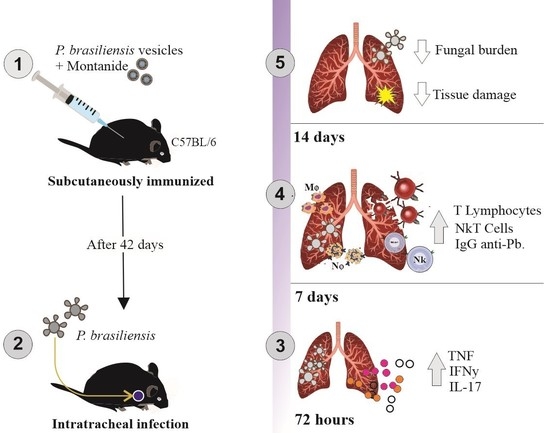
Protective Response in Experimental Paracoccidioidomycosis Elicited by Extracellular Vesicles Containing Antigens of Paracoccidioides brasiliensis
Abstract
:1. Introduction
2. Material and Methods
2.1. Fungal Strain
2.2. Extracellular Vesicles Purification
2.3. Mice and Ethics Statement
2.4. Immunization Protocol
2.5. Splenocyte Response Assay
2.6. Mice Infection
2.7. Enzyme Linked Immunosorbent Assay (ELISA) for IgM and IgG
2.8. Fungal Burden Quantification
2.9. Myeloperoxidase (MPO) and N-Acetylglucosaminidase (NAG) Activity Evaluation
2.10. Cytokine Analysis
2.11. Histopathological Analysis
2.12. Flow Cytometry Analysis
2.13. Statistical Analysis
3. Results
3.1. Immunization with P. brasiliensis-Derived EVs Induces Antibody Production In Vivo and Cytokine Release upon Ex Vivo Restimulation
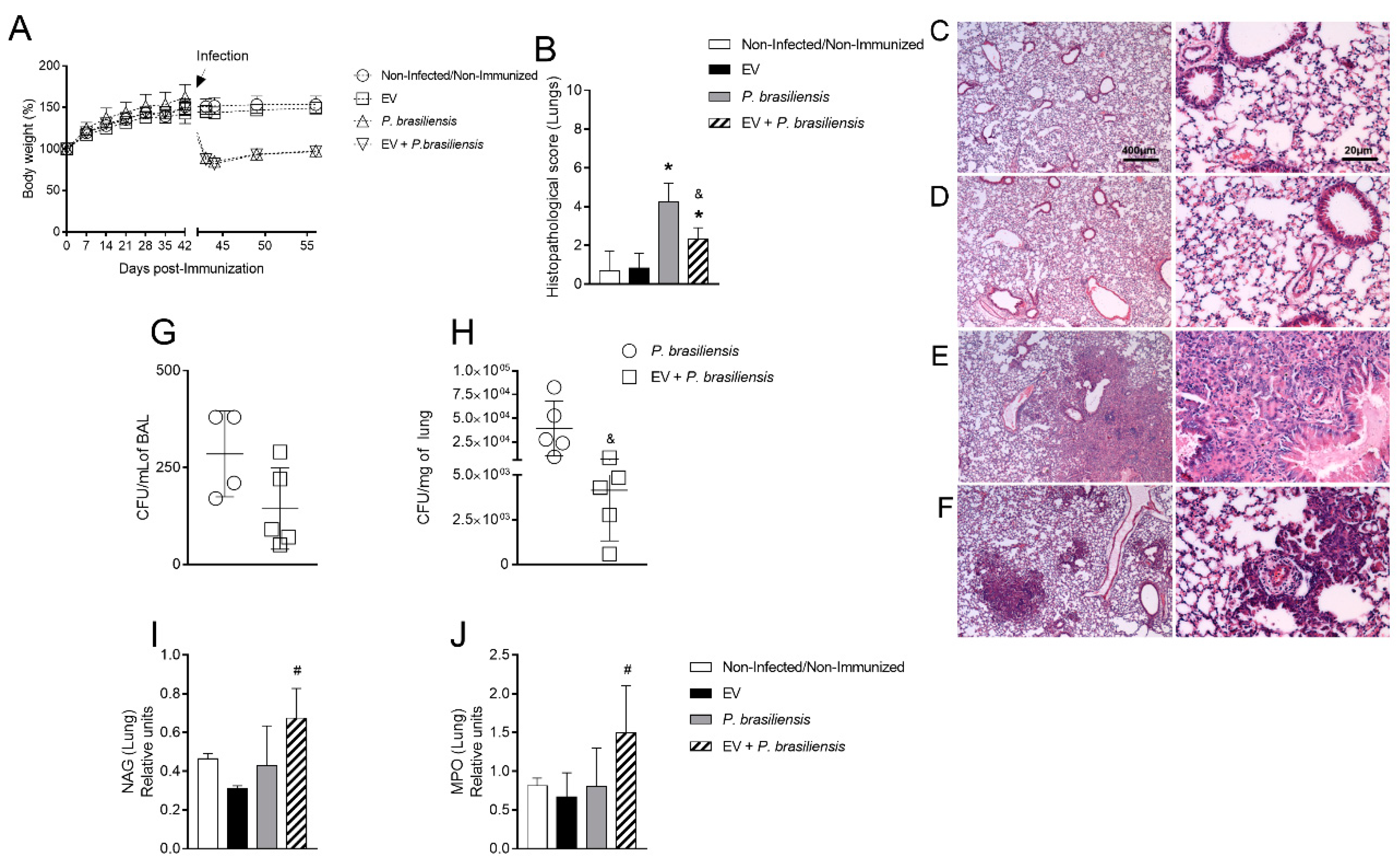
3.2. Immunization with P. brasiliensis-Derived EVs Ameliorates Histopathological Parameters and Reduces Fungal Burden in Lung Tissue 72 h Post-Infection by P. brasiliensis
3.3. Immunization with P. brasiliensis-Derived EVs Ameliorates Histopathological Parameters and Reduces Fungal Burden in Lung Tissue 14 Days Post-Infection with P. brasiliensis Yeast Cells
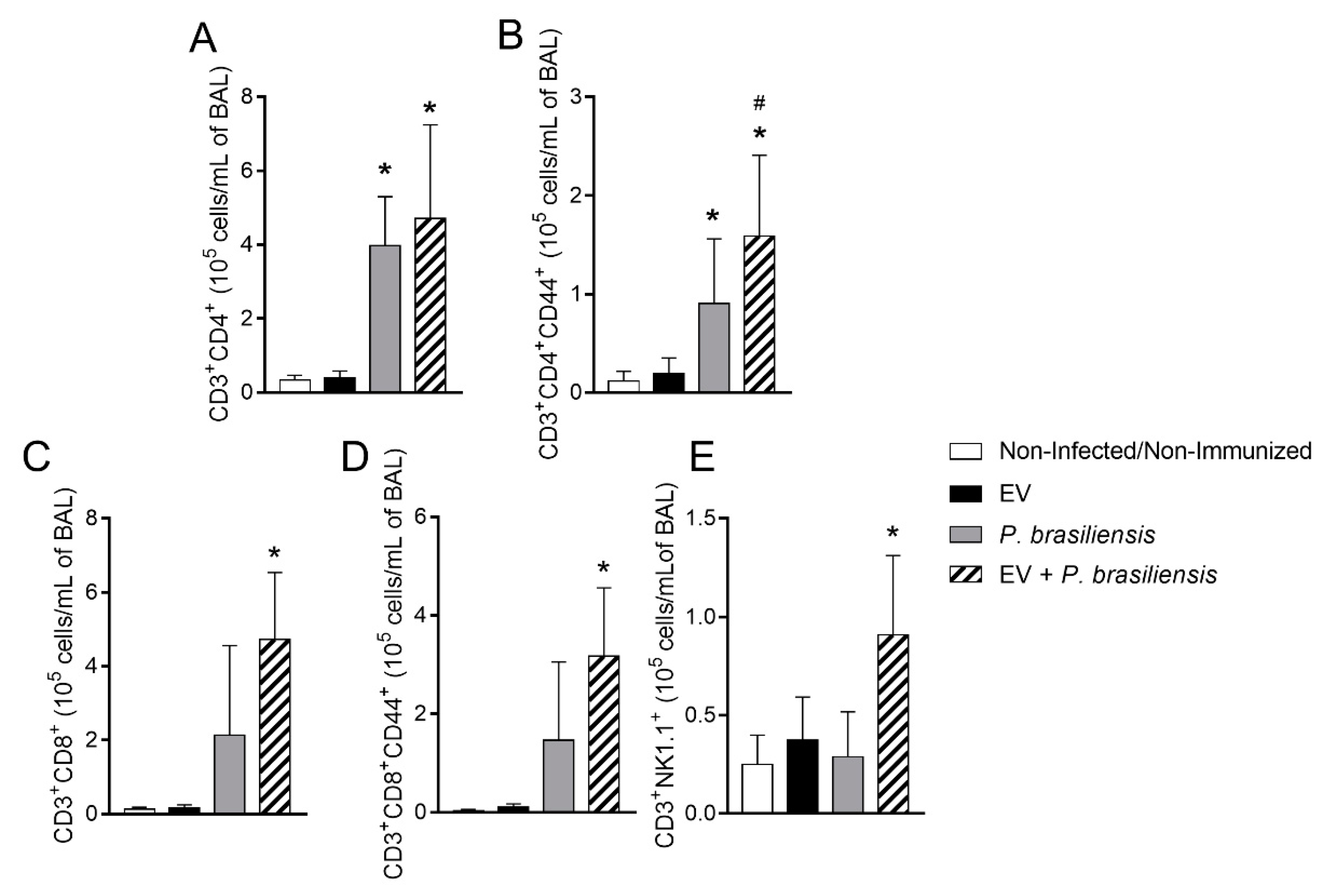
3.4. Immunization with P. brasiliensis-Derived EVs Induces Mobilization of Activated T lymphocytes and NKT Cells upon Challenge
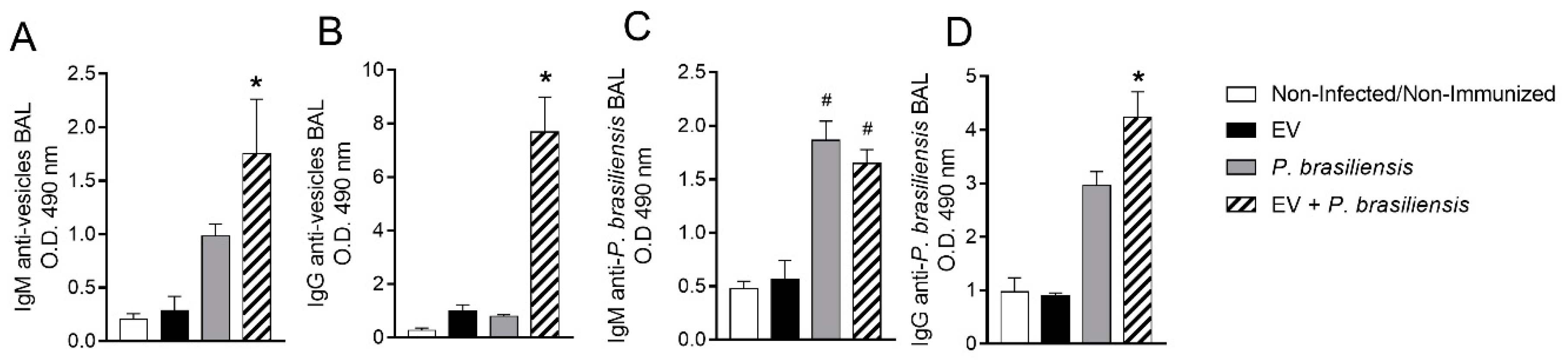
3.5. Mice Immunized with EVs from P. brasiliensis Had Higher Production of Antibodies Specific to Both EV-Derived Protein and P. brasiliensis Cells
3.6. Mice Immunized with EVs from P. brasiliensis Had Higher Levels of Cytokines Important to Control P. brasiliensis Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef]
- Mendes, R.P.; Cavalcante, R.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, H.C.; Assato, P.A.; Marcos, C.M.; Scorzoni, L.; de Paula, E.S.A.C.; Da Silva Jde, F. Paracoccidioides-host Interaction: An Overview on Recent Advances in the Paracoccidioidomycosis. Front. Microbiol. 2015, 6, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, J.E.; Luft, V.D.; Amorim, J.; Magalhaes, A.; Thomaz, L.; Nosanchuk, J.D. Immunization with P10 peptide increases specific immunity and protects immunosuppressed BALB/c mice infected with virulent yeasts of Paracoccidioides brasiliensis. Mycopathologia 2014, 178, 177–188. [Google Scholar] [CrossRef]
- de Amorim, J.; Magalhaes, A.; Munoz, J.E.; Rittner, G.M.; Nosanchuk, J.D.; Travassos, L.R. DNA vaccine encoding peptide P10 against experimental paracoccidioidomycosis induces long-term protection in presence of regulatory T cells. Microbes Infect. 2013, 15, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Souza, I.E.L.; Fernandes, F.F.; Schiavoni, M.C.L.; Silva, C.L.; Panunto-Castelo, A. Therapeutic effect of DNA vaccine encoding the 60-kDa-heat shock protein from Paracoccidoides brasiliensis on experimental paracoccidioidomycosis in mice. Vaccine 2019, 37, 5607–5613. [Google Scholar] [CrossRef] [PubMed]
- Holanda, R.A.; Munoz, J.E.; Dias, L.S.; Silva, L.B.R.; Santos, J.R.A.; Pagliari, S. Recombinant vaccines of a CD4+ T-cell epitope promote efficient control of Paracoccidioides brasiliensis burden by restraining primary organ infection. PLoS Negl. Trop. Dis. 2017, 11, e0005927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I. Biological properties of extracellular vesicles and their physiological functions. J. Extracell Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, M.L.; Casadevall, A. A two-way road: Novel roles for fungal extracellular vesicles. Mol. Microbiol. 2018, 110, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Han, Y.; Sun, Y.Z.; Jiang, H.H.; Liu, M.; Qi, R.Q. Extracellular vesicles derived from Malassezia furfur stimulate IL-6 production in keratinocytes as demonstrated in in vitro and in vivo models. J. Dermatol. Sci. 2019, 93, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Baltazar, L.M.; Zamith-Miranda, D.; Burnet, M.C.; Choi, H.; Nimrichter, L.; Nakayasu, E.S. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci. Rep. 2018, 8, 8065. [Google Scholar] [CrossRef]
- Matos Baltazar, L.; Nakayasu, E.S.; Sobreira, T.J.; Choi, H.; Casadevall, A.; Nimrichter, L. Antibody Binding Alters the Characteristics and Contents of Extracellular Vesicles Released by Histoplasma capsulatum. mSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, M.C.; Nakayasu, E.S.; Matsuo, A.L.; Sobreira, T.J.; Longo, L.V.; Ganiko, L. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: Comparative analysis with other pathogenic fungi. J. Proteome Res. 2012, 11, 1676–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, T.A.; Roque-Barreira, M.C.; Casadevall, A.; Almeida, F. Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Sci. Rep. 2016, 24, 35867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer-Vermes, L.M.; Burger, E.; Franco, M.F.; Moscar Di-Bacchi, M.; Mendes-Giannini, M.J.S.; Calich, V.L.G. Evaluation of the pathogenicity and immunogenicity of seven Paracoccidioides brasiliensis isolates in susceptible inbred mice. Med. Mycol. 1989, 27, 71–82. [Google Scholar] [CrossRef]
- Desjardins, C.A.; Champion, M.D.; Holder, J.W.; Muszewska, A.; Goldberg, J.; Bailão, A.M.; Brigido, M.M.; Ferreira, M.E.S.; Garcia, A.M.; Grynberg, M.; et al. Comparative genomic analysis of human fungal pathogens causing paracoccidioidomycosis. PLoS Genet. 2011, 7, e1002345. [Google Scholar] [CrossRef] [Green Version]
- Morais, E.A.; Chame, D.F.; Melo, E.M.; de Carvalho Oliveira, J.A.; de Paula, A.C.; Peixoto, A.C. TLR 9 involvement in early protection induced by immunization with rPb27 against Paracoccidioidomycosis. Microbes Infect. 2016, 18, 137–147. [Google Scholar] [CrossRef]
- Souza, D.G.; Cara, D.C.; Cassali, G.D.; Coutinho, S.F.; Silveira, M.R.; Andrade, S.P. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 2000, 131, 1800–1808. [Google Scholar] [CrossRef] [Green Version]
- Barcelos, L.S.; Talvani, A.; Teixeira, A.S.; Vieira, L.Q.; Cassali, G.D.; Andrade, S.P. Impaired inflammatory angiogenesis, but not leukocyte influx, in mice lacking TNFR1. J. Leukoc. Biol. 2005, 78, 352–358. [Google Scholar] [CrossRef]
- Santos, P.C.; Santos, D.A.; Ribeiro, L.S.; Fagundes, C.T.; de Paula, T.P.; Avila, T.V. The pivotal role of 5-lipoxygenase-derived LTB4 in controlling pulmonary paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2013, 7, e2390. [Google Scholar] [CrossRef] [Green Version]
- Fodey, T.L.; Delahaut, P.; Charlier, C.; Elliott, C.T. Comparison of three adjuvants used to produce polyclonal antibodies to veterinary drugs. Vet. Immunol. Immunopathol. 2008, 122, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.F.; Vilela, A.; Coura, L.A.; Rodrigues, F.T.; Nagem, R.A.; Chavez-Olortegui, C. Protective antibodies against a sphingomyelinase D from Loxosceles intermedia spider venom elicited in mice with different genetic background. Vaccine 2016, 34, 3828–3834. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-gamma): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Meng, G. Pathogenic Fungal Infection in the Lung. Front. Immunol. 2019, 10, 1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calich, V.L.; da Costa, T.A.; Felonato, M.; Arruda, C.; Bernardino, S.; Loures, F.V. Innate immunity to Paracoccidioides brasiliensis infection. Mycopathologia 2008, 165, 223–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, T.A.; Di Gangi, R.; Martins, P.; Longhini, A.L.; Zanucoli, F.; de Oliveira, A.L. Protection against Paracoccidioides brasiliensis infection in mice treated with modulated dendritic cells relies on inhibition of interleukin-10 production by CD8+ T cells. Immunology 2015, 146, 486–495. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Watchmaker, P.; Urban, J.; Sheridan, B.; Giermasz, A.; Nishimura, F. Helper function of memory CD8+ T cells: Heterologous CD8+ T cells support the induction of therapeutic cancer immunity. Cancer Res. 2007, 67, 10012–10018. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, D.I.; Pellicci, D.G.; Rossjohn, J. NKT cells: The smoking gun in fungal-induced asthma? Nat. Med. 2013, 19, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Albacker, L.A.; Chaudhary, V.; Chang, Y.J.; Kim, H.Y.; Chuang, Y.T.; Pichavant, M. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat. Med. 2013, 19, 1297–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuthrich, M.; Deepe, G.S., Jr.; Klein, B. Adaptive immunity to fungi. Annu. Rev. Immunol. 2012, 30, 115–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, V.; Rivera, A. Cytokines and the regulation of fungus-specific CD4 T cell differentiation. Cytokine 2012, 58, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, K.; Pierau, M.; Arra, A.; Lampe, K.; Schlueter, D.; Arens, C. Developmental induction of human T-cell responses against Candida albicans and Aspergillus fumigatus. Sci. Rep. 2018, 8, 16904. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.J.; Klein, B.S. Helper T-cell responses and pulmonary fungal infections. Immunology 2018, 155, 155–163. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltazar, L.M.; Ribeiro, G.F.; Freitas, G.J.; Queiroz-Junior, C.M.; Fagundes, C.T.; Chaves-Olórtegui, C.; Teixeira, M.M.; Souza, D.G. Protective Response in Experimental Paracoccidioidomycosis Elicited by Extracellular Vesicles Containing Antigens of Paracoccidioides brasiliensis. Cells 2021, 10, 1813. https://doi.org/10.3390/cells10071813
Baltazar LM, Ribeiro GF, Freitas GJ, Queiroz-Junior CM, Fagundes CT, Chaves-Olórtegui C, Teixeira MM, Souza DG. Protective Response in Experimental Paracoccidioidomycosis Elicited by Extracellular Vesicles Containing Antigens of Paracoccidioides brasiliensis. Cells. 2021; 10(7):1813. https://doi.org/10.3390/cells10071813
Chicago/Turabian StyleBaltazar, Ludmila Matos, Gabriela Fior Ribeiro, Gustavo J. Freitas, Celso Martins Queiroz-Junior, Caio Tavares Fagundes, Carlos Chaves-Olórtegui, Mauro Martins Teixeira, and Daniele G. Souza. 2021. "Protective Response in Experimental Paracoccidioidomycosis Elicited by Extracellular Vesicles Containing Antigens of Paracoccidioides brasiliensis" Cells 10, no. 7: 1813. https://doi.org/10.3390/cells10071813
APA StyleBaltazar, L. M., Ribeiro, G. F., Freitas, G. J., Queiroz-Junior, C. M., Fagundes, C. T., Chaves-Olórtegui, C., Teixeira, M. M., & Souza, D. G. (2021). Protective Response in Experimental Paracoccidioidomycosis Elicited by Extracellular Vesicles Containing Antigens of Paracoccidioides brasiliensis. Cells, 10(7), 1813. https://doi.org/10.3390/cells10071813