Mitochondrial Dysfunction and Mitophagy in Fuchs Endothelial Corneal Dystrophy
Abstract
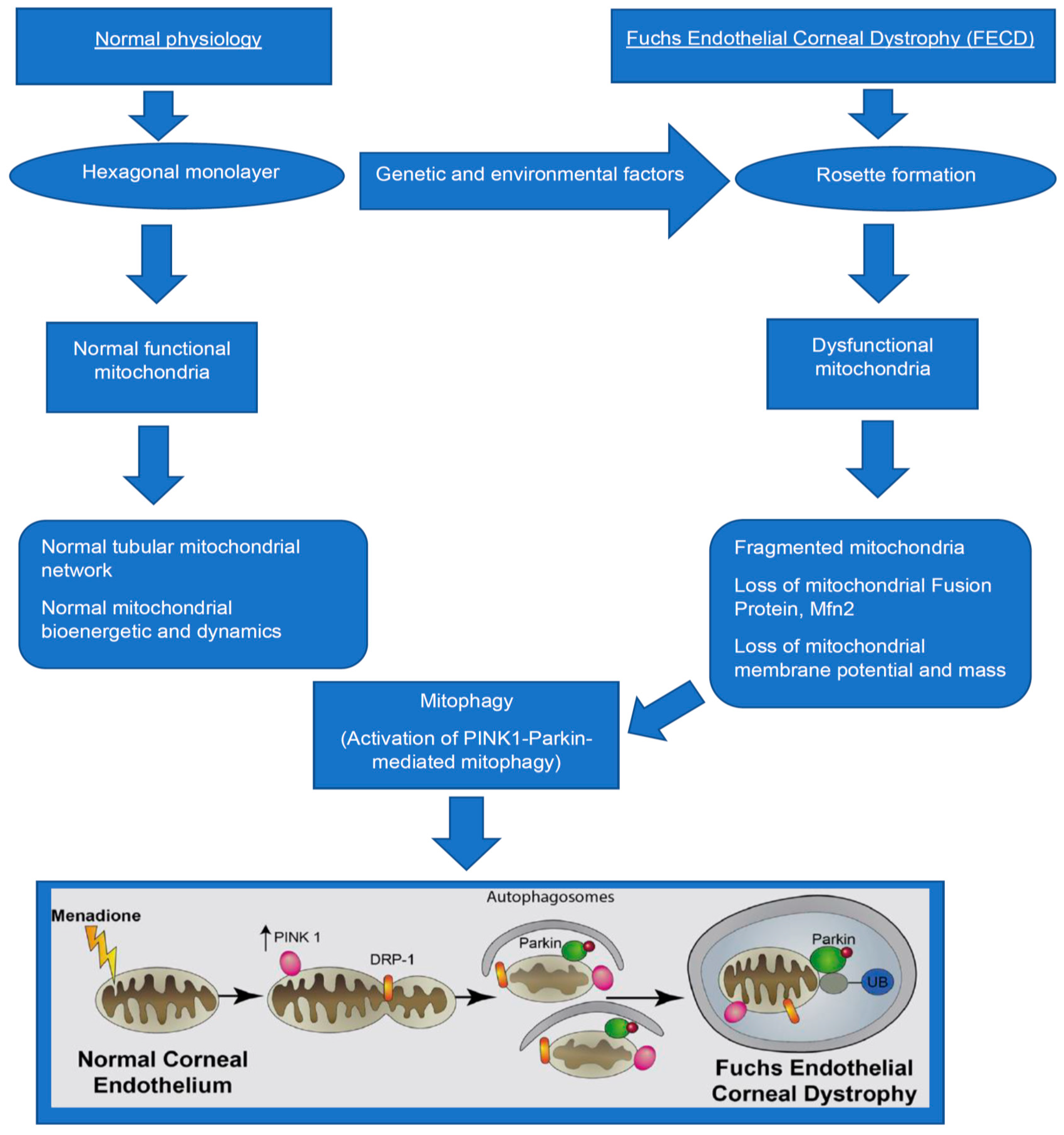
1. Introduction
2. Mitochondria in CEnCs
3. Mitochondrial DNA Damage in FECD
4. Mitochondrial Dysfunction in FECD
5. Autophagy and Mitophagy
6. Mechanisms of Mitophagy in FECD
7. Role of Mitophagy in FECD and Other Neurodegenerative Diseases
8. Unanswered Questions
- (a)
- Is the activation of mitophagy beneficial or detrimental to CEnC survival, thereby playing a bidirectional role in FECD?
- (b)
- How does lysosome play a role in the clearance of autophagic structures containing mitochondria in FECD?
- (c)
- How are the mechanisms of mitophagy in FECD different from neurodegenerative diseases or cancer?
- (d)
- Does mitophagy play a context-dependent role at various stages of FECD?
- (e)
- How can we use mitophagy as a therapeutic option for FECD?
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Bell, W.R.; Sundin, O.H.; de La Cruz, Z.; Stark, W.J.; Green, W.R.; Gottsch, J.D. Immunohistochemistry and electron microscopy of early-onset Fuchs corneal dystrophy in three cases with the same L450W COL8A2 mutation. Trans. Am. Oph-Thalmol. Soc. 2006, 104, 85–97. [Google Scholar]
- Krachmer, J.H.; Purcell, J.J., Jr.; Young, C.W.; Bucher, K.D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 1978, 96, 2036–2039. [Google Scholar] [CrossRef]
- Rosenblum, P.; Stark, W.J.; Maumenee, I.H.; Hirst, L.W.; Maumenee, A.E. Hereditary Fuchs’ dystrophy. Am. J. Ophthalmol. 1980, 90, 455–462. [Google Scholar] [CrossRef]
- Bahn, C.F.; Falls, H.F.; Varley, G.A.; Meyer, R.F.; Edelhauser, H.F.; Bourne, W.M. Classification of corneal endothelial disorders based on neural crest origin. Ophthalmology 1984, 91, 558–563. [Google Scholar] [CrossRef]
- Goyer, B.; Thériault, M.; Gendron, S.P.; Brunette, I.; Rochette, P.; Proulx, S. Extracellular matrix and integrin expression profiles in Fuchs endothelial corneal dystrophy cells and tissue model. Tissue Eng. Part A 2018, 24, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.M.; Zenkel, M.; Schlötzer-Schrehardt, U.; Bachmann, B.O.; Tourtas, T.; Kruse, F.E. Extracellular matrix alterations in late-onset Fuchs’ corneal dystrophy. Investig. Opthalmol. Vis. Sci. 2014, 55, 3700–3708. [Google Scholar] [CrossRef]
- Poulsen, E.T.; Dyrlund, T.F.; Runager, K.; Scavenius, C.; Krogager, T.P.; Højrup, P.; Thøgersen, I.B.; Sanggaard, K.W.; Vorum, H.; Hjortdal, J.; et al. Proteomics of Fuchs’ endothelial corneal dystrophy support that the extracellular matrix of descemet’s membrane is disordered. J. Proteome Res. 2014, 13, 4659–4667. [Google Scholar] [CrossRef]
- Matthaei, M.; Meng, H.; Meeker, A.K.; Eberhart, C.G.; Jun, A.S. Endothelial Cdkn1a (p21) overexpression and accelerated se-nescence in a mouse model of Fuchs endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6718–6727. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Bitar, M.S.; Funaki, T.; Azizi, B. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. Am. J. Pathol. 2010, 177, 2278–2289. [Google Scholar] [CrossRef]
- Liu, C.; Miyajima, T.; Melangath, G.; Miyai, T.; Vasanth, S.; Deshpande, N.; Kumar, V.; Tone, S.O.; Gupta, R.; Zhu, S.; et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. USA 2020, 117, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Halilovic, A.; Schmedt, T.; Benischke, A.-S.; Hamill, C.; Chen, Y.; Santos, J.H.; Jurkunas, U.V. Menadione-induced DNA damage leads to mitochondrial dysfunction and fragmentation during rosette formation in Fuchs endothelial corneal dystrophy. Antioxid. Redox Signal. 2016, 24, 1072–1083. [Google Scholar] [CrossRef]
- Engler, C.; Kelliher, C.; Spitze, A.R.; Speck, C.L.; Eberhart, C.G.; Jun, A.S. Unfolded protein response in Fuchs endothelial corneal dystrophy: A unifying pathogenic pathway? Am. J. Ophthalmol. 2010, 149, 194–202.e2. [Google Scholar] [CrossRef]
- Okumura, N.; Hashimoto, K.; Kitahara, M.; Okuda, H.; Ueda, E.; Watanabe, K.; Nakahara, M.; Sato, T.; Kinoshita, S.; Tourtas, T.; et al. Activation of TGF-beta signaling induces cell death via the unfolded protein response in Fuchs endothelial corneal dys-trophy. Sci. Rep. 2017, 7, 6801. [Google Scholar] [CrossRef]
- Okumura, N.; Kitahara, M.; Okuda, H.; Hashimoto, K.; Ueda, E.; Nakahara, M.; Kinoshita, S.; Young, R.D.; Quantock, A.J.; Tourtas, T.; et al. Sustained activation of the unfolded protein response induces cell death in Fuchs’ endothelial corneal dystrophy. Investig. Opthalmol. Vis. Sci. 2017, 58, 3697–3707. [Google Scholar] [CrossRef]
- Benischke, A.S.; Vasanth, S.; Miyai, T.; Katikireddy, K.R.; White, T.; Chen, Y.; Halilovic, A.; Price, M.; Price, F., Jr.; Liton, P.B.; et al. Activation of mitophagy leads to decline in Mfn2 and loss of mitochondrial mass in Fuchs endothelial corneal dystrophy. Sci. Rep. 2017, 7, 6656. [Google Scholar] [CrossRef]
- Méthot, S.J.; Proulx, S.; Brunette, I.; Rochette, P.J. Chronology of cellular events related to mitochondrial burnout leading to cell death in Fuchs endothelial corneal dystrophy. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Li, Y.J.; Minear, M.A.; Qin, X.; Rimmler, J.; Hauser, M.A.; Allingham, R.R.; Igo, R.P.; Lass, J.H.; Iyengar, S.K.; Klintworth, G.K.; et al. Mitochondrial polymorphism A10398G and Haplogroup I are associated with Fuchs’ endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4577–4584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyai, T.; Vasanth, S.; Melangath, G.; Deshpande, N.; Kumar, V.; Benischke, A.-S.; Chen, Y.; Price, M.O.; Price, F.W.; Jurkunas, U.V. Activation of PINK1-parkin–mediated mitophagy degrades mitochondrial quality control proteins in Fuchs endothelial corneal dystrophy. Am. J. Pathol. 2019, 189, 2061–2076. [Google Scholar] [CrossRef] [PubMed]
- Barfort, P.; Maurice, D. Electrical potential and fluid transport across the corneal endothelium. Exp. Eye Res. 1974, 19, 11–19. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Hogan, M.J. Histology of the Human Eye: An Atlas and Textbook; Saunders: Philadelphia, PA, USA, 1971. [Google Scholar]
- Suomalainen, A.; Battersby, B.J. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef]
- Tzameli, I. The evolving role of mitochondria in metabolism. Trends Endocrinol. Metab. 2012, 23, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Weissman, L.; Bohr, V.A.; Mattson, M.P. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair 2008, 7, 1110–1120. [Google Scholar] [CrossRef]
- Jarrett, S.; Lin, H.; Godley, B.F.; Boulton, M.E. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog. Retin. Eye Res. 2008, 27, 596–607. [Google Scholar] [CrossRef]
- Vallabh, N.A.; Romano, V.; Willoughby, C.E. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion 2017, 36, 103–113. [Google Scholar] [CrossRef]
- Czarny, P.; Seda, A.; Wielgorski, M.; Binczyk, E.; Markiewicz, B.; Kasprzak, E.; Jimenez-Garcia, M.P.; Grabska-Liberek, I.; Pawlowska, E.; Blasiak, J.; et al. Mutagenesis of mitochondrial DNA in Fuchs endothelial corneal dystrophy. Mutat. Res. Mol. Mech. Mutagen. 2014, 760, 42–47. [Google Scholar] [CrossRef]
- Gendron, S.P.; Thériault, M.; Proulx, S.; Brunette, I.; Rochette, P.J. Restoration of mitochondrial integrity, telomere length, and sensitivity to oxidation by in vitro culture of Fuchs’ endothelial corneal dystrophy cells. Investig. Opthalmol. Vis. Sci. 2016, 57, 5926–5934. [Google Scholar] [CrossRef]
- Miyajima, T.; Melangath, G.; Zhu, S.; Deshpande, N.; Vasanth, S.; Mondal, B.; Kumar, V.; Chen, Y.; Price, M.O.; Price, F.W.; et al. Loss of NQO1 generates genotoxic estrogen-DNA adducts in Fuchs endothelial corneal dystrophy. Free Radic. Biol. Med. 2020, 147, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Wieben, E.D.; Aleff, R.A.; Tang, X.; Kalari, K.R.; Maguire, L.J.; Patel, S.V.; Baratz, K.H.; Fautsch, M.P. Gene expression in the corneal endothelium of Fuchs endothelial corneal dystrophy patients with and without expansion of a trinucleotide repeat in TCF4. PLoS ONE 2018, 13, e0200005. [Google Scholar] [CrossRef]
- Hu, F.; Liu, F. Mitochondrial stress: A bridge between mitochondrial dysfunction and metabolic diseases? Cell. Signal. 2011, 23, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Sataranatarajan, K.; van Remmen, H. Role of signaling molecules in mitochondrial stress response. Front. Genet. 2018, 9, 225. [Google Scholar] [CrossRef]
- Hill, S.; van Remmen, H. Mitochondrial stress signaling in longevity: A new role for mitochondrial function in aging. Redox Biol. 2014, 2, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Payne, B.A.I.; Chinnery, P.F. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 1347–1353. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.-M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Borboli, S.; Colby, K. Mechanisms of disease: Fuchs’ endothelial dystrophy. Ophthalmol. Clin. N. Am. 2002, 15, 17–25. [Google Scholar] [CrossRef]
- Tuberville, A.W.; Wood, T.O.; McLaughlin, B.J. Cytochrome oxidase activity of Fuchs’ endothelial dystrophy. Curr. Eye Res. 1986, 5, 939–947. [Google Scholar] [CrossRef]
- Xavier, R.J.; Huett, A.; Rioux, J.D. Autophagy as an important process in gut homeostasis and Crohn’s disease pathogenesis. Gut 2008, 57, 717–720. [Google Scholar] [CrossRef]
- Meng, H.; Matthaei, M.; Ramanan, N.; Grebe, R.; Chakravarti, S.; Speck, C.L.; Kimos, M.; Vij, N.; Eberhart, C.G.; Jun, A.S. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1887–1897. [Google Scholar] [CrossRef]
- Kim, E.C.; Meng, H.; Jun, A.S. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br. J. Ophthalmol. 2013, 97, 1068–1073. [Google Scholar] [CrossRef]
- Lahiri, V.; Klionsky, D.J. Functional impairment in RHOT1/Miro1 degradation and mitophagy is a shared feature in familial and sporadic Parkinson disease. Autophagy 2017, 13, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkiran, J.A.; Reddy, P.H. Defective mitophagy in Alzheimer’s disease. Ageing Res. Rev. 2020, 64, 1–101191. [Google Scholar] [CrossRef]
- Hwang, S.; Disatnik, M.H.; Mochly-Rosen, D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO Mol. Med. 2015, 7, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.S.; Tungtur, S.; Tanaka, T.; Nadeau, L.L.; Badawi, Y.; Wang, H.; Ni, H.-M.; Ding, W.-X.; Nishimune, H. Impaired mitophagy plays a role in denervation of neuromuscular junctions in ALS mice. Front. Neurosci. 2017, 11, 473. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Khalil, B.D.; El Fissi, N.; Aouane, A.; Cabirol-Pol, M.-J.; Rival, T.; Liévens, J.-C. PINK1-induced mitophagy promotes neuroprotection in Huntington’s disease. Cell Death Dis. 2015, 6, e1617. [Google Scholar] [CrossRef]
- Koentjoro, B.; Park, J.-S.; Sue, C.M. Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson’s disease. Sci. Rep. 2017, 7, srep44373. [Google Scholar] [CrossRef]
- Subramaniam, S. Exaggerated mitophagy: A weapon of striatal destruction in the brain? Biochem. Soc. Trans. 2020, 48, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-H.; Wu, Y.-F.; Wang, D.-P.; Hai, J. Inhibition of excessive autophagy and mitophagy mediates neuroprotective effects of URB597 against chronic cerebral hypoperfusion. Cell Death Dis. 2018, 9, 733. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Jurkunas, U.V. Mitochondrial Dysfunction and Mitophagy in Fuchs Endothelial Corneal Dystrophy. Cells 2021, 10, 1888. https://doi.org/10.3390/cells10081888
Kumar V, Jurkunas UV. Mitochondrial Dysfunction and Mitophagy in Fuchs Endothelial Corneal Dystrophy. Cells. 2021; 10(8):1888. https://doi.org/10.3390/cells10081888
Chicago/Turabian StyleKumar, Varun, and Ula V. Jurkunas. 2021. "Mitochondrial Dysfunction and Mitophagy in Fuchs Endothelial Corneal Dystrophy" Cells 10, no. 8: 1888. https://doi.org/10.3390/cells10081888
APA StyleKumar, V., & Jurkunas, U. V. (2021). Mitochondrial Dysfunction and Mitophagy in Fuchs Endothelial Corneal Dystrophy. Cells, 10(8), 1888. https://doi.org/10.3390/cells10081888





