Rate in the Nematode. J. Gerontol. 1993, 48, B57–B61. [Google Scholar] [CrossRef]
- Adachi, H.; Ishii, N. Effects of Tocotrienols on Life Span and Protein Carbonylation in Caenorhabditis elegans. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2000, 55, B280–B285. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, E.M.; Hoyt, J.M.; Johnson, J.; Miller, D. Hypoxia disrupts proteostasis in Caenorhabditis elegans. Aging Cell 2014, 14, 92–101. [Google Scholar] [CrossRef]
- Menuz, V.; Howell, K.S.; Gentina, S.; Epson, S.; Riezman, I.; Fornallaz-Mulhourser, M.; Hengartner, M.; Gomez, M.; Riezman, H.; Martinou, J.-C. Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science 2009, 324, 381–384. [Google Scholar] [CrossRef]
- Hartman, P.S.; Herman, R.K. Radiation-sensitive mutants of Caenorhabditis elegans. Genetics 1982, 102, 159–178. [Google Scholar] [CrossRef]
- Kai, M.; Suzuki, K.; Imaoka, T.; Sasatani, M.; Tanaka, S.; Yamada, Y.; Kakinuma, S. Estimation of dose-rate effectiveness factor for malignant tumor mortality: Joint analysis of mouse data exposed to chronic and acute radiation. Radiat. Res. 2020, 194, 500–510. [Google Scholar]
- Wu, H.; Huff, J.L.; Casey, R.; Kim, M.H.; Cucinotta, F.A. Risk of acute radiation syndromes due to solar particle events. In The Human Health and Performance Risks for Space Explorations; NASA Human Research Program: Houston, TX, USA, 2009; pp. 171–190. [Google Scholar]
- Cekanaviciute, E.; Rosi, S.; Costes, S.V. Central nervous system responses to simulated galactic cosmic rays. Int. J. Mol. Sci. 2018, 19, 3669. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E.; Hartman, P.S. Radiation Effects on Life Span in Caenorhabditis elegans. J. Gerontol. 1988, 43, B137–B141. [Google Scholar] [CrossRef]
- Nelson, G.; Schubert, W.; Kazarians, G.; Richards, G.; Benton, E.; Henke, R. Radiation effects in nematodes: Results from IML-1 experiments. Adv. Space Res. 1994, 14, 87–91. [Google Scholar] [CrossRef]
- Hartman, P.S.; Hlavacek, A.; Wilde, H.; Lewicki, D.; Schubert, W.; Kern, R.G.; Kazarians, G.A.; Benton, E.V.; Benton, E.R.; Nelson, G.A. A comparison of mutations induced by accelerated iron particles versus those induced by low earth orbit space radiation in the FEM-3 gene of Caenorhabditis elegans. Mutat. Res. Mol. Mech. Mutagen. 2001, 474, 47–55. [Google Scholar] [CrossRef]
- Zhao, Y.; Lai, K.; Cheung, I.; Youds, J.; Tarailo, M.; Tarailo, S.; Rose, A. A mutational analysis of Caenorhabditis elegans in space. Mutat. Res. 2006, 601, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jones, M.; Baillie, D.; Rose, A. Developing an integrating biological dosimeter for spaceflight. Microgravity Sci. Technol. 2007, 19, 201–204. [Google Scholar] [CrossRef]
- Yi, S.; Kim, S.; Song, J. Analysis of the Effect of Space Radiations on the Nematode, Caenorhabditis elegans, through the Simulated Space Radiation. Int. J. Astron. Astrophys. 2013, 03, 291–302. [Google Scholar] [CrossRef]
- Reitz, G.; Beaujean, R.; Benton, E.; Burmeister, S.; Dachev, T.; Deme, S.; Luszik-Bhadra, M.; Olko, P. Space radiation measurements on-board ISS—The DOSMAP experiment. Radiat. Prot. Dosim. 2005, 116, 374–379. [Google Scholar] [CrossRef]
- Guo, X.; Sun, J.; Bian, P.; Chen, L.; Zhan, F.; Wang, J.; Xu, A.; Wang, Y.; Hei, T.K.; Wu, L. Radiation-Induced Bystander Signaling from Somatic Cells to Germ Cells in Caenorhabditis elegans. Radiat. Res. 2013, 180, 268–275. [Google Scholar] [CrossRef]
- Tang, H.; Chen, L.; Chen, L.; Chen, B.; Wang, T.; Yang, A.; Zhang, F.; Wu, L.; Bian, P. Interaction between radioadaptive response and radiation-induced bystander effect in Caenorhabditis elegans: A unique role of the DNA damage checkpoint. Radiat. Res. 2016, 186, 662–668. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Liu, M.; Lu, Y.; Yue, Y.; Liu, Y.; Chen, H.; Xiu, F.; Zhang, C. High-throughput transcriptome sequencing reveals extremely high doses of ionizing radiation-response genes in Caenorhabditis elegans. Toxicol. Res. 2019, 8, 754–766. [Google Scholar] [CrossRef]
- Sakashita, T.; Hamada, N.; Ikeda, D.D.; Yanase, S.; Suzuki, M.; Ishii, N.; Kobayashi, Y. Modulatory effect of ionizing radiation on food—NaCl associative learning: The role of γ subunit of G protein in Caenorhabditis elegans. FASEB J. 2008, 22, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Kuzmic, M.; Galas, S.; Lecomte-Pradines, C.; Dubois, C.; Dubourg, N.; Frelon, S. Interplay between ionizing radiation effects and aging in C. elegans. Free. Radic. Biol. Med. 2019, 134, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Pophillat, M.; Audebert, S.; Fourquet, P.; Lecomte, C.; Dubourg, N.; Galas, S.; Camoin, C.; Frelon, S. Differential modification of the C. elegans proteome in response to acute and chronic gamma radiation: Link with reproduction decline. Sci. Total Environ. 2019, 676, 767–781. [Google Scholar] [CrossRef]
- Suzuki, M.; Hattori, Y.; Sakashita, T.; Yokota, Y.; Kobayasi, Y.; Funayama, T. Region-specific irradiation system with heavy-ion microbeam for active individuals of Caenorhabditis elegans. J. Radiat. Res. 2017, 58, 881–886. [Google Scholar] [CrossRef]
- Suzuki, M.; Soh, Z.; Yamashita, H.; Tsuji, T.; Funayama, T. Targeted Central Nervous System Irradiation of Caenorhabditis elegans Induces a Limited Effect on Motility. Biology 2020, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Funayama, T.; Sakashita, T.; Suzuki, M.; Yokota, Y.; Miyawaki, N.; Kashiwagi, H.; Satoh, T.; Kurashima, S. An irradiation device for biological targets using focused microbeams of cyclotron-accelerated heavy ion. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 465, 101–109. [Google Scholar] [CrossRef]
- Min, H.; Sung, M.; Son, M.; Kawasaki, I.; Shim, Y.-H. Transgenerational effects of proton beam irradiation on Caenorhabditis elegans germline apoptosis. Biochem. Biophys. Res. Commun. 2017, 490, 608–615. [Google Scholar] [CrossRef]
- Sugimoto, T.; Dazai, K.; Sakashita, T.; Funayama, T.; Wada, S.; Hamada, N.; Kakizaki, T.; Kobayashi, Y.; Higashitani, A. Cell cycle arrest and apoptosis in Caenorhabditis elegans germline cells following heavy-ion microbeam irradiation. Int. J. Radiat. Biol. 2006, 82, 31–38. [Google Scholar] [CrossRef]
- Greer, E.; Maures, T.J.; Hauswirth, A.G.; Green, E.; Leeman, D.S.; Maro, G.S.; Han, S.; Banko, M.R.; Gozani, O.; Brunet, A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nat. Cell Biol. 2010, 466, 383–387. [Google Scholar] [CrossRef]
- Greer, E.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nat. Cell Biol. 2011, 479, 365–371. [Google Scholar] [CrossRef]
- Kishimoto, S.; Uno, M.; Okabe, E.; Nono, M.; Nishida, E. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat. Commun. 2017, 8, 14031. [Google Scholar] [CrossRef]
- Alpi, A.; Pasierbek, P.; Gartner, A.; Loidl, J. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 2003, 112, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.R.; Milstein, S.; Boulton, S.J.; Ye, M.; Hofmann, J.; Sergiou, L.; Gartner, A.; Vidal, M.; Hengartner, M. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 2002, 12, 1908–1918. [Google Scholar] [CrossRef]
- Ryu, S.J.; Kang, S.J.; Koo, H.-S. The 53BP1 homolog in C. elegans influences DNA repair and promotes apoptosis in response to ionizing radiation. PLoS ONE 2013, 8, e64028. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, S.; Wang, B.; Meier, B.; Hong, Y.; Gartner, A. Caenorhabditis elegans BUB-3 and SAN-1/MAD3 spindle assembly checkpoint components are required for genome stability in response to treatment with ionizing radiation. G3 Genes Genomes Genet. 2017, 7, 3875–3885. [Google Scholar] [CrossRef] [PubMed]
- Vujin, A.; Jones, S.; Zetka, M. NHJ-1 Is Required for Canonical Nonhomologous End Joining in Caenorhabditis elegans. Genetics 2020, 215, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, G.; Xu, Y.; Cheng, X.; Xu, S.; Chen, S.; Wu, L. Ceramide mediates radiation-induced germ cell apoptosis via regulating mitochondria function and MAPK factors in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2020, 208, 111579. [Google Scholar] [CrossRef]
- Buisset-Goussen, A.; Goussen, B.; Della-Vedova, C.; Galas, S.; Adam-Guillermin, C.; Lecomte-Pradines, C. Effects of chronic gamma irradiation: A multigenerational study using Caenorhabditis elegans. J. Environ. Radioact. 2014, 137, 190–197. [Google Scholar] [CrossRef]
- Barber, R.C.; Hickenbotham, P.; Hatch, T.; Kelly, D.; Topchiy, N.; Almeida, G.; Jones, G.; Johnson, G.; Parry, J.M.; Rothkamm, K.; et al. Radiation-induced transgenerational alterations in genome stability and DNA damage. Oncogene 2006, 25, 7336–7342. [Google Scholar] [CrossRef]
- Dubois, C.; Lecomte, C.; Dit Ruys, S.P.; Kuzmic, M.; Della-Vedova, C.; Dubourg, N.; Glasa, S.; Frelin, S. Precoce and opposite response of proteasome activity after acute or chronic exposure of C. elegans to γ-radiation. Sci. Rep. 2018, 8, 11349. [Google Scholar] [CrossRef]
- Maremonti, E.; Eide, D.M.; Oughton, D.H.; Salbu, B.; Grammes, F.; Kassaye, Y.; Guedon, R.; Lecomte-Pradines, C.; Brede, D. Gamma radiation induces life stage-dependent reprotoxicity in Caenorhabditis elegans via impairment of spermatogenesis. Sci. Total Environ. 2019, 695, 133835. [Google Scholar] [CrossRef]
- Maremonti, E.; Brede, D.A.; Olsen, A.K.; Eide, D.M.; Berg, E.S. Ionizing radiation, genotoxic stress, and mitochondrial DNA copy-number variation in Caenorhabditis elegans: Droplet digital PCR analysis. Mutat. Res. 2020, 858–860, 503277. [Google Scholar] [CrossRef]
- Maremonti, E.; Eide, D.M.; Rossbach, L.M.; Lind, O.C.; Salbu, B.; Brede, D.A. In vivo assessment of reactive oxygen species production and oxidative stress effects induced by chronic exposure to gamma radiation in Caenorhabditis elegans. Free. Radic. Biol. Med. 2020, 152, 583–596. [Google Scholar] [CrossRef]
- Weidhaas, J.B.; Eisenmann, D.M.; Holub, J.M.; Nallur, S.V. A Caenorhabditis elegans tissue model of radiation-induced reproductive cell death. Proc. Natl. Acad. Sci. USA 2006, 103, 9946–9951. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, L.; Liu, J.; Shi, J.; Li, Q.; Wang, T.; Wu, L.; Zhan, F.; Bian, P. Radioadaptive Response for Reproductive Cell Death Demonstrated in In Vivo Tissue Model of Caenorhabditis elegans. Radiat. Res. 2016, 185, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, H.; Du, Y.; Dai, Z.; Wang, T.; Wu, L.; Zhou, L.; Bian, P. Induction of reproductive cell death in Caenorhabditis elegans across entire linear-energy-transfer range of carbon-ion irradiation. DNA Repair 2018, 63, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A. Using C. elegans for aging research. Invertebr. Reprod. Dev. 2015, 59 (Suppl. S1), 59–63. [Google Scholar] [CrossRef]
- Liu, M.; Xiong, Y.; Shan, S.; Zhu, Y.; Zeng, D.; Shi, Y.; Zhang, Y.; Lu, W. Eleutheroside E Enhances the Long-Term Memory of Radiation-Damaged C. elegans through G-Protein-Coupled Receptor and Neuropeptide Signaling Pathways. J. Nat. Prod. 2020, 83, 3315–3323. [Google Scholar] [CrossRef] [PubMed]
- Altintas, O.; Park, S.; Lee, S.-J.V. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Zhang, Z. Leucine Exerts Lifespan Extension and Improvement in Three Types of Stress Resistance (Thermotolerance, Anti-Oxidation and Anti-UV Irradiation) in C. elegans. J. Food Nutr. Res. 2018, 6, 665–673. [Google Scholar] [CrossRef][Green Version]
- Edwards, C.; Canfield, J.; Copes, N.; Brito, A.; Rehan, M.; Lipps, D.; Brunquell, J.; Westerheide, S.D.; Bradshaw, P.C. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015, 16, 1–24. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, X.; Jiang, L.; Wang, L.; Bai, S.; Jiao, Y.; Xing, S.; Li, W.; Ma, J. Arbutin increases Caenorhabditis elegans longevity and stress resistance. Peer J. 2017, 5, e4170. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, X.; Luo, Y.; Du, F.; Wang, H.; Xing, S.; Li, W.; Ma, J. 2-SeCD treatment extends lifespan, improves healthspan and enhances resistance to stress in Caenorhabditis elegans. RSC Adv. 2017, 7, 48245–48252. [Google Scholar] [CrossRef]
- Kato, M.; Hamazaki, Y.; Sun, S.; Nishikawa, Y.; Kage-Nakadai, E. Clostridium butyricum MIYAIRI 588 Increases the Lifespan and Multiple-Stress Resistance of Caenorhabditis elegans. Nutrients 2018, 10, 1921. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018, 9, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Park, S.-K. Supplementation of S-allyl cysteine improves health span in Caenorhabditis elegans. Biosci. J. 2017, 33, 411–421. [Google Scholar] [CrossRef]
- Vayndorf, E.M.; Lee, S.S.; Liu, H. Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society Medical and Editorial Content Team. Ultraviolet (UV) Radiation. 2019. Available online: https://www.cancer.org/cancer/cancer-causes/radiation-exposure/uv-radiation.html (accessed on 20 November 2020).
- Hung, J.-Y.; Hsu, Y.-L.; Ko, Y.-C.; Tsai, Y.-M.; Yang, C.-J.; Huang, M.-S.; Kuo, P.-L. Didymin, a dietary flavonoid glycoside from citrus fruits, induces Fas-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo. Lung Cancer 2010, 68, 366–374. [Google Scholar] [CrossRef]
- Miccadei, S.; Masella, R.; Mileo, A.M.; Gessani, S. ω3 Polyunsaturated Fatty Acids as Immunomodulators in Colorectal Cancer: New Potential Role in Adjuvant Therapies. Front. Immunol. 2016, 7, 486. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Liu, M.; Lu, Y.; Lin, G.; Chen, L.; Zhang, C.; Liu, Z. VA and PUFA protection against 60Co γ-ray-induced abnormal expression of DNA damage-related genes in C. elegans. Int. J. Clin. Exp. Med. 2019, 12, 5675–5681. [Google Scholar]
- Vanfleteren, J.R. Oxidative stress and ageing in Caenorhabditis elegans. Biochem. J. 1993, 292, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Senoo-Matsuda, N.; Miyake, K.; Yasuda, K.; Ishii, T.; Hartman, P.S.; Furukawa, S. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech. Ageing Dev. 2004, 125, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Ji, C.-B.; Lu, X.-W.; Ni, Y.-H.; Gao, C.-L.; Chen, X.-H.; Zhao, Y.-P.; Gu, G.-X.; Guo, X.-R. Resveratrol Attenuates Radiation Damage in Caenorhabditis elegans by Preventing Oxidative Stress. J. Radiat. Res. 2010, 51, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of resveratrol on oxidative stress resistance and life span in C. aenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, R.; Yosofvand, M.; Yavari, M.; Abdulrahman, R.; Schurr, R.; Moustaid-Moussa, N.; Moussa, H. Review of Biological Effects of Acute and Chronic Radiation Exposure on Caenorhabditis elegans. Cells 2021, 10, 1966. https://doi.org/10.3390/cells10081966
Dhakal R, Yosofvand M, Yavari M, Abdulrahman R, Schurr R, Moustaid-Moussa N, Moussa H. Review of Biological Effects of Acute and Chronic Radiation Exposure on Caenorhabditis elegans. Cells. 2021; 10(8):1966. https://doi.org/10.3390/cells10081966
Chicago/Turabian StyleDhakal, Rabin, Mohammad Yosofvand, Mahsa Yavari, Ramzi Abdulrahman, Ryan Schurr, Naima Moustaid-Moussa, and Hanna Moussa. 2021. "Review of Biological Effects of Acute and Chronic Radiation Exposure on Caenorhabditis elegans" Cells 10, no. 8: 1966. https://doi.org/10.3390/cells10081966
APA StyleDhakal, R., Yosofvand, M., Yavari, M., Abdulrahman, R., Schurr, R., Moustaid-Moussa, N., & Moussa, H. (2021). Review of Biological Effects of Acute and Chronic Radiation Exposure on Caenorhabditis elegans. Cells, 10(8), 1966. https://doi.org/10.3390/cells10081966










