Age-Dependent Distinct Distributions of Dendritic Cells in Autoimmune Dry Eye Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
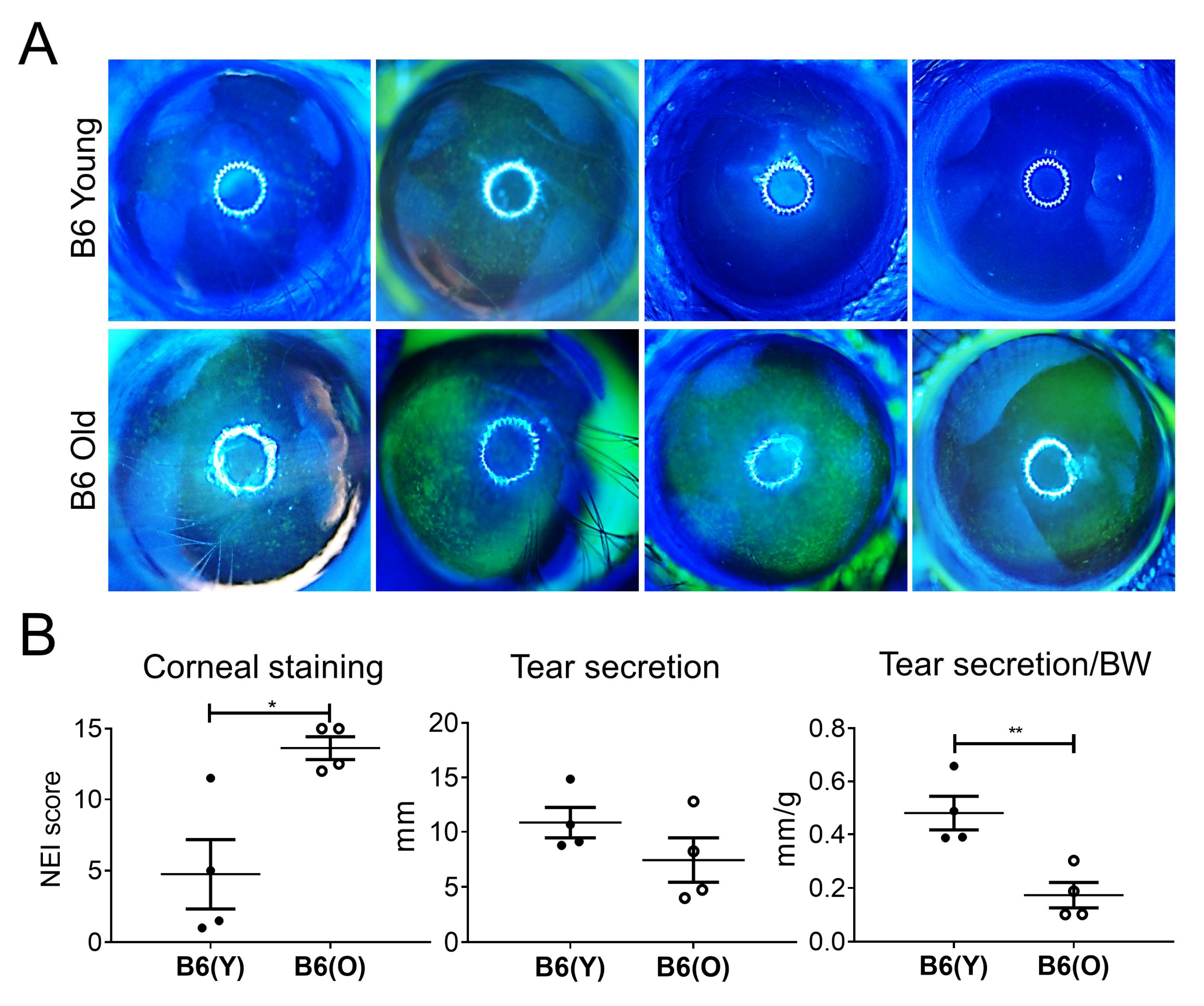
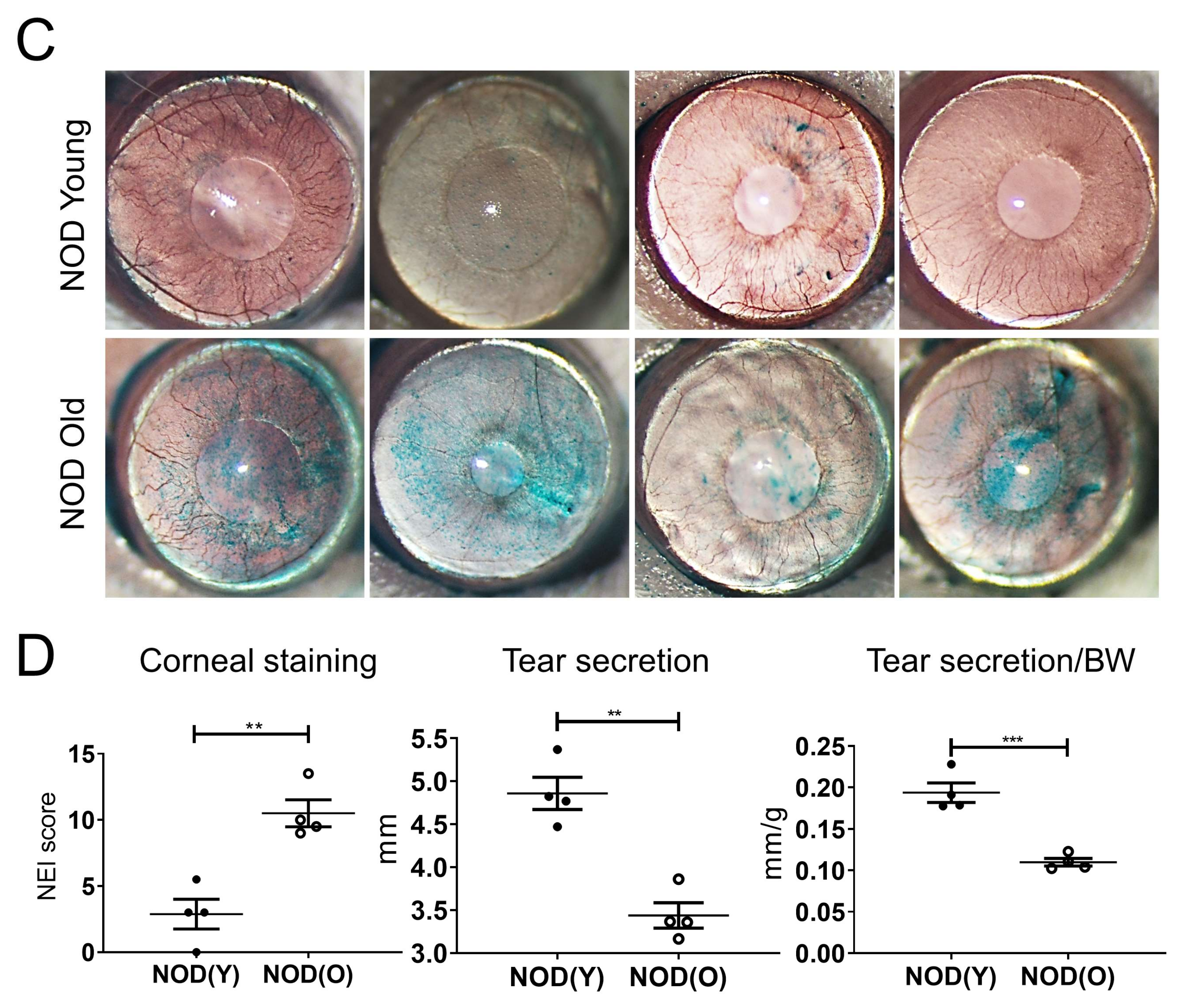
2.2. Clinical Evaluation of the Dry Eye
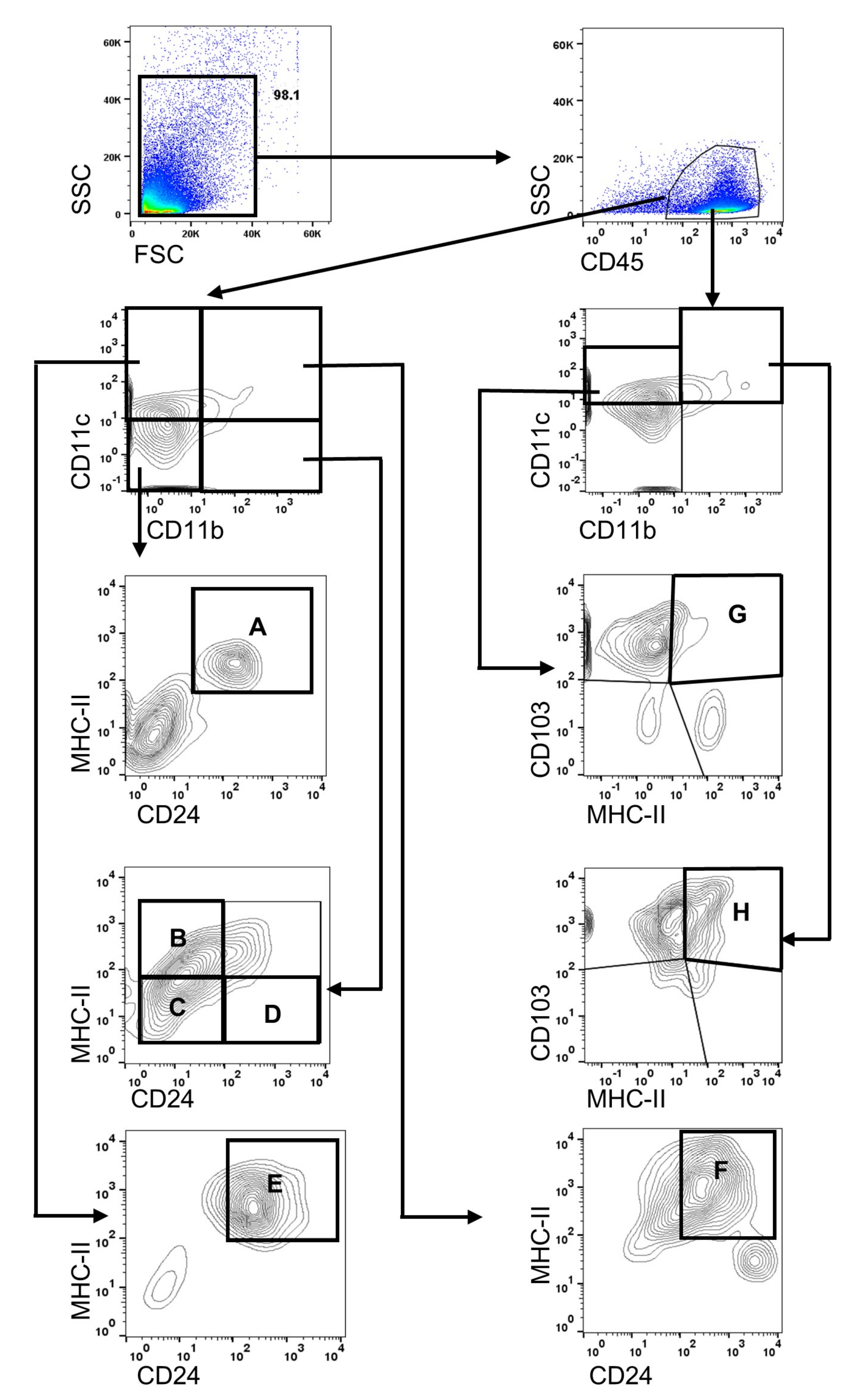
2.3. Flow Cytometry for Analyzing Immune Cells
2.4. Statistical Analyses
3. Results
3.1. Dry Eye Is Manifest in Aged B6 and NOD.B10.H2b Mice
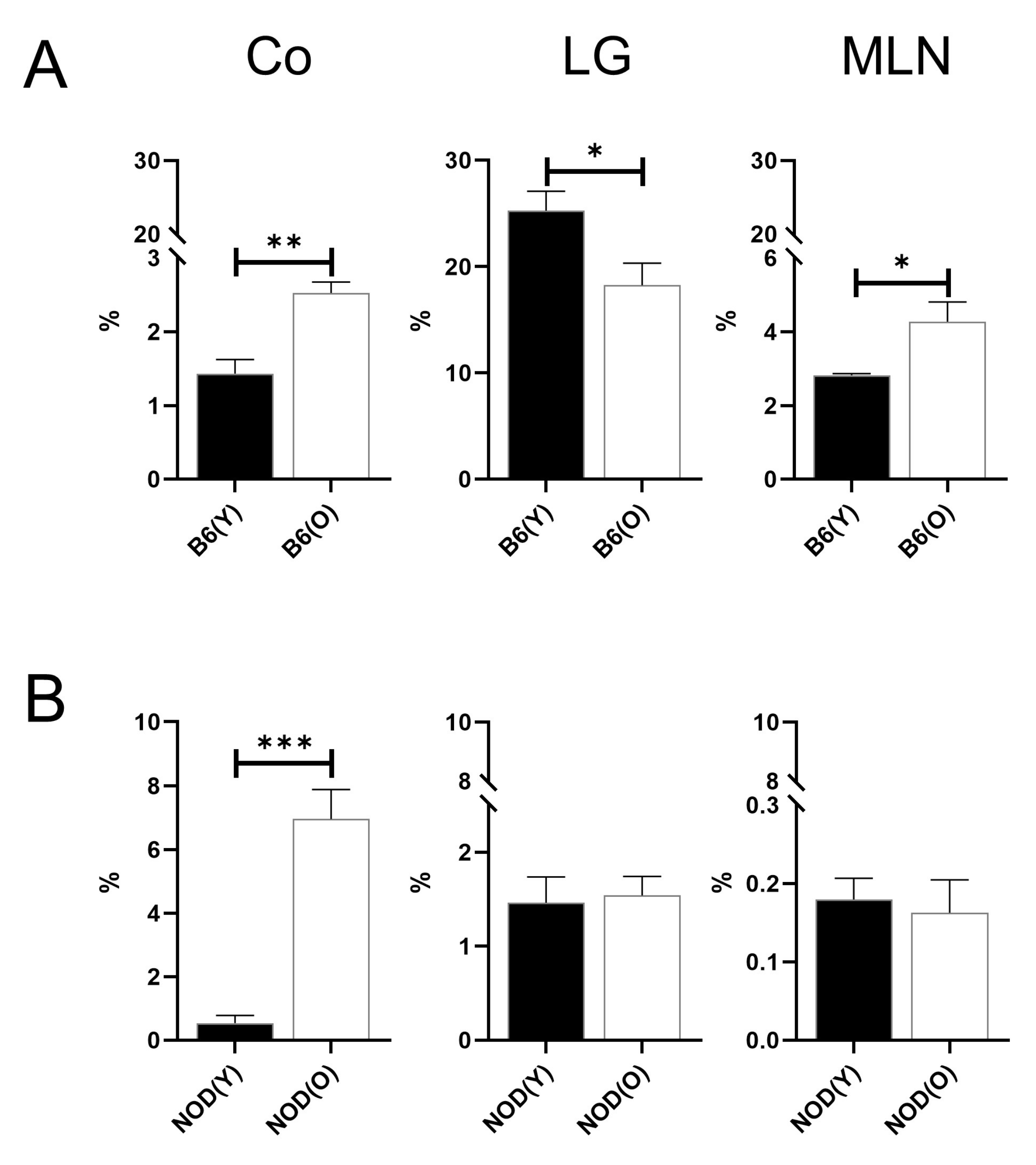
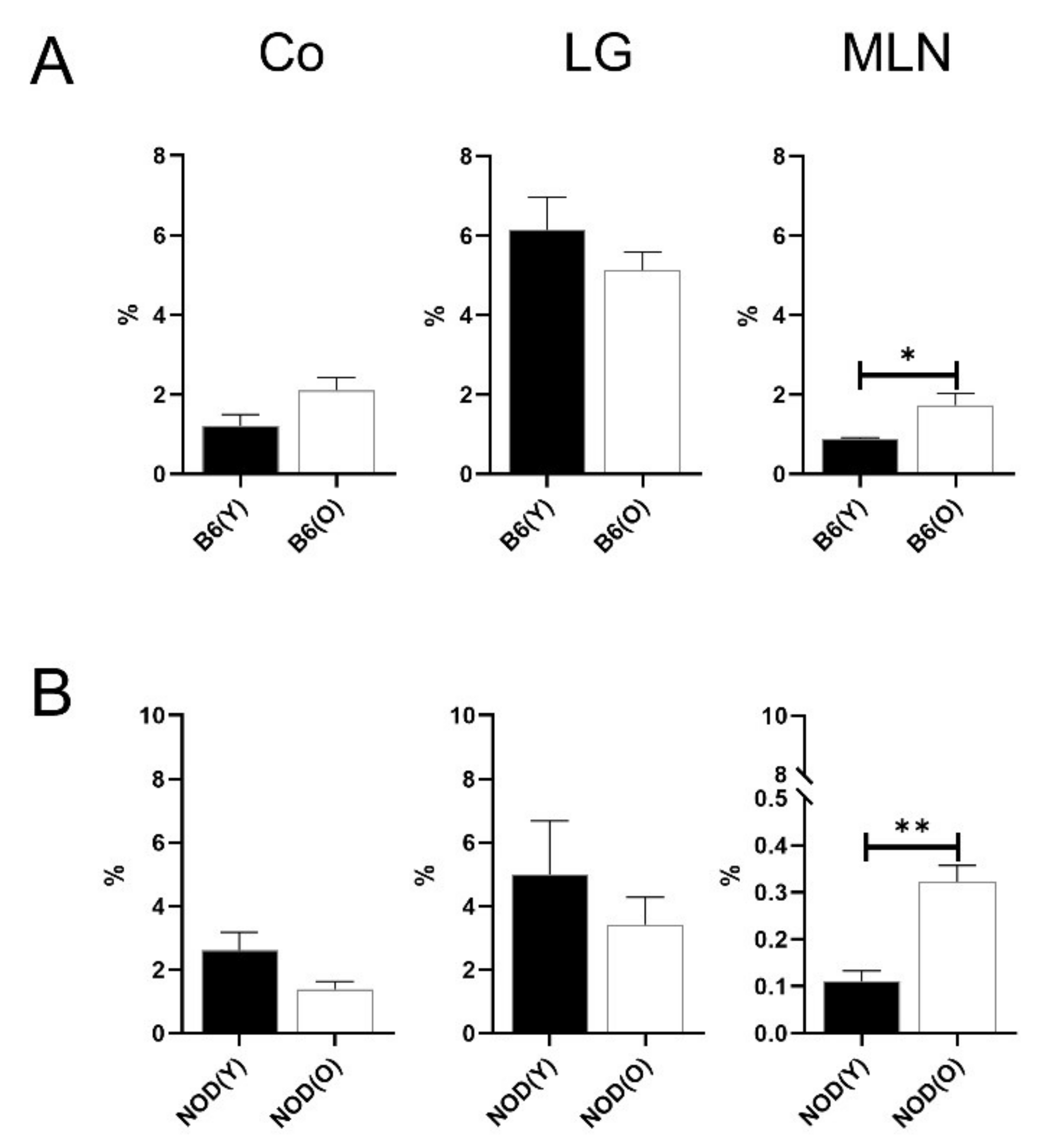
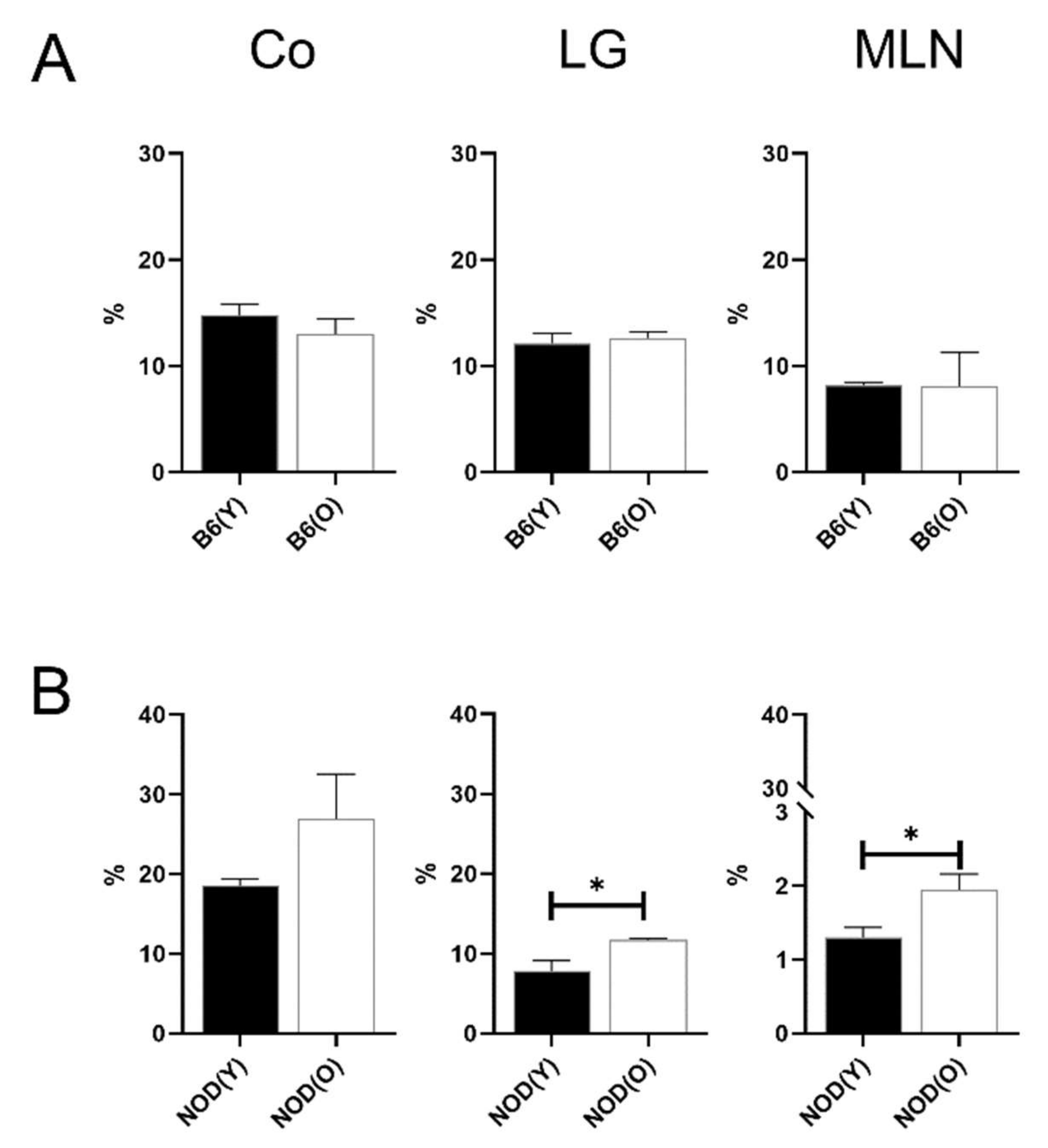
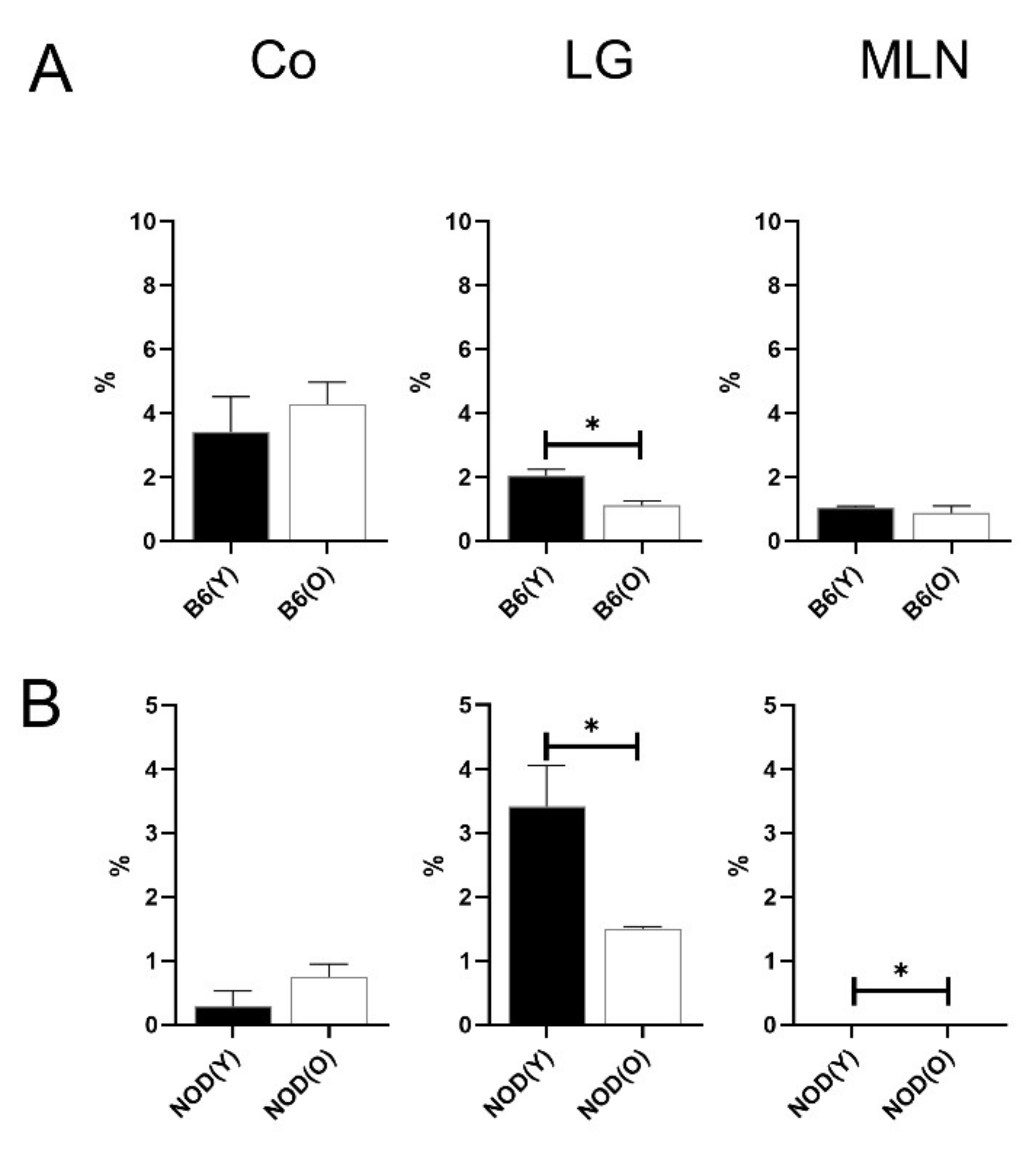
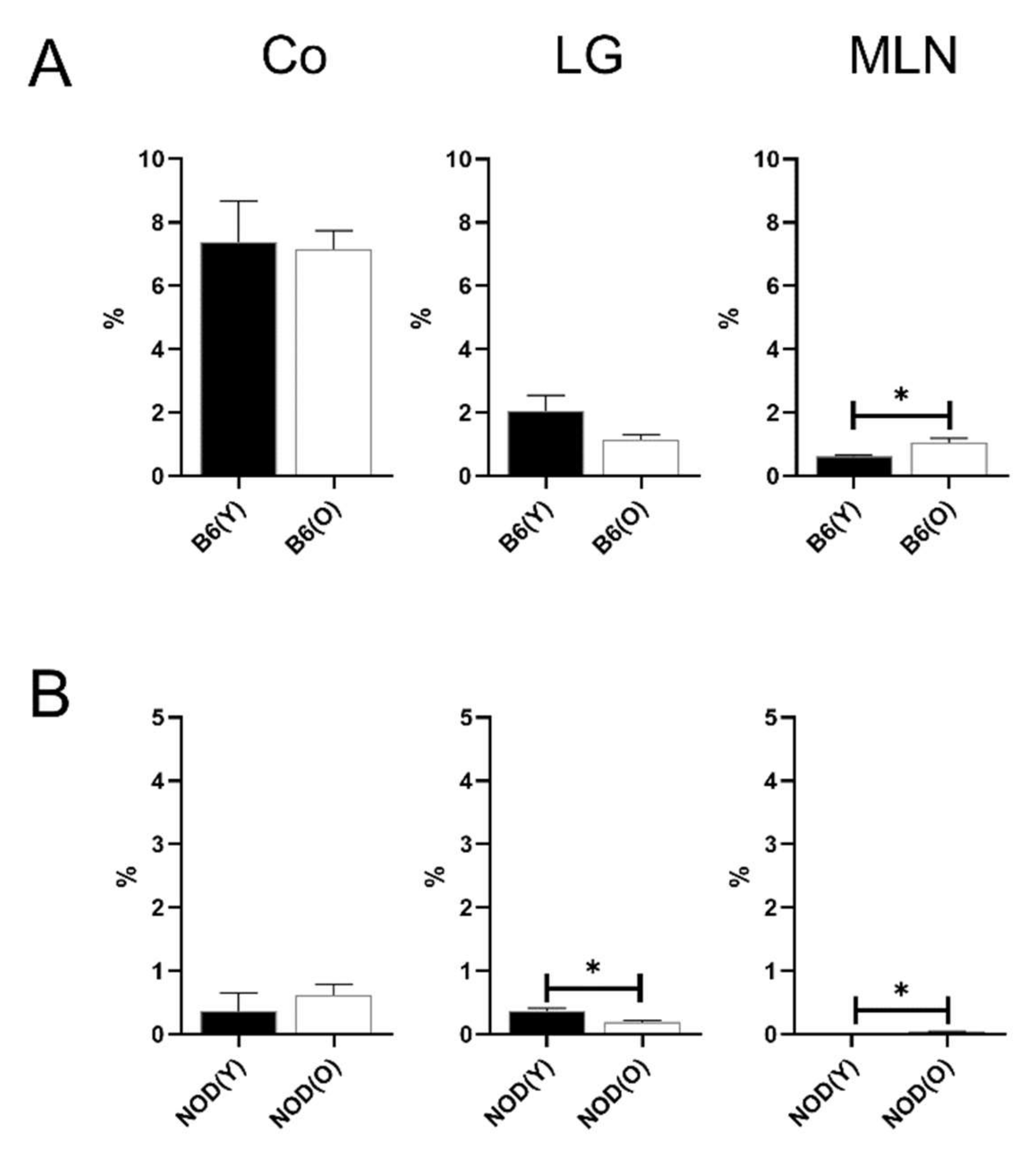
3.2. Changes of Distribution CD11b+DC Are Distinct in Aged Mice
3.3. Changes of Distribution CD103+CD11b−DC Subsets in Aged Mice
3.4. Changes of Distribution CD103+CD11b+DC Subsets in Aged Mice
3.5. Distributions of MHC-IIhi B Cells in Aged Mice
3.6. Distributions of Other Antigen Presenting Cell (APC) Subsets in Aged Mice
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med 1973, 137, 1142–1162. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Lasker Basic Medical Research Award. Dendritic cells: Versatile controllers of the immune system. Nat. Med. 2007, 13, 1155–1159. [Google Scholar] [CrossRef]
- Sojka, D.K.; Huang, Y.H.; Fowell, D.J. Mechanisms of regulatory T-cell suppression—A diverse arsenal for a moving target. Immunology 2008, 124, 13–22. [Google Scholar] [CrossRef]
- Hasegawa, H.; Matsumoto, T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Rius, A.; Desai, A.; Yuen, D.; Johnston, A.P.R.; Voelcker, N.H. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2020. [Google Scholar] [CrossRef]
- Agrawal, A.; Gupta, S. Impact of aging on dendritic cell functions in humans. Ageing Res. Rev. 2011, 10, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Xu, H.; Kuffová, L.; Dick, A.D.; McMenamin, P.G. Dendritic cell physiology and function in the eye. Immunol. Rev. 2010, 234, 282–304. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, H.R.; Humphries, T.; Clare, A.; Dixon, A.E.; Howes, K.; Moran, C.B.; Scott, D.; Zakrzewski, M.; Pearlman, E.; McMenamin, P.G. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology 2008, 125, 541–548. [Google Scholar] [CrossRef]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch. Ophthalmol. 2003, 121, 1132–1140. [Google Scholar] [CrossRef]
- Liu, Y.; Hamrah, P.; Zhang, Q.; Taylor, A.W.; Dana, M.R. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J. Exp. Med. 2002, 195, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Liu, Y.; Zhang, Q.; Dana, M.R. The corneal stroma is endowed with a significant number of resident dendritic cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Schonberg, A.; Hamdorf, M.; Bock, F. Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 280. [Google Scholar] [CrossRef]
- Maruoka, S.; Inaba, M.; Ogata, N. Activation of Dendritic Cells in Dry Eye Mouse Model. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3269–3277. [Google Scholar] [CrossRef]
- Vogelsang, P.; Jonsson, M.V.; Dalvin, S.T.; Appel, S. Role of dendritic cells in Sjögren’s syndrome. Scand. J. Immunol. 2006, 64, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Zhang, Q.; Liu, Y.; Dana, M.R. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 639–646. [Google Scholar]
- Dana, M.R. Corneal antigen-presenting cells: Diversity, plasticity, and disguise: The Cogan lecture. Investig. Ophthalmol. Vis. Sci. 2004, 45, 722–727. [Google Scholar] [CrossRef][Green Version]
- Egan, R.M.; Yorkey, C.; Black, R.; Loh, W.K.; Stevens, J.L.; Storozynsky, E.; Lord, E.M.; Frelinger, J.G.; Woodward, J.G. In vivo behavior of peptide-specific T cells during mucosal tolerance induction: Antigen introduced through the mucosa of the conjunctiva elicits prolonged antigen-specific T cell priming followed by anergy. J. Immunol. 2000, 164, 4543–4550. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef]
- Stern, M.E.; Gao, J.; Schwalb, T.A.; Ngo, M.; Tieu, D.D.; Chan, C.C.; Reis, B.L.; Whitcup, S.M.; Thompson, D.; Smith, J.A. Conjunctival T-cell subpopulations in Sjögren’s and non-Sjögren’s patients with dry eye. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2609–2614. [Google Scholar]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.H.; Klein, B.E.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef]
- de Paiva, C.S. Effects of Aging in Dry Eye. Int. Ophthalmol. Clin. 2017, 57, 47–64. [Google Scholar] [CrossRef]
- Lee, H.S.; Amouzegar, A.; Dana, R. Kinetics of Corneal Antigen Presenting Cells in Experimental Dry Eye Disease. BMJ Open Ophthalmol. 2017, 1, e000078. [Google Scholar] [CrossRef]
- Perez, V.L.; Stern, M.E.; Pflugfelder, S.C. Inflammatory basis for dry eye disease flares. Exp. Eye Res. 2020, 108294. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.; Chao, C.; Jamali, A.; Seyed-Razavi, Y.; Kenyon, B.; Harris, D.L.; Zoukhri, D.; Hamrah, P. Effect of Dry Eye Disease on the Kinetics of Lacrimal Gland Dendritic Cells as Visualized by Intravital Multi-Photon Microscopy. Front. Immunol. 2020, 11, 1713. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Oh, J.W.; Ryu, J.S.; Kim, H.M.; Im, S.H.; Kim, K.P.; Kim, M.K. IRT5 Probiotics Changes Immune Modulatory Protein Expression in the Extraorbital Lacrimal Glands of an Autoimmune Dry Eye Mouse Model. Investig. Ophthalmol. Vis. Sci. 2020, 61, 42. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Yoon, C.H.; Choi, S.H.; Kim, M.K. Can Gut Microbiota Affect Dry Eye Syndrome? Int. J. Mol. Sci. 2020, 21, 8443. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Choi, S.H.; Yoon, C.H.; Kim, M.K. Gut dysbiosis is prevailing in Sjögren’s syndrome and is related to dry eye severity. PLoS ONE 2020, 15, e0229029. [Google Scholar] [CrossRef] [PubMed]
- Messner, A.; Fischak, C.; Pfister, M.; Schutzenberger, K.; Garreis, F.; Paulsen, F.; Stegmann, H.; Aranha Dos Santos, V.; Garhofer, G.; Schmetterer, L.; et al. Characterization of dry eye disease in a mouse model by optical coherence tomography and fluorescein staining. Biomed. Opt. Express 2019, 10, 4884–4895. [Google Scholar] [CrossRef] [PubMed]
- Burade, V.; Zalawadia, R.; Patel, A.; Ogundele, A. Preclinical Efficacy Comparison of Cyclosporine Ophthalmic Solution 0.09% vs Cyclosporine Ophthalmic Emulsion 0.05% vs Ciclosporin Ophthalmic Emulsion 0.1% in a NOD Mouse Model of Dry Eye Disease. Clin. Ophthalmol. 2020, 14, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, D.H.; Ryu, J.S.; Ko, A.Y.; Ko, J.H.; Kim, M.K.; Wee, W.R.; Khwarg, S.I.; Oh, J.Y. Topical TSG-6 Administration Protects the Ocular Surface in Two Mouse Models of Inflammation-Related Dry Eye. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5175–5181. [Google Scholar] [CrossRef]
- Yoon, C.H.; Ryu, J.S.; Hwang, H.S.; Kim, M.K. Comparative Analysis of Age-Related Changes in Lacrimal Glands and Meibomian Glands of a C57BL/6 Male Mouse Model. Int. J. Mol. Sci. 2020, 21, 4169. [Google Scholar] [CrossRef]
- Moon, J.; Ryu, J.S.; Kim, J.Y.; Im, S.H.; Kim, M.K. Effect of IRT5 probiotics on dry eye in the experimental dry eye mouse model. PLoS ONE 2020, 15, e0243176. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Ryu, J.S.; Moon, J.; Kim, M.K. Association between aging-dependent gut microbiome dysbiosis and dry eye severity in C57BL/6 male mouse model: A pilot study. BMC Microbiol. 2021, 21, 106. [Google Scholar] [CrossRef]
- Kojima, T.; Wakamatsu, T.H.; Dogru, M.; Ogawa, Y.; Igarashi, A.; Ibrahim, O.M.; Inaba, T.; Shimizu, T.; Noda, S.; Obata, H.; et al. Age-related dysfunction of the lacrimal gland and oxidative stress: Evidence from the Cu,Zn-superoxide dismutase-1 (Sod1) knockout mice. Am. J. Pathol. 2012, 180, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Zwingenberger, A.L.; Park, S.A.; Murphy, C.J. Computed tomographic imaging characteristics of the normal canine lacrimal glands. BMC Vet. Res. 2014, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.G.; de Paiva, C.S.; Alves, M.R. Age-related Autoimmune Changes in Lacrimal Glands. Immune. Netw. 2019, 19, e3. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharm. 2020, 127, 110182. [Google Scholar] [CrossRef]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- del Rio, M.L.; Bernhardt, G.; Rodriguez-Barbosa, J.I.; Forster, R. Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 2010, 234, 268–281. [Google Scholar] [CrossRef]
- Hillen, M.R.; Pandit, A.; Blokland, S.L.M.; Hartgring, S.A.Y.; Bekker, C.P.J.; van der Heijden, E.H.M.; Servaas, N.H.; Rossato, M.; Kruize, A.A.; van Roon, J.A.G.; et al. Plasmacytoid DCs from Patients With Sjögren’s Syndrome Are Transcriptionally Primed for Enhanced Pro-inflammatory Cytokine Production. Front. Immunol. 2019, 10, 2096. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Cagnard, N.; Lucchesi, C.; Letourneur, F.; Mistou, S.; Lazure, T.; Jacques, S.; Ba, N.; Ittah, M.; Lepajolec, C.; et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 2770–2775. [Google Scholar] [CrossRef]
- Alonso-Ramirez, R.; Loisel, S.; Buors, C.; Pers, J.O.; Montero, E.; Youinou, P.; Renaudineau, Y. Rationale for Targeting CD6 as a Treatment for Autoimmune Diseases. Arthritis 2010, 2010, 130646. [Google Scholar] [CrossRef]
- Rock, K.L.; Benacerraf, B.; Abbas, A.K. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J. Exp. Med. 1984, 160, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. B cells in the pathogenesis of primary Sjogren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Dana, M.R. Corneal antigen-presenting cells. Chem. Immunol. Allergy 2007, 92, 58–70. [Google Scholar] [CrossRef]
- Saban, D.R. The chemokine receptor CCR7 expressed by dendritic cells: A key player in corneal and ocular surface inflammation. Ocul. Surf. 2014, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.; Hu, K.; Sendra, V.G.; Blanco, T.; Lopez, M.J.; Ortiz, G.; Qazi, Y.; Zheng, L.; Turhan, A.; Harris, D.L.; et al. Characterization of Resident Corneal Plasmacytoid Dendritic Cells and Their Pivotal Role in Herpes Simplex Keratitis. Cell Rep. 2020, 32, 108099. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.; Kenyon, B.; Ortiz, G.; Abou-Slaybi, A.; Sendra, V.G.; Harris, D.L.; Hamrah, P. Plasmacytoid dendritic cells in the eye. Prog. Retin. Eye Res. 2020, 100877. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.; Seyed-Razavi, Y.; Chao, C.; Ortiz, G.; Kenyon, B.; Blanco, T.; Harris, D.L.; Hamrah, P. Intravital Multiphoton Microscopy of the Ocular Surface: Alterations in Conventional Dendritic Cell Morphology and Kinetics in Dry Eye Disease. Front. Immunol. 2020, 11, 742. [Google Scholar] [CrossRef]
- Sun, T.; Nguyen, A.; Gommerman, J.L. Dendritic Cell Subsets in Intestinal Immunity and Inflammation. J. Immunol. 2020, 204, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Hattori, T.; Park, E.Y.; Stevenson, W.; Chauhan, S.K.; Dana, R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5632–5640. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, P.; Blanco-Mezquita, T.; Emami, P.; Lee, H.S.; Reyes, N.J.; Mathew, R.; Huang, R.; Saban, D.R. Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PLoS ONE 2013, 8, e64193. [Google Scholar] [CrossRef]
- Hua, J.; Stevenson, W.; Dohlman, T.H.; Inomata, T.; Tahvildari, M.; Calcagno, N.; Pirmadjid, N.; Sadrai, Z.; Chauhan, S.K.; Dana, R. Graft Site Microenvironment Determines Dendritic Cell Trafficking Through the CCR7-CCL19/21 Axis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1457–1467. [Google Scholar] [CrossRef]
- Nguyen, C.; Cornelius, J.; Singson, E.; Killedar, S.; Cha, S.; Peck, A.B. Role of complement and B lymphocytes in Sjogren’s syndrome-like autoimmune exocrinopathy of NOD.B10-H2b mice. Mol. Immunol. 2006, 43, 1332–1339. [Google Scholar] [CrossRef]
- Fasano, S.; Mauro, D.; Macaluso, F.; Xiao, F.; Zhao, Y.; Lu, L.; Guggino, G.; Ciccia, F. Pathogenesis of primary Sjogren’s syndrome beyond B lymphocytes. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 315–323. [Google Scholar] [PubMed]
- Zhao, Q.; Elson, C.O. Adaptive immune education by gut microbiota antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef]
- Sundling, C.; Ronnberg, C.; Yman, V.; Asghar, M.; Jahnmatz, P.; Lakshmikanth, T.; Chen, Y.; Mikes, J.; Forsell, M.N.; Sonden, K.; et al. B cell profiling in malaria reveals expansion and remodelling of CD11c+ B cell subsets. JCI Insight 2019, 5. [Google Scholar] [CrossRef]
- McClellan, A.J.; Volpe, E.A.; Zhang, X.; Darlington, G.J.; Li, D.Q.; Pflugfelder, S.C.; de Paiva, C.S. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am. J. Pathol. 2014, 184, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E.; Boggs, L.; Attia, R.; Cooper, L.R.; Saban, D.R.; Nguyen, C.Q.; Peck, A.B. Autoimmune dacryoadenitis of NOD/LtJ mice and its subsequent effects on tear protein composition. Am. J. Pathol. 2007, 171, 1224–1236. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Rocha, E.M.; Aragona, P.; Clayton, J.A.; Ding, J.; Golebiowski, B.; Hampel, U.; McDermott, A.M.; Schaumberg, D.A.; Srinivasan, S.; et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul. Surf. 2017, 15, 284–333. [Google Scholar] [CrossRef] [PubMed]
- Paula, C.; Motta, A.; Schmitz, C.; Nunes, C.P.; Souza, A.P.; Bonorino, C. Alterations in dendritic cell function in aged mice: Potential implications for immunotherapy design. Biogerontology 2009, 10, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Godoy, G.J.; Paira, D.A.; Olivera, C.; Breser, M.L.; Sanchez, L.R.; Motrich, R.D.; Rivero, V.E. Differences in T regulatory cells between mouse strains frequently used in immunological research: Treg cell quantities and subpopulations in NOD, B6 and BALB/c mice. Immunol. Lett. 2020, 223, 17–25. [Google Scholar] [CrossRef]
- Daley, J.M.; Thomay, A.A.; Connolly, M.D.; Reichner, J.S.; Albina, J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008, 83, 64–70. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.-H.; Ryu, J.-S.; Yoon, C.-H.; Kim, M.-K. Age-Dependent Distinct Distributions of Dendritic Cells in Autoimmune Dry Eye Murine Model. Cells 2021, 10, 1857. https://doi.org/10.3390/cells10081857
Jung Y-H, Ryu J-S, Yoon C-H, Kim M-K. Age-Dependent Distinct Distributions of Dendritic Cells in Autoimmune Dry Eye Murine Model. Cells. 2021; 10(8):1857. https://doi.org/10.3390/cells10081857
Chicago/Turabian StyleJung, Young-Ho, Jin-Suk Ryu, Chang-Ho Yoon, and Mee-Kum Kim. 2021. "Age-Dependent Distinct Distributions of Dendritic Cells in Autoimmune Dry Eye Murine Model" Cells 10, no. 8: 1857. https://doi.org/10.3390/cells10081857
APA StyleJung, Y.-H., Ryu, J.-S., Yoon, C.-H., & Kim, M.-K. (2021). Age-Dependent Distinct Distributions of Dendritic Cells in Autoimmune Dry Eye Murine Model. Cells, 10(8), 1857. https://doi.org/10.3390/cells10081857





