Distinctive Prognostic Value and Cellular Functions of Osteopontin Splice Variants in Human Gastric Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Specimens
2.2. Cell Lines
2.3. Plasmid Construction and Cell Transfection
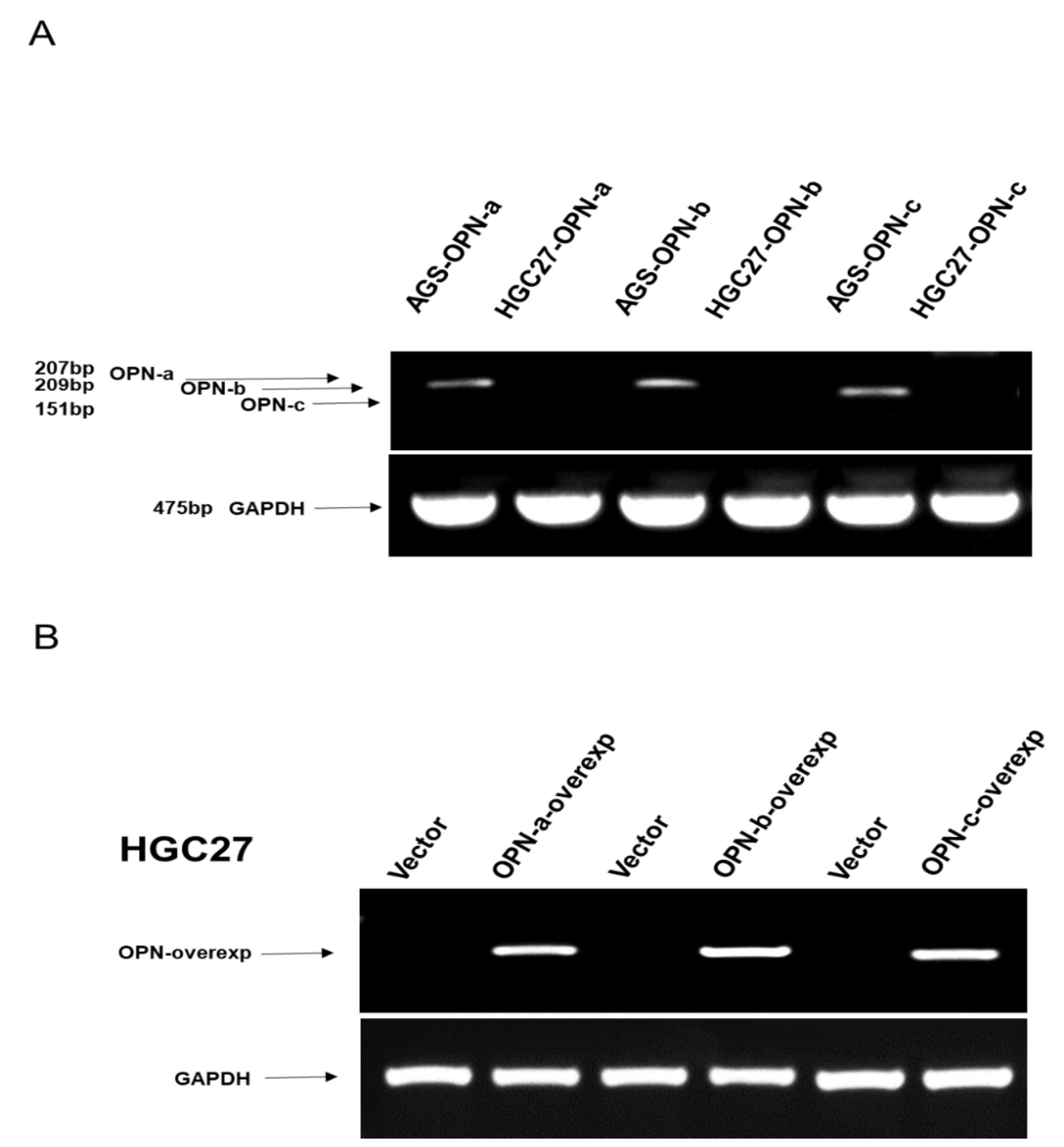
2.4. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.5. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-QPCR)
2.6. In Vitro Cell Growth Assay
2.7. In Vitro Invasion Assay
2.8. In Vitro Cell–Matrix Adhesion Assay
2.9. Electrical Cell Substrate Impedance Sensing (ECIS)-Based Cell Migration Assay
2.10. Measurement of ROS Production
2.11. Protein Prediction
2.12. Statistical Analysis
3. Results
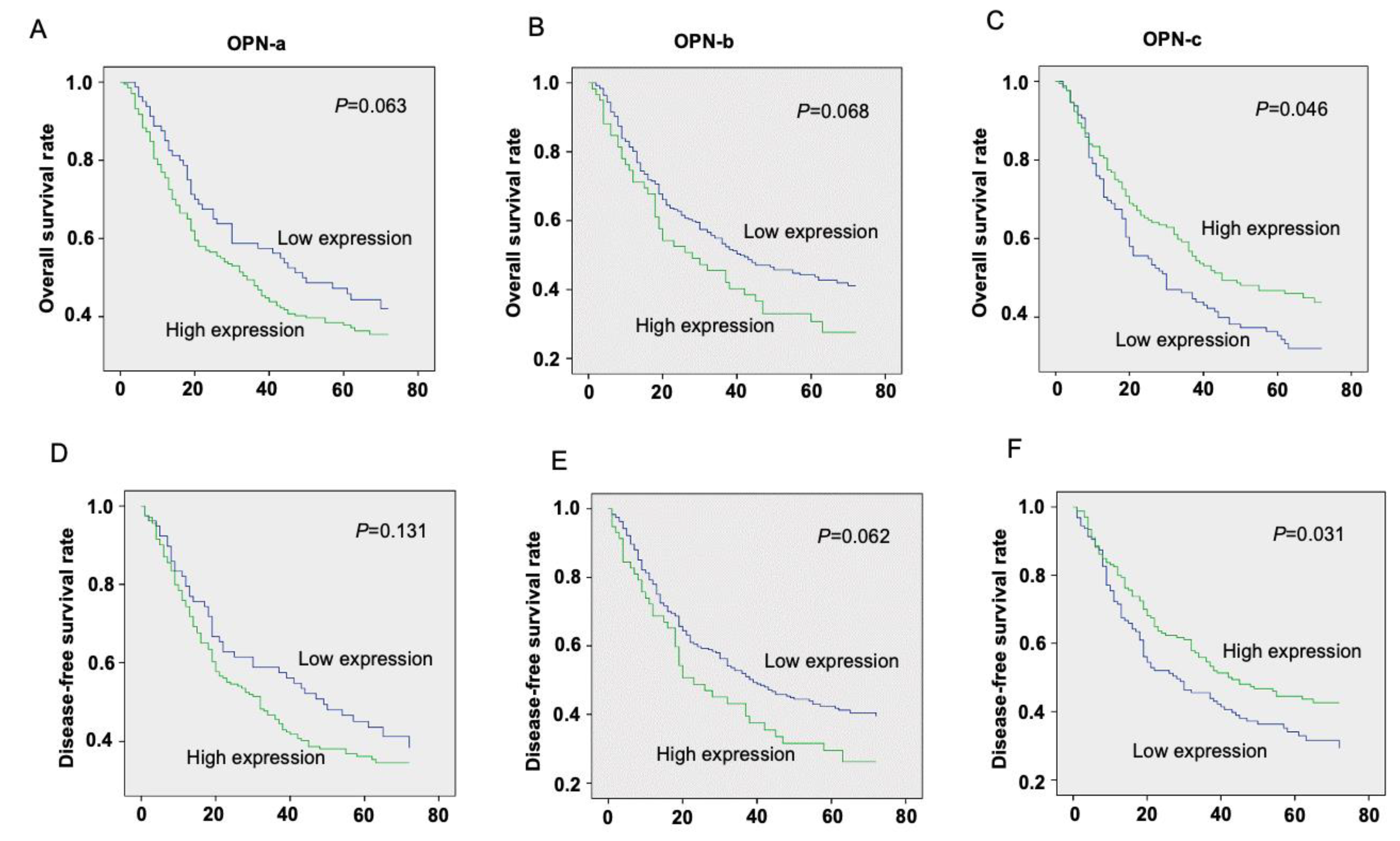
3.1. The Prognostic Value of OPN Splice Variants in Gastric Cancer Patients
3.2. Association of OPN Splice variants’ Expression with Clinicopathological Features in Gastric Cancer
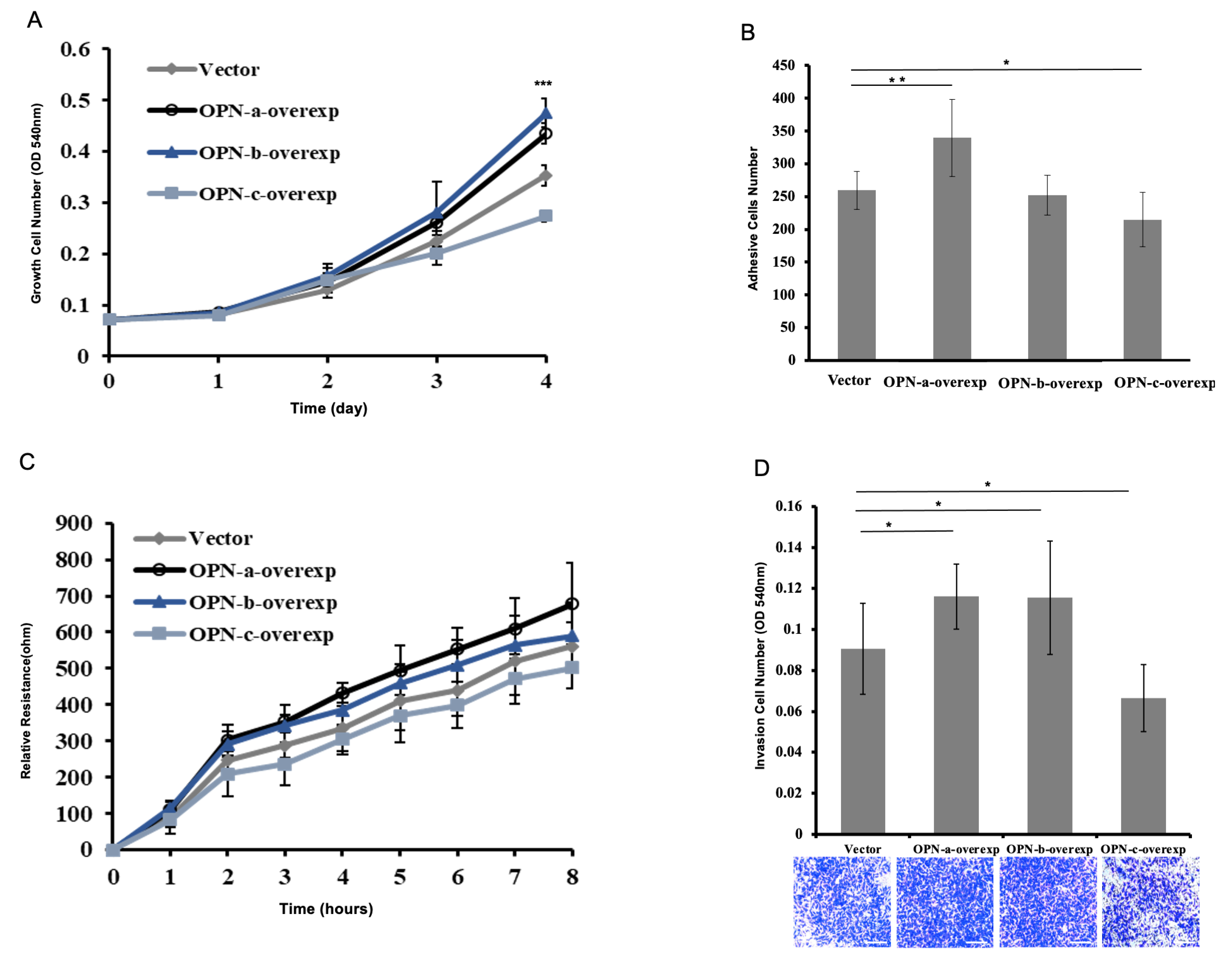
3.3. Effects of OPN Splice Variants on In Vitro Functions of Gastric Cancer Cell Line HGC-27, Respectively
3.4. Effect of OPN Splice Variants on the Proliferation of HGC-27 Cells
3.5. Effect of OPN Splice Variants on the Adhesion of HGC-27 Cells
3.6. Effect of OPN Splice Variants on the Migration of HGC-27 Cells
3.7. Effect of OPN Splice Variants on the Invasion of HGC-27 Cells
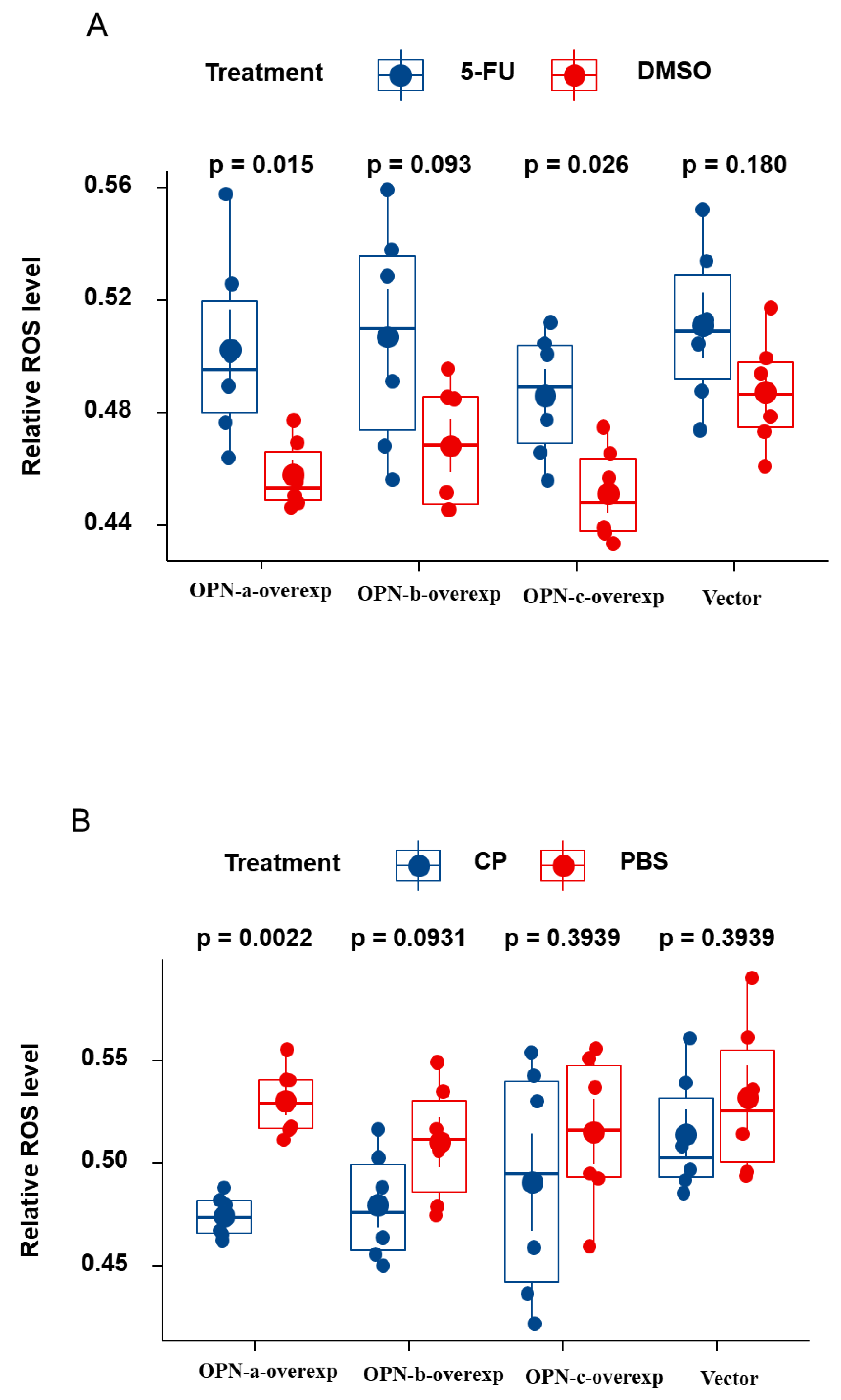
3.8. Effect of OPN Splice Variants on ROS Generation of HGC-27 Cells Following Treatment with 5-Fu and Cisplatin, Respectively
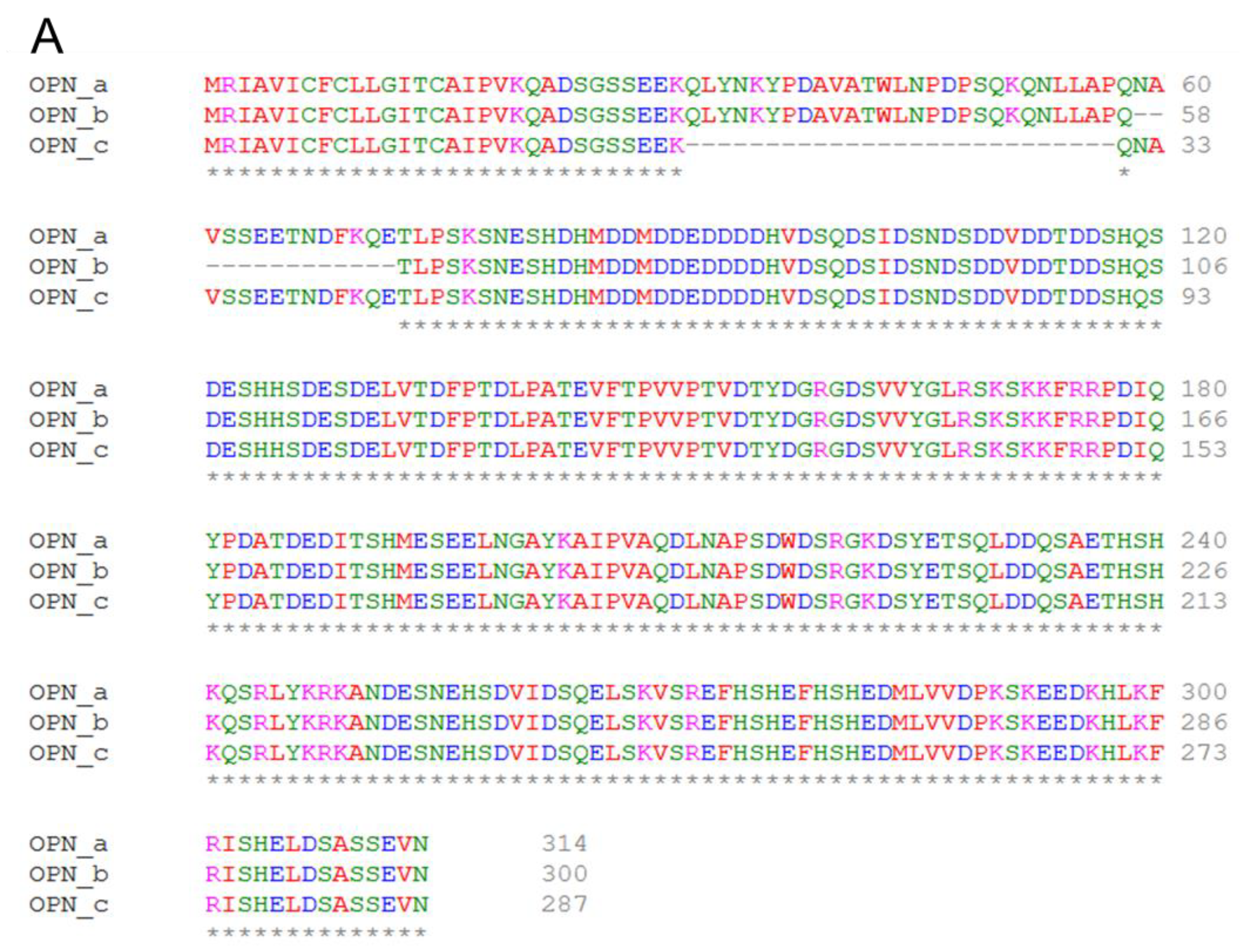
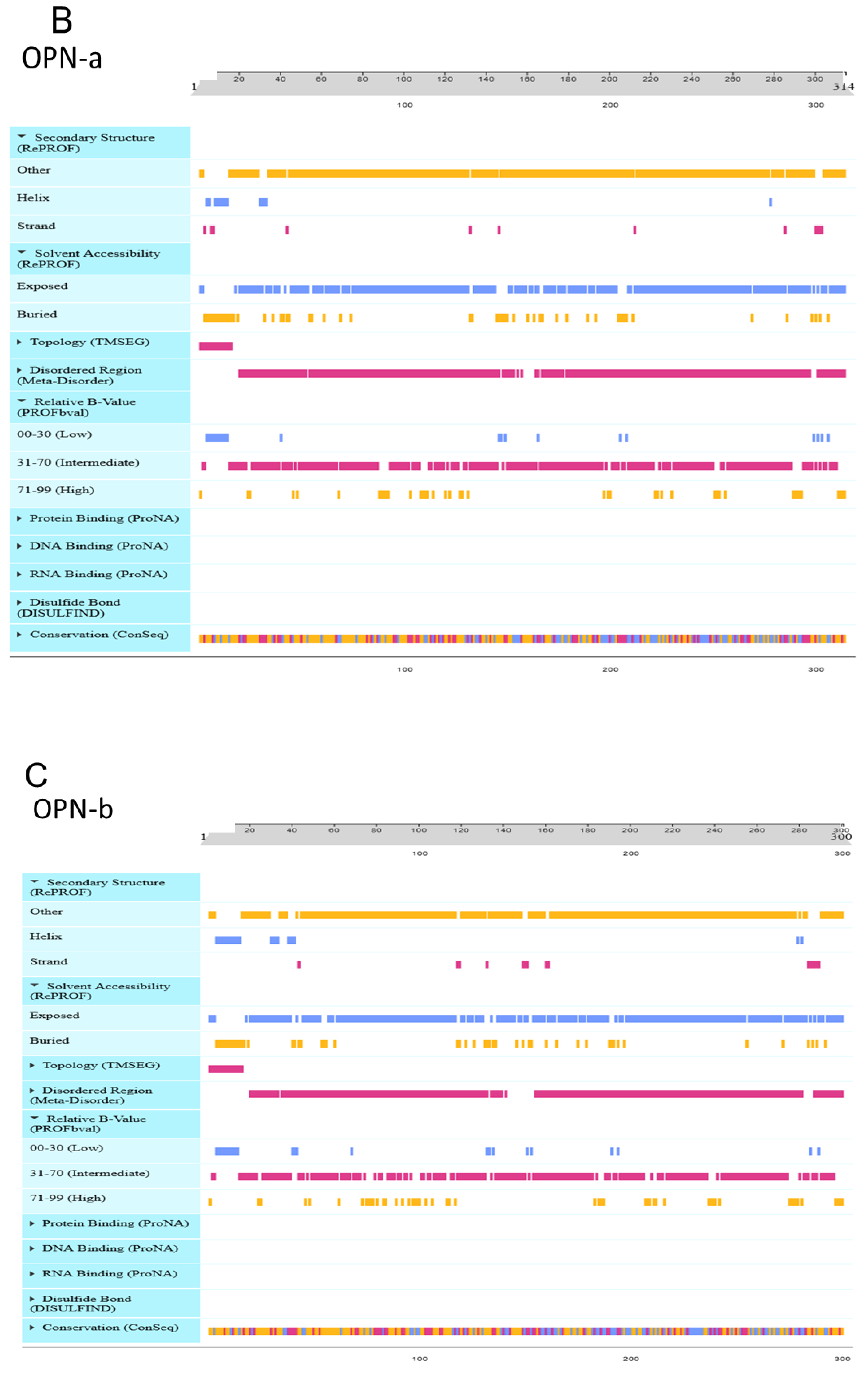
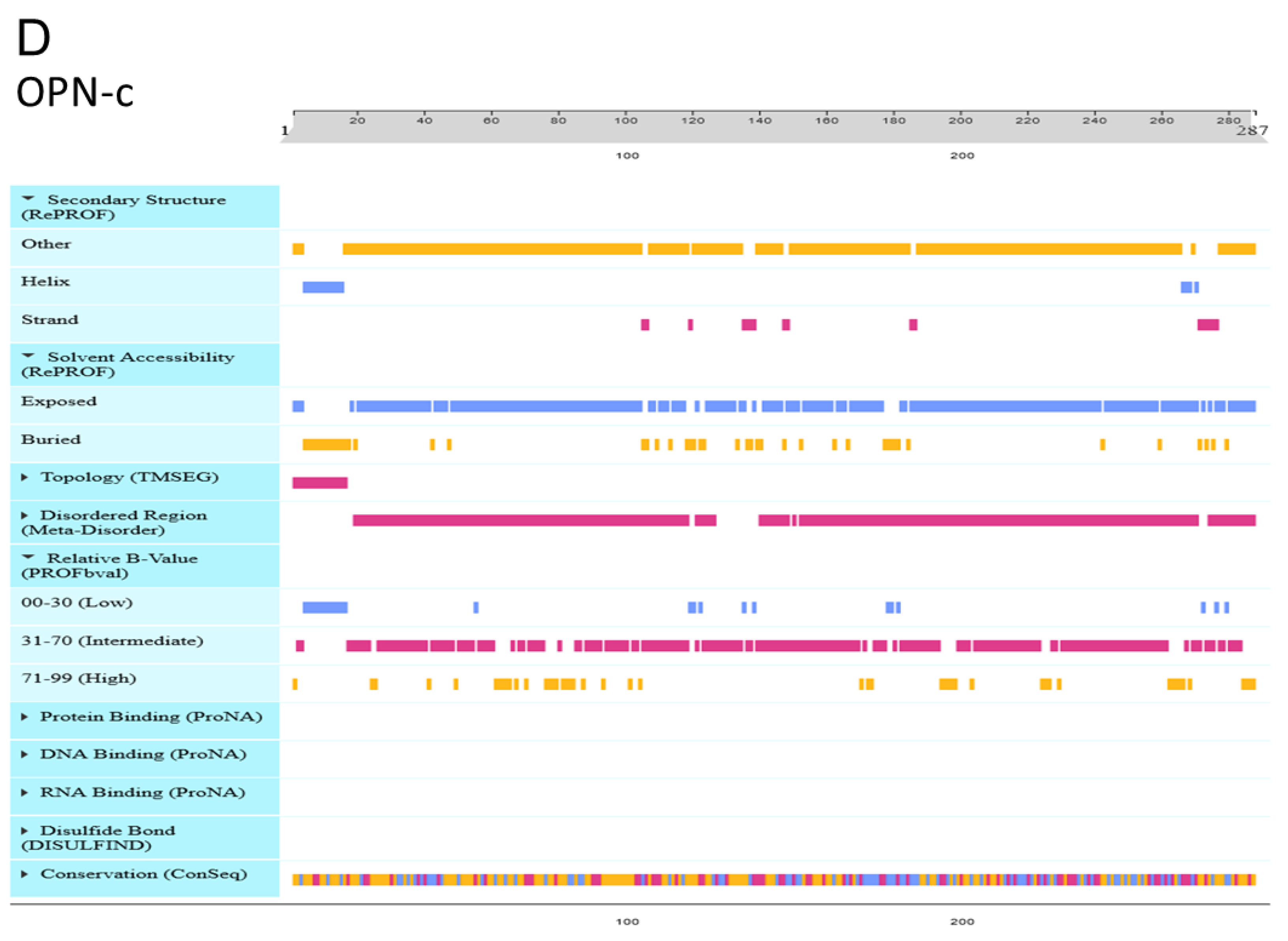
3.9. Effect of OPN Splice Variants on Protein Structural Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakrishnan, M.; George, R.; Sharma, A.; Graham, D.Y. Changing trends in stomach cancer throughout the world. Curr. Gastroenterol. Rep. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Ricci, A.; Rizzo, A.; Llimpe, F.R.; di Fabio, F.; de Biase, D.; Rihawi, K. Novel HER2-directed treatments in advanced gastric carcinoma: AnotHER Paradigm Shift? Cancers 2021, 13, 1664. [Google Scholar] [CrossRef]
- Joharatnam-Hogan, N.; Shiu, K.K.; Khan, K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat. Rev. 2020, 85, 101980. [Google Scholar] [CrossRef]
- Bandopadhyay, M.; Bulbule, A.; Butti, R.; Chakraborty, G.; Ghorpade, P.; Ghosh, P.; Gorain, M.; Kale, S.; Kumar, D.; Kumar, S.; et al. Osteopontin as a therapeutic target for cancer. Expert Opin. Ther. Targets 2014, 18, 883–895. [Google Scholar] [CrossRef]
- Fu, D.-J.; Wang, L.; Chouairi, F.K.; Rose, I.M.; Abetov, D.A.; Miller, A.D.; Yamulla, R.; Schimenti, J.C.; Flesken-Nikitin, A.; Nikitin, A.Y. Gastric squamous-columnar junction contains a large pool of cancer-prone immature osteopontin responsive Lgr5−CD44+ cells. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yazici, O.; Dogan, M.; Ozal, G.; Aktas, S.H.; Demirkazik, A.; Utkan, G.; Senler, F.C.; Icli, F.; Akbulut, H. Osteopontin is a prognostic factor in patients with advanced gastric cancer. Comb. Chem. High Throughput Screen. 2021, 24, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Simeone, D.M.; van Golen, K.; Logsdon, C.D. S100P promotes pancreatic cancer growth, survival, and invasion. Clin. Cancer Res. 2005, 11, 5356–5364. [Google Scholar] [CrossRef] [Green Version]
- Briones-Orta, M.; Avendaño-Vázquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.-K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim. Biophys Acta Rev. Cancer 2017, 1868. [Google Scholar] [CrossRef] [PubMed]
- Anborgh, P.H.; Mutrie, J.C.; Tuck, A.B.; Chambers, A.F. Pre- and post-translational regulation of osteopontin in cancer. J. Cell Commun. Signal. 2011, 5, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, C.; Cui, Y.; Chang, S.; Huang, J.; Birkin, E.; Hu, M.; Zhi, X.; Li, W.; Zhang, L.; Cheng, S.; et al. OPN promotes the aggressiveness of non-small-cell lung cancer cells through the activation of the RON tyrosine kinase. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Hiscox, S.; Hallett, M.B.; Horrobin, D.F.; Mansel, R.E.; Puntis, M.C. Regulation of the expression of E-cadherin on human cancer cells by gamma-linolenic acid (GLA). Cancer Res. 1995, 55, 5043–5048. [Google Scholar]
- Parr, C.; Hiscox, S.; Nakamura, T.; Matsumoto, K.; Jiang, W.G.; Parr, C.; Hiscox, S.; Nakamura, T.; Matsumoto, K.; Jiang, W.G. Nk4, a new HGF/SF variant, is an antagonist to the influence of HGF/SF on the motility and invasion of colon cancer cells. Int. J. Cancer 2000, 85, 563–570. [Google Scholar] [CrossRef]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef] [Green Version]
- Giopanou, I.; Kanellakis, N.I.; Giannou, A.D.; Lilis, I.; Marazioti, A.; Spella, M.; Papaleonidopoulos, V.; Simoes, D.C.M.; Zazara, D.E.; Agalioti, T.; et al. Osteopontin drives KRAS-mutant lung adenocarcinoma. Carcinogenesis 2019, 41, 1134–1144. [Google Scholar] [CrossRef]
- Lamort, A.-S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a link between inflammation and cancer: The thorax in the spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef] [Green Version]
- Gan, N.; Zou, S.; Hang, W.; Yang, D.; Zhang, X.; Yin, Y. Osteopontin is critical for hyperactive mTOR-induced tumorigenesis in oral squamous cell carcinoma. J. Cancer 2017, 8, 1362–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Ruhland, M.K.; Pazolli, E.; Lind, A.C.; Stewart, S.A. Osteopontin stimulates preneoplastic cellular proliferation through activation of the MAPK pathway. Mol. Cancer Res. 2011, 9, 1018–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, J.; Chang, Y.-C.; Tsai, H.-F.; Lin, Y.-F.; Huang, M.-S.; Yang, C.-J.; Hsiao, M. Follistatin-like protein 1 inhibits lung cancer metastasis by preventing proteolytic activation of osteopontin. Cancer Res. 2019, 79, 6113–6125. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.C.S.F.; Blunck, C.B.; Emerenciano, M.; Gimba, E.R.P. Osteopontin and their roles in hematological malignancies: Splice variants on the new avenues. Cancer Lett. 2017, 408, 138–143. [Google Scholar] [CrossRef]
- Hahnel, A.; Wichmann, H.; Greither, T.; Kappler, M.; Würl, P.; Kotzsch, M.; Taubert, H.; Vordermark, D.; Bache, M. Prognostic impact of mRNA levels of osteopontin splice variants in soft tissue sarcoma patients. BMC Cancer 2012, 12, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Mirza, M.; Weber, G.F. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene 2005, 25, 2192–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Li, J.; Yu, B.; Su, L.; Yu, Y.; Yan, M.; Liu, B.; Zhu, Z. Osteopontin splice variants differentially exert clinicopathological features and biological functions in gastric cancer. Int. J. Biol. Sci. 2013, 9, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-W.; Chae, S.; Jun, H.-O.; Lee, E.G.; Yang, S.-J.; Lee, D.C.; Jung, J.K.; Park, K.C.; Yeom, Y.I. Osteopontin splice variants differentially modulate the migratory activity of hepatocellular carcinoma cell lines. Int. J. Oncol. 2009, 35, 1409–1416. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.-F.; Huang, C.C.; Bin Dabil, N.; Chang, P.-L. Assessing SPP1/osteopontin (OPN) splice variants and their association to nonmelanoma skin cancer by absolute quantification: Identification of OPN-5 subvariants and their protein coding potential. Cancer Investig. 2021, 1–28. [Google Scholar] [CrossRef]
- Walaszek, K.; Lower, E.E.; Ziolkowski, P.; Weber, G.F. Breast cancer risk in premalignant lesions: Osteopontin splice variants indicate prognosis. Br. J. Cancer 2018, 119, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Ghaffari, S.H.; Nikbakht, M.; Foumani, H.K.; Vaezi, M.; Mohammadi, S.; Alimoghaddam, K.; Ghavamzadeh, A. OPN b and c isoforms doubtless veto anti-angiogenesis effects of curcumin in combination with conventional aml regiment. Asian Pac. J. Cancer Prev. 2017, 18, 2591–2599. [Google Scholar] [CrossRef]
- Nakamura, K.D.M.; Tilli, T.M.; Wanderley, J.L.; Palumbo, A., Jr.; Mattos, R.M.; Ferreira, A.C.; Klumb, C.E.; Nasciutti, L.E.; Gimba, E.R. Osteopontin splice variants expression is involved on docetaxel resistance in PC3 prostate cancer cells. Tumor Biol. 2015, 37, 2655–2663. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef]
- Carbone, M.; Arron, S.T.; Beutler, B.; Bononi, A.; Cavenee, W.; Cleaver, J.E.; Croce, C.M.; D’Andrea, A.; Foulkes, W.D.; Gaudino, G.; et al. Tumour predisposition and cancer syndromes as models to study gene-environment interactions. Nat. Rev. Cancer 2020, 20, 533–549. [Google Scholar] [CrossRef]
- Junior, P.L.D.S.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The roles of ROS in cancer heterogeneity and therapy. Oxidative Med. Cell. Longev. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Chen, Y.; Gibson, S.B. Regulation of autophagy by Reactive Oxygen Species (ROS): Implications for cancer progression and treatment. Antioxid. Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; DeNicola, G.M.; Nixon, C.; Blyth, K.; Labuschagne, C.F.; Tuveson, D.A.; Vousden, K.H. Dynamic ROS control by TIGAR regulates the initiation and progression of pancreatic cancer. Cancer Cell 2020, 37, 168–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Myers, A.L.; Wang, Z.; Nancarrow, D.J.; Ferrer-Torres, D.; Handlogten, A.; Leverenz, K.; Bao, J.; Thomas, D.G.; Wang, T.D.; et al. Osteopontin (OPN/SPP1) isoforms collectively enhance tumor cell invasion and dissemination in esophageal adenocarcinoma. Oncotarget 2015, 6, 22239–22257. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-Y.; Wu, M.-S.; Chiang, E.; Chen, Y.-J.; Chi, N.-H.; Chen, G.-H.; Lin, J.-T. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut 2007, 56, 782–789. [Google Scholar] [CrossRef] [Green Version]
| RT-QPCR |
| Primers for OPN-a(NM:001040058.2; Length:105 bp) |
| 5′-ACAACAAATACCCAGATGCT-3′ |
| 5′-ACTGAACCTGACCGTACACATTGGTTTCTTCAGAGGAC-3′ |
| Primers for OPN-b(NM:000582.3; Length:95 bp) |
| 5′-ACAACAAATACCCAGATGCT-3′ |
| 5′-ACTGAACCTGACCGTACAGGACTTACTTGGAAGGGTCT-3′ |
| Primers for OPN-c(NM:001040060.2; Length:87 bp) |
| 5′-AAGTTCTGAGGAAAAGCAGA-3′ |
| 5′-ACTGAACCTGACCGTACACTTTCGTTGGACTTACTTGG-3′ |
| Primers for GAPDH(NM: 002046.7; Length:93 bp) |
| 5′-CTGAGTACGTCGTGGAGTC-3′ |
| 5′-ACTGAACCTGACCGTACACAGAGATGATGACCCTTTTG-3′ |
| RT-PCR |
| Primers for OPN-a(NM:001040058.2; Length:207 bp) |
| 5′-ATCTCCTAGCCCCACAGAAT-3′ |
| 5′-CATCAGACTGGTGAGAATCATC-3′ |
| Primers for OPN-b(NM: 000582.3; Length:209 bp) |
| 5′-ATCTCCTAGCCCCACAGAC-3′ |
| 5′-AAAATCAGTGACCAGTTCATCAG-3′ |
| Primers for OPN-c(NM: 001040060.2; Length:151 bp) |
| 5′-TGAGGAAAAGCAGAATGCTG-3′ |
| 5′-GTCAATGGAGTCCTGGCTGT-3′ |
| Primers for GAPDH(NM: 002046.7; Length:475 bp) |
| 5′- GGCTGCTTTTAACTCTGGTA -3′ |
| 5′- GACTGTGGTCATGAGTCCTT -3′ |
| Characteristics | n | OPN-a Median (IQ Range) | p-Value | OPN-b Median (IQ Range) | p-Value | OPN-c Median (IQ Range) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Sample type | Tumor | 324 | 19110 (513, 681494) | <0.0001 | 22 (1, 303) | <0.0001 | 338 (9, 8715) | 0.0002 |
| Normal | 324 | 491 (129, 2346) | 0 (0, 1) | 28 (7, 223) | ||||
| Gender | Male | 231 | 15781 (490, 509248) | 0.4447 | 18 (1, 255) | 0.7282 | 280 (6, 6031) | 0.5666 |
| Female | 93 | 52657 (550, 1549284) | 40 (0, 505) | 601 (12, 14579) | ||||
| Infiltration depth | T1 + T2 | 42 | 5502 (48, 399965) | 0.1116 | 8 (0, 402) | 0.2819 | 74 (7,5 713) | 0.4678 |
| T3 + T4 | 274 | 23948 (603, 762375) | 25 (1, 297) | 358 (9, 10974) | ||||
| Nodal status | N0 | 71 | 2967 (59, 579536) | 0.0346 | 8 (0, 274) | 0.2856 | 552 (9, 14259) | 0.0226 |
| N1 + 2 + 3 | 247 | 29723 (1032, 850931) | 25 (1, 363) | 336 (6, 9106) | ||||
| M-staging | M0 | 282 | 13518 (323, 546163) | 0.0076 | 19 (1, 275) | 0.097 | 287 (6, 8292) | 0.139 |
| M1 | 41 | 214420 (9820, 4845742) | 44 (3, 1402) | 439 (33, 15523) | ||||
| TNM staging | TNM1 + 2 | 85 | 5502 (63, 546163) | 0.0385 | 8 (1, 287) | 0.3448 | 433 (8, 10485) | 0.0551 |
| TNM3 + 4 | 230 | 30629 (983, 758385) | 25 (1, 338) | 336 (9, 7539) | ||||
| Differentiation | High | 1 | ||||||
| High-Medium | 6 | 51281(9947, 547813) | 14.84(1.13, 41.01) | 1932 (79, 82560) | ||||
| Medium | 62 | 11039 (55, 360955) | 0.4234 | 41(2, 1584) | 0.258 | 687 (4, 14259) | 0.7000 | |
| Medium-Low | 82 | 54626 (989, 1141029) | 0.8892 | 44 (1, 493) | 0.2533 | 540 (14, 13658) | 0.6071 | |
| Low | 138 | 15546 (734, 381642) | 0.7801 | 11 (0, 231) | 0.751 | 120 (6, 2892) | 0.4134 | |
| Clinical outcome | Alive | 120 | 16689 (305, 550306) | 0.5139 | 22 (1, 248) | 0.9832 | 1004 (24, 20290) | 0.375 |
| Died | 177 | 23867 (601, 842154) | 22 (0, 607) | 113 (3, 5596) | ||||
| Disease-Free | 106 | 16139 (239, 519513) | 0.4325 | 20 (1, 246) | 0.4857 | 724 (26, 13547) | 0.3237 | |
| Metastasis | 14 | 118027 (1201, 8879,44) | 83 (2, 731) | 5044 (2, 65,790) | ||||
| Multivariate vs. OS | Univariate vs. OS | |||
|---|---|---|---|---|
| p Value | Exp(B) | p Value | Exp(B) | |
| Sex | 0.34 | 0.718 | 0.882 | 0.963 |
| Diagnosis | 0.088 | 2.06 | 0.38 | 1.269 |
| TNM | <0.001 | 5.322 | <0.001 | 5.294 |
| Location | 0.478 | 1.071 | 0.836 | 1.013 |
| Histology | 0.106 | 1.329 | 0.296 | 0.924 |
| Differentiation | 0.464 | 0.863 | 0.312 | 1.145 |
| Invasion | 0.611 | 1.143 | <0.001 | 0.591 |
| Embolism | 0.296 | 1.424 | <0.001 | 3.095 |
| OPN-a | 0.855 | 1.066 | 0.38 | 1.255 |
| OPN-b | 0.026 | 2.675 | 0.064 | 1.753 |
| OPN-c | 0.014 | 0.446 | 0.009 | 0.54 |
| Multivariate vs. RFS | Univariate vs. RFS | |||
|---|---|---|---|---|
| Sig. | Exp(B) | Sig. | Exp(B) | |
| Sex | 0.456 | 0.766 | 0.92 | 1.026 |
| Diagnosis | 0.146 | 1.892 | 0.111 | 1.59 |
| TNM | <0.001 | 5.916 | <0.001 | 4.908 |
| Location | 0.488 | 1.072 | 0.725 | 1.024 |
| Histology | 0.172 | 1.276 | 0.946 | 1.005 |
| Differentiation | 0.468 | 0.861 | 0.203 | 1.193 |
| Invasion | 0.422 | 1.242 | 0.001 | 0.607 |
| Embolism | 0.335 | 1.401 | <0.001 | 3.147 |
| OPN-a | 0.685 | 1.158 | 0.173 | 1.43 |
| OPN-b | 0.046 | 2.503 | 0.09 | 1.698 |
| OPN-c | 0.013 | 0.428 | 0.008 | 0.522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, C.; Cui, Y.; Lane, J.; Jia, S.; Ji, J.; Jiang, W.G. Distinctive Prognostic Value and Cellular Functions of Osteopontin Splice Variants in Human Gastric Cancer. Cells 2021, 10, 1820. https://doi.org/10.3390/cells10071820
Hao C, Cui Y, Lane J, Jia S, Ji J, Jiang WG. Distinctive Prognostic Value and Cellular Functions of Osteopontin Splice Variants in Human Gastric Cancer. Cells. 2021; 10(7):1820. https://doi.org/10.3390/cells10071820
Chicago/Turabian StyleHao, Chengcheng, Yuxin Cui, Jane Lane, Shuqin Jia, Jiafu Ji, and Wen G. Jiang. 2021. "Distinctive Prognostic Value and Cellular Functions of Osteopontin Splice Variants in Human Gastric Cancer" Cells 10, no. 7: 1820. https://doi.org/10.3390/cells10071820
APA StyleHao, C., Cui, Y., Lane, J., Jia, S., Ji, J., & Jiang, W. G. (2021). Distinctive Prognostic Value and Cellular Functions of Osteopontin Splice Variants in Human Gastric Cancer. Cells, 10(7), 1820. https://doi.org/10.3390/cells10071820







