Diclofenac Alters the Cell Cycle Progression of the Green Alga Chlamydomonas reinhardtii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions and Exposure to Toxicant
2.2. Determination of Starch Content
2.3. Photosynthetic Pigment Contents in the Cells
2.4. Chlorophyll a Fluorescence
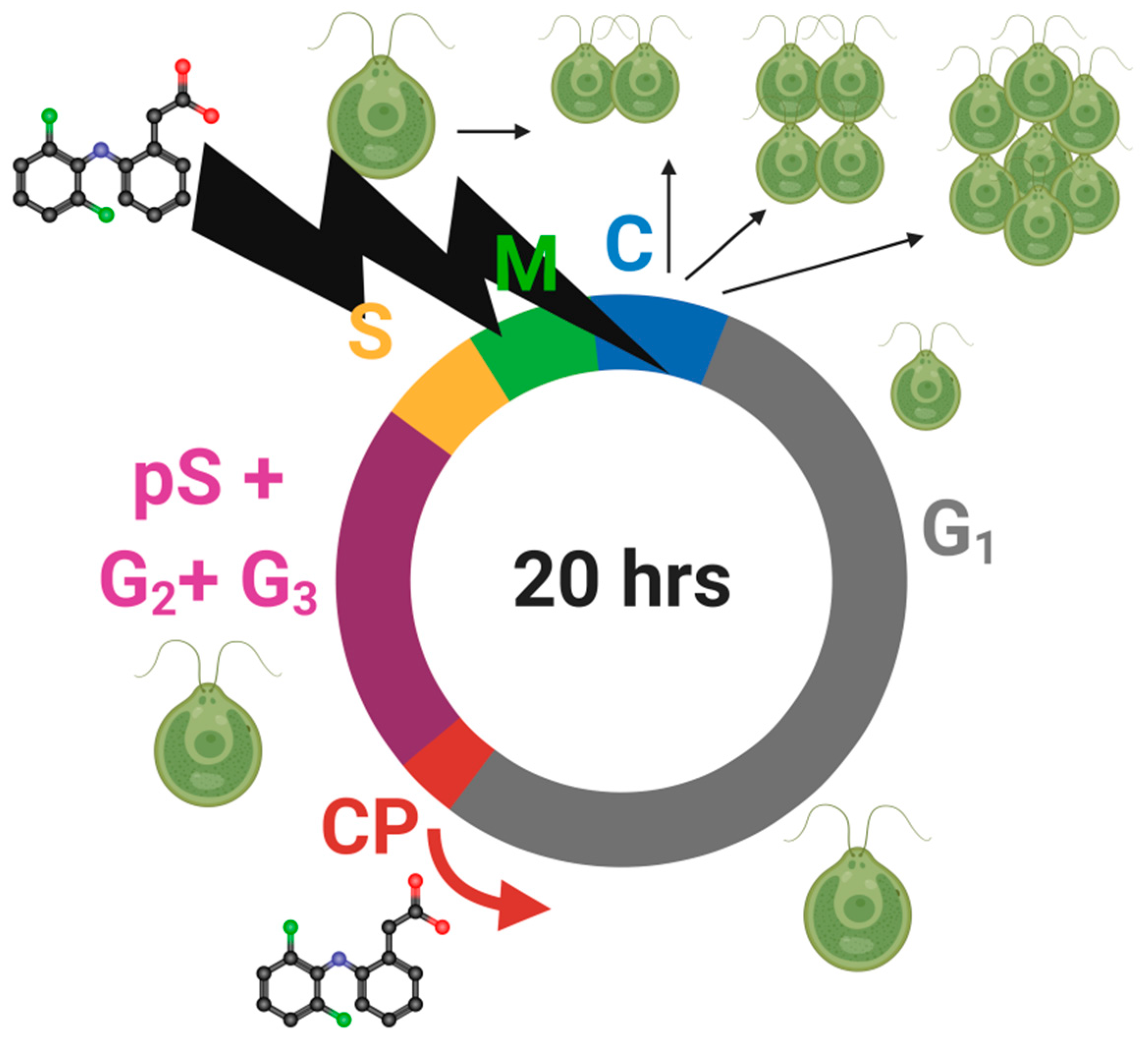
2.5. Cell Cycle Analyses
2.6. Cell Volume
2.7. Nucleic Acid Analyses
2.8. Microscopy Observation and 4′,6-Diamidine-2′-Phenylindole Dihydrochloride Staining
2.9. Statistical Analyses
3. Results
3.1. Analysis of Cell Size
3.2. RNA Content and Protein Accumulation
3.3. Analysis of Starch Content in the Cells
3.4. Analysis of Photosynthetic Pigments Content
3.5. Analysis of Photosynthetic Parameters by OJIP Method
3.6. Cell Cycle Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidoo, V.; Swan, G.E. Diclofenac toxicity in Gyps vulture is associated with decreased uric acid excretion and not renal portal vasoconstriction. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef]
- Proia, L.; Osorio, V.; Soley, S.; Köck-Schulmeyer, M.; Pérez, S.; Barceló, D.; Romaní, A.M.; Sabater, S. Effects of pesticides and pharmaceuticals on biofilms in a highly impacted river. Environ. Pollut. 2013, 178, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Fleming, J. Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Arch. Environ. Contam. Toxicol. 2008, 54, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Jansen, M.; Bartels, P.; Adler, N.; Altenburger, R. Phytotoxicity assessment of diclofenac and its phototransformation products. Anal. Bioanal. Chem. 2007, 387, 1389–1396. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Majewska, M.; Harshkova, D.; Guściora, M.; Aksmann, A. Phytotoxic activity of diclofenac: Evaluation using a model green alga Chlamydomonas reinhardtii with atrazine as a reference substance. Chemosphere 2018, 209, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Pokora, W.; Aksmann, A.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Rykaczewski, M.; Gappa, M.; Tukaj, Z. Changes in nitric oxide/hydrogen peroxide content and cell cycle progression: Study with synchronized cultures of green alga Chlamydomonas reinhardtii. J. Plant Physiol. 2017, 208, 84–93. [Google Scholar] [CrossRef]
- Pokora, W.; Aksmann, A.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Tukaj, Z. Exogenously applied hydrogen peroxide modifies the course of the Chlamydomonas reinhardtii cell cycle. J. Plant Physiol. 2018, 230, 61–72. [Google Scholar] [CrossRef]
- Čížková, M.; Slavková, M.; Vítová, M.; Zachleder, V.; Bišová, K. Response of the Green Alga Chlamydomonas reinhardtii to the DNA Damaging Agent Zeocin. Cells 2019, 8, 735. [Google Scholar] [CrossRef] [Green Version]
- Lien, T.; Knutsen, G. Synchronous growth of Chlamydomonas reinhardtii (Chlorophyceae): A review of optimal conditions. J. Phycol. 1979, 15, 191–200. [Google Scholar] [CrossRef]
- Coleman, A.W. The nuclear-cell cycle in Chlamydomonas (Chlorophyceae). J. Phycol. 1982, 18, 192–195. [Google Scholar] [CrossRef]
- Craigie, R.A.; Cavalier-Smith, T. Cell Volume and the Control of the Chlamydomonas Cell Cycle. J. Cell Sci. 1982, 54, 173–191. [Google Scholar] [CrossRef]
- Lien, T.; Knutsen, G. Synchronized cultures of a cell wall-less mutant of Chlamydomonas reinhardii. Arch. Microbiol. 1976, 108, 189–194. [Google Scholar] [CrossRef]
- Donnan, L.; John, P.C.L. CeII cycle control by timer and sizer in Chlamydomonas. Nature 1983, 304, 630–633. [Google Scholar] [CrossRef]
- Donnan, L.; John, P.C.L. Timer and sizer controls in the cell cycles of Chlamydomonas and Chlorella. In The Microbial Cell Cycle; Nurse, P., Streiblová, E., Eds.; CRC Press: Boca Raton, FL, USA, 1984; pp. 231–251. [Google Scholar]
- John, P.C.L. Control points in the Chlamydomonas cell cycle. In Algal Development; Wiesnar, W., Robinson, D.G., Starr, R.C., Eds.; Springer: Berlin, Germany, 1987; pp. 9–16. [Google Scholar]
- Bišová, K.; Hendrychová, J.; Cepák, V.; Zachleder, V. Cell growth and division processes are differentially sensitive to cadmium in Scenedesmus quadricauda. Folia Microbiol. 2003, 48, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.H. The Chlamydomonas Sourcebook: Introduction to Chlamydomonas and Its Laboratory Use, 2nd ed.; Harrisduke, E., Ed.; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780080919553. [Google Scholar]
- McCready, R.M.; Guggolz, J.; Silviera, V.; Owens, H.S. Determination of Starch and Amylose in Vegetables. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae-novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef]
- Řezanka, T.; Kaineder, K.; Mezricky, D.; Řezanka, M.; Bišová, K.; Zachleder, V.; Vítová, M. The effect of lanthanides on photosynthesis, growth, and chlorophyll profile of the green alga Desmodesmus quadricauda. Photosynth. Res. 2016, 130, 335–346. [Google Scholar] [CrossRef]
- Mackinney, G. Absorption of Light By Chlorophyll Solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Lukavský, J.; Simmer, J.; Kubín, Š. Buchbesprechungen. In Algal Essays and Monitoring Eutrophication; Marvan, P., Přibil, S., Lhotský, O., Eipieper, E., Eds.; Schweizerbart’sche Verlagsbuchhandlung (Nagele u. Obermiller): Stuttgart, Germany, 1979; pp. 77–85. [Google Scholar]
- Aksmann, A.; Pokora, W.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Tukaj, Z. High hydrogen peroxide production and antioxidative enzymes expression in the Chlamydomonas reinhardtii cia3 mutant with an increased tolerance to cadmium and anthracene. Phycol. Res. 2016, 64, 300–311. [Google Scholar] [CrossRef]
- Hlavová, M.; Vítová, M.; Bišová, K. Synchronization of Green Algae by Light and Dark Regimes for Cell Cycle and Cell Division Studies. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1370, pp. 3–16. ISBN 9781493931422. [Google Scholar]
- Neumann, G.; Teras, R.; Monson, L.; Kivisaar, M.; Schauer, F.; Heipieper, H.J. Simultaneous Degradation of Atrazine and Phenol by Pseudomonas sp. Strain ADP: Effects of Toxicity and Adaptation. Appl. Environ. Microbiol. 2004, 70, 1907–1912. [Google Scholar] [CrossRef] [Green Version]
- Wanka, F. Die Bestimmung der Nucleinsäuren in Chlorella-Kulturen. Planta 1962, 58, 594–609. [Google Scholar] [CrossRef]
- Decallonne, J.R.; Weyns, J.C. A shortened procedure of the diphenylamine reaction for the measurement of deoxyribonucleic acid by using light activation. Anal. Biochem. 1976, 74, 448–456. [Google Scholar] [CrossRef]
- Zachleder, V. Optimization of nucleic acids assay in green and blue-green algae: Extraction procedures and the light-activated diphenylamine reaction for DNA. Algol. Stud. Hydrobiol. Suppl. Vol. 1984, 67, 313–328. [Google Scholar] [CrossRef]
- Lowry, O.H.; Randall, R.J. Protein Measurement byt the Folin Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Majewska, M.; Harshkova, D.; Pokora, W.; Baścik-Remisiewicz, A.; Tułodziecki, S.; Aksmann, A. Does diclofenac act like a photosynthetic herbicide on green algae? Chlamydomonas reinhardtii synchronous culture-based study with atrazine as reference. Ecotoxicol. Environ. Saf. 2021, 208, 111630. [Google Scholar] [CrossRef] [PubMed]
- Harshkova, D.; Majewska, M.; Pokora, W.; Baścik-Remisiewicz, A.; Tułodziecki, S.; Aksmann, A. Diclofenac and atrazine restrict the growth of a synchronous Chlamydomonas reinhardtii population via various mechanisms. Aquat. Toxicol. 2021, 230, 105698. [Google Scholar] [CrossRef]
- Brain, R.A.; Hanson, M.L.; Solomon, K.R.; Brooks, B.W. Aquatic Plants Exposed to Pharmaceuticals: Effects and Risks. In Reviews of Environmental Contamination and Toxicology; Springer: Amsterdam, The Netherlands, 2008; Volume 192, pp. 67–115. ISBN 9780387717234. [Google Scholar]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the marine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Liang, Y.; Kashdan, T.; Sterner, C.; Dombrowski, L.; Petrick, I.; Kröger, M.; Höfer, R. Algal Biorefineries. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 35–90. ISBN 9780444634535. [Google Scholar]
- Torres-Romero, I.; Kong, F.; Légeret, B.; Beisson, F.; Peltier, G.; Li-Beisson, Y. Chlamydomonas cell cycle mutant crcdc5 over-accumulates starch and oil. Biochimie 2020, 169, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Vítová, M.; Bišová, K.; Hlavová, M.; Kawano, S.; Zachleder, V.; Čížková, M. Chlamydomonas reinhardtii: Duration of its cell cycle and phases at growth rates affected by temperature. Planta 2011, 234, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Ye, Q.; Zhang, J.; Richards, J.; Borchardt, D.; Gan, J. Diclofenac in Arabidopsis cells: Rapid formation of conjugates. Environ. Pollut. 2017, 222, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Pluciński, B.; Kuczyńska, P.; Kruk, J. Physiological characterization of Chlamydomonas reinhardtii acclimated to chronic stress induced by Ag, Cd, Cr, Cu and Hg ions. Ecotoxicol. Environ. Saf. 2016, 130, 133–145. [Google Scholar] [CrossRef]
- Khona, D.K.; Shirolikar, S.M.; Gawde, K.K.; Hom, E.; Deodhar, M.A.; D’Souza, J.S. Characterization of salt stress-induced palmelloids in the green alga, Chlamydomonas reinhardtii. Algal Res. 2016, 16, 434–448. [Google Scholar] [CrossRef]
- Valle, B.L.; D’Souza, T.; Becker, K.G.; Wood, W.H.; Zhang, Y.; Wersto, R.P.; Morin, P.J. Non-Steroidal Anti-inflammatory Drugs Decrease E2F1 Expression and Inhibit Cell Growth in Ovarian Cancer Cells. PLoS ONE 2013, 8, e61836. [Google Scholar] [CrossRef] [Green Version]
- Zachleder, V.; Van Den Ende, H. Cell cycle events in the green alga Chlamydomonas eugametos and their control by environmental factors. J. Cell Sci. 1992, 102, 469–474. [Google Scholar] [CrossRef]
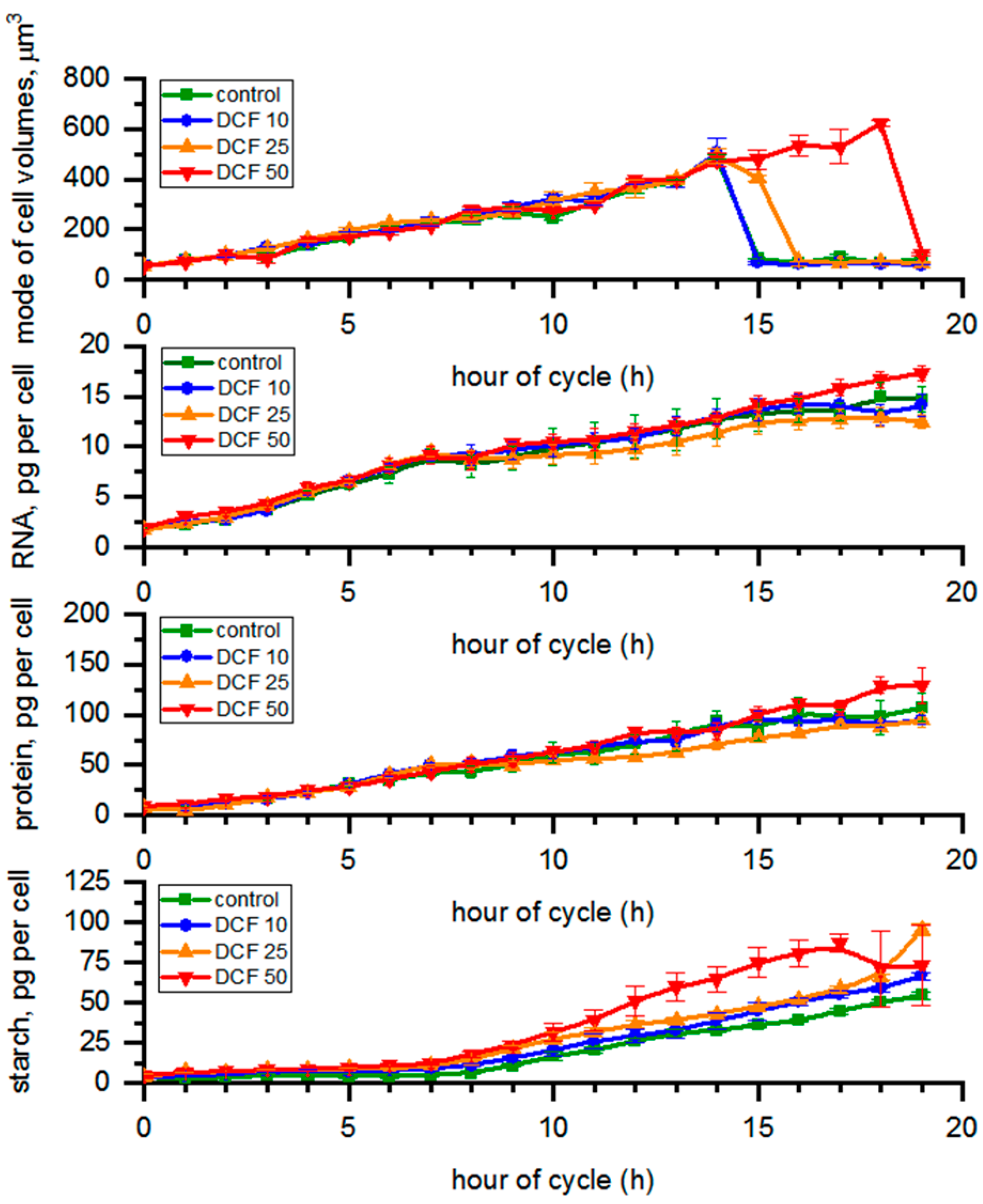
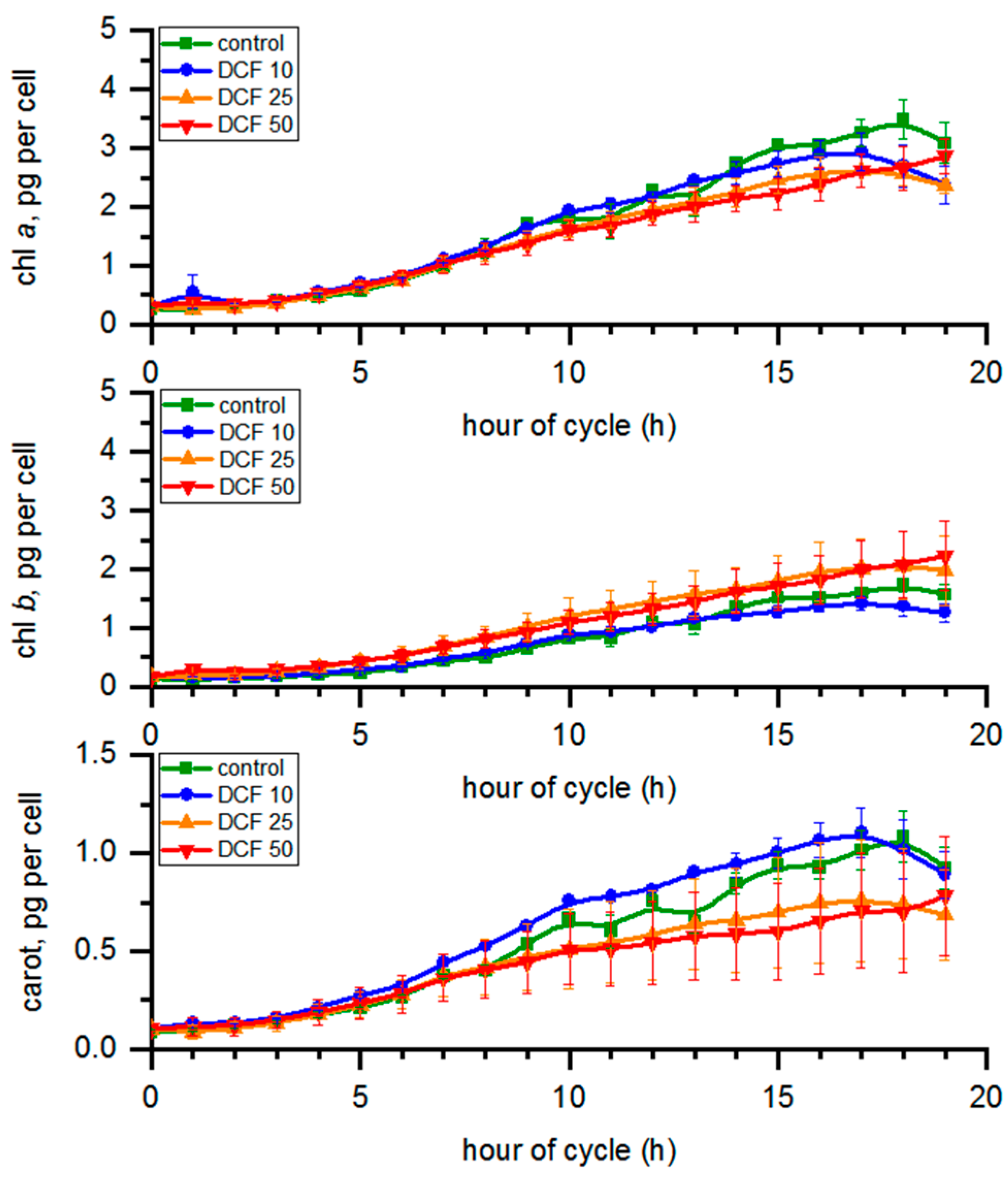
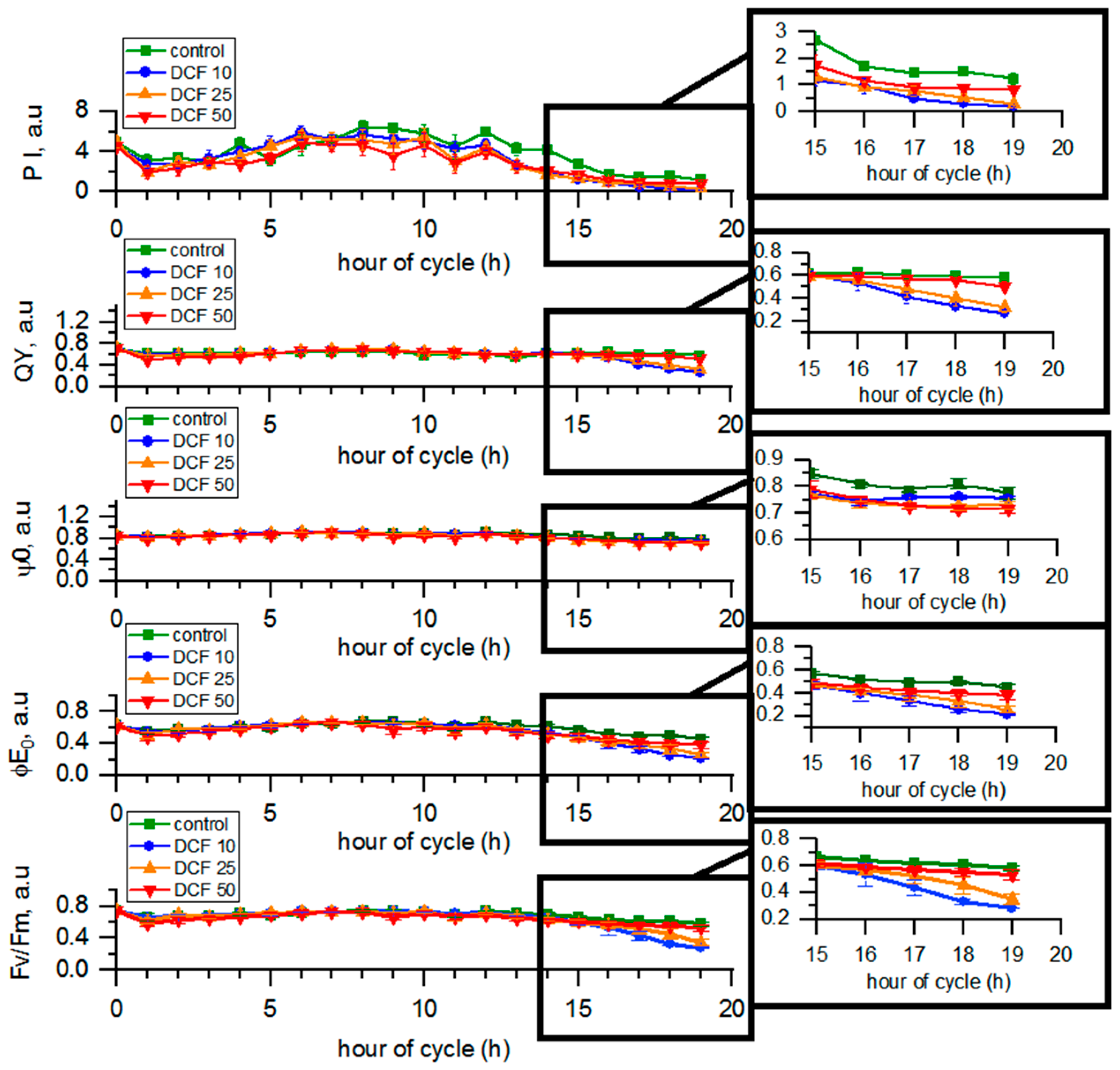
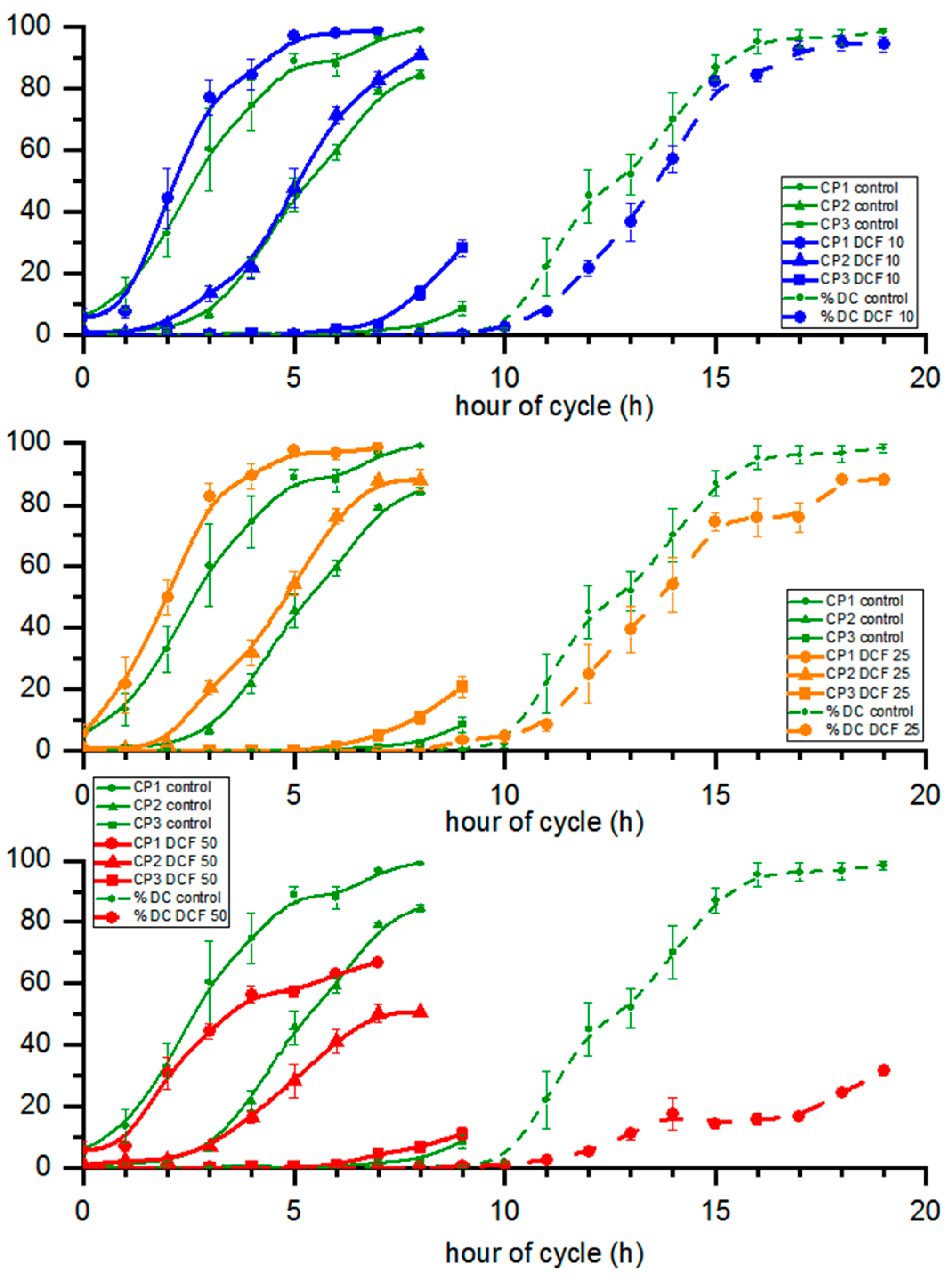

| Td (h) | DN | VMC (µm3) | VDC (µm3) | |
|---|---|---|---|---|
| control | 10.42 ± 0.40 | 6.20 ± 0.44 | 461.30 ± 23.77 | 39.88 ± 2.64 |
| DCF10 | 12.61 * ± 0.54 | 4.51 * ± 0.26 | 505.87 ± 55.46 | 44.62 ± 2.80 |
| DCF25 | 12.70 * ± 0.25 | 4.46 * ± 0.13 | 491.83 ± 31.67 | 51.20 * ± 3.86 |
| DCF50 | 16.08 * ± 1.16 | 3.26 * ± 0.30 | 616.20 * ± 10.45 | 32.06 ± 6.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harshkova, D.; Liakh, I.; Bialevich, V.; Ondrejmišková, K.; Aksmann, A.; Bišová, K. Diclofenac Alters the Cell Cycle Progression of the Green Alga Chlamydomonas reinhardtii. Cells 2021, 10, 1936. https://doi.org/10.3390/cells10081936
Harshkova D, Liakh I, Bialevich V, Ondrejmišková K, Aksmann A, Bišová K. Diclofenac Alters the Cell Cycle Progression of the Green Alga Chlamydomonas reinhardtii. Cells. 2021; 10(8):1936. https://doi.org/10.3390/cells10081936
Chicago/Turabian StyleHarshkova, Darya, Ivan Liakh, Vitali Bialevich, Kamila Ondrejmišková, Anna Aksmann, and Kateřina Bišová. 2021. "Diclofenac Alters the Cell Cycle Progression of the Green Alga Chlamydomonas reinhardtii" Cells 10, no. 8: 1936. https://doi.org/10.3390/cells10081936
APA StyleHarshkova, D., Liakh, I., Bialevich, V., Ondrejmišková, K., Aksmann, A., & Bišová, K. (2021). Diclofenac Alters the Cell Cycle Progression of the Green Alga Chlamydomonas reinhardtii. Cells, 10(8), 1936. https://doi.org/10.3390/cells10081936









