Obesity-Induced Dysbiosis Exacerbates IFN-γ Production and Pulmonary Inflammation in the Mycobacterium tuberculosis Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. LFD and HFD
2.3. Glycemia
2.4. Adipose Tissue Weight
2.5. M. tuberculosis Infection
2.6. Colony-Forming Unit Assay
2.7. Histopathology
2.8. Gene Expression
2.9. Cytokine Production
2.10. Flow Cytometry
2.11. Bacterial DNA Analysis
2.12. Short-Chain Fatty Acids’ Quantification
2.13. In Vivo Intestinal Permeability Assay
2.14. Antibiotic Treatment and Fecal Transplantation
2.15. Gut Microbiota Profiling
2.16. Statistical Analysis
3. Results
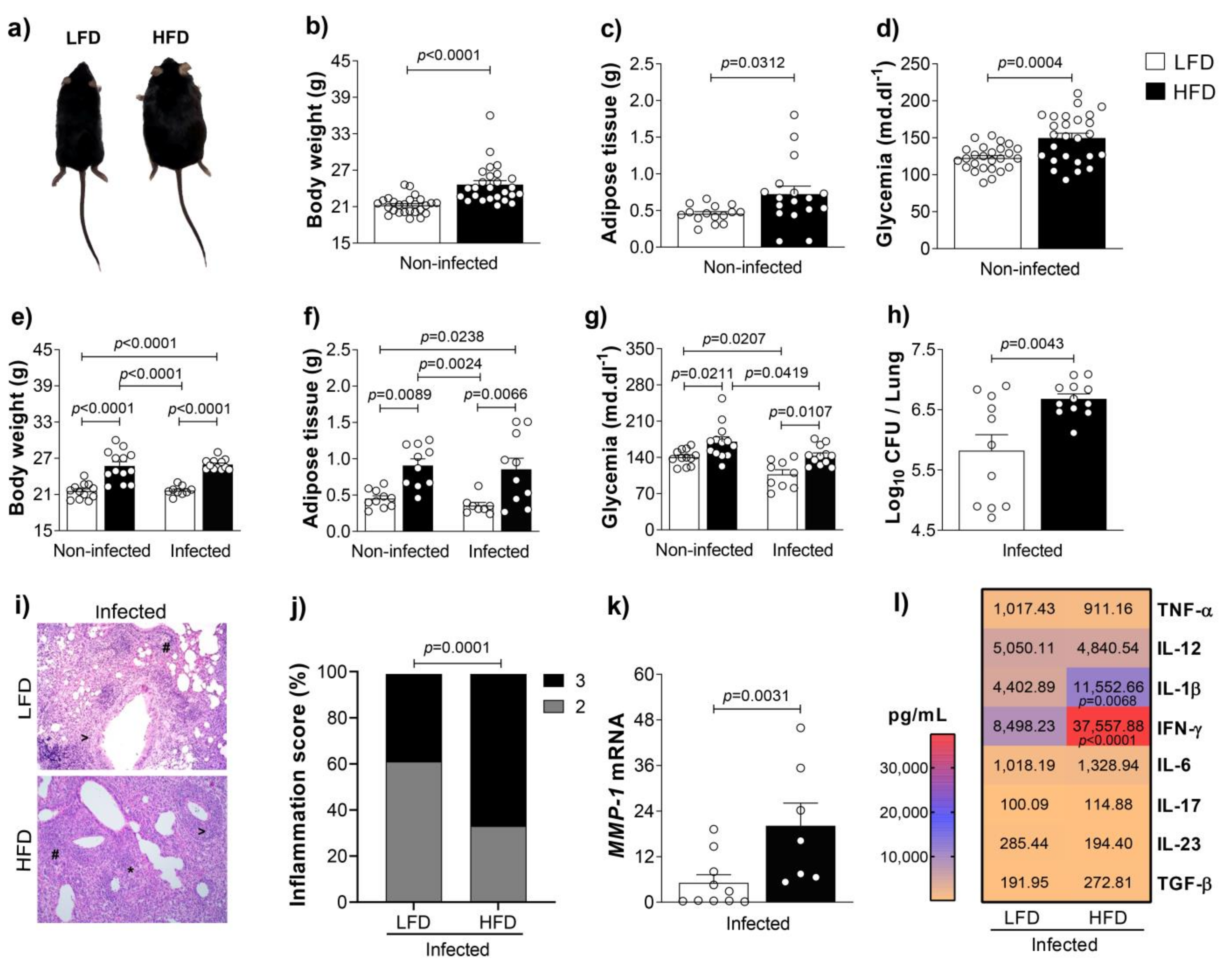
3.1. Obesity Increases Inflammation and Bacterial Loads in the Lungs of M. tuberculosis-Infected Mice
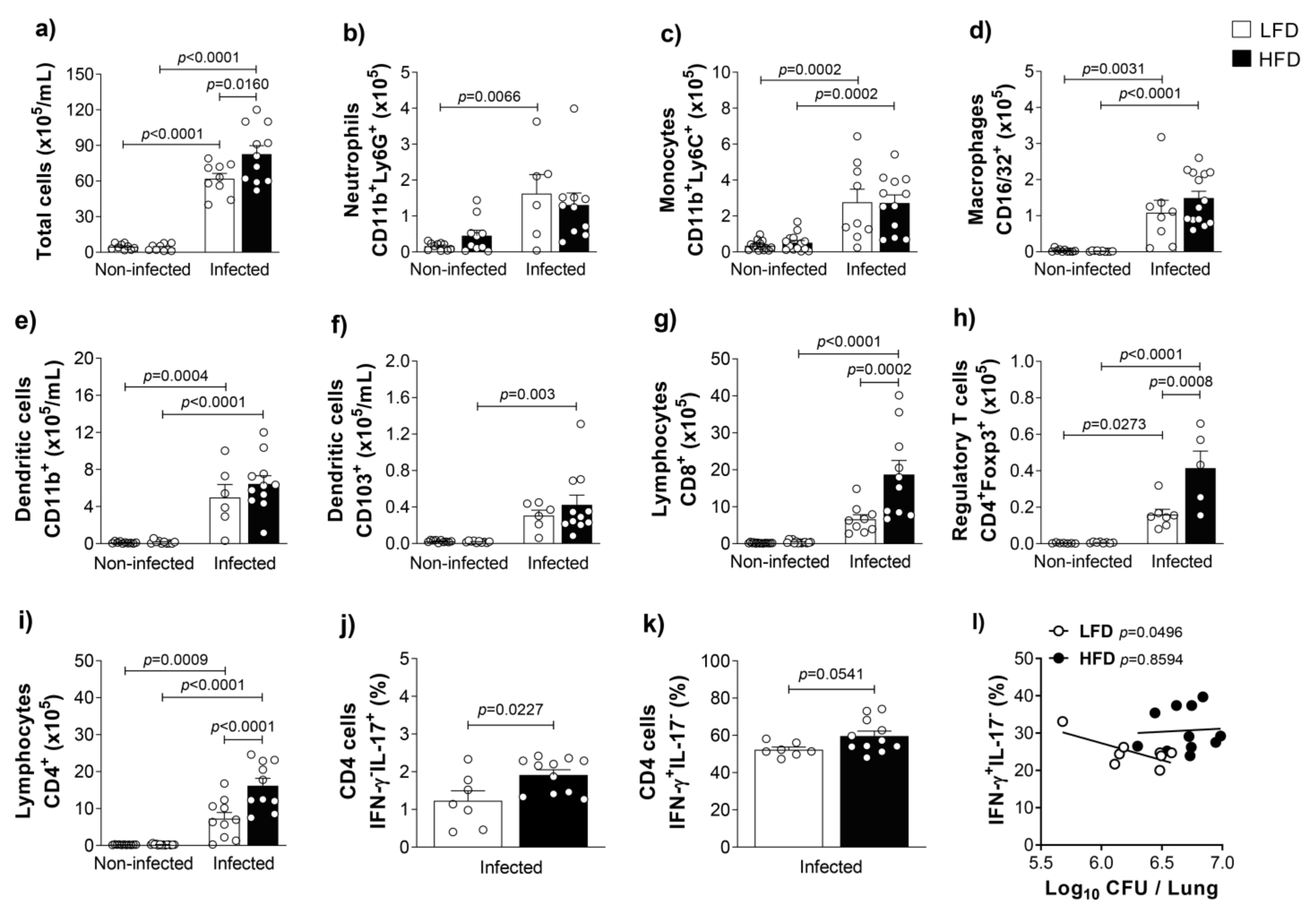
3.2. Obesity Accentuates the Activation and the Influx of Adaptive Immune Leukocytes to the Lungs of M. tuberculosis-Infected Mice
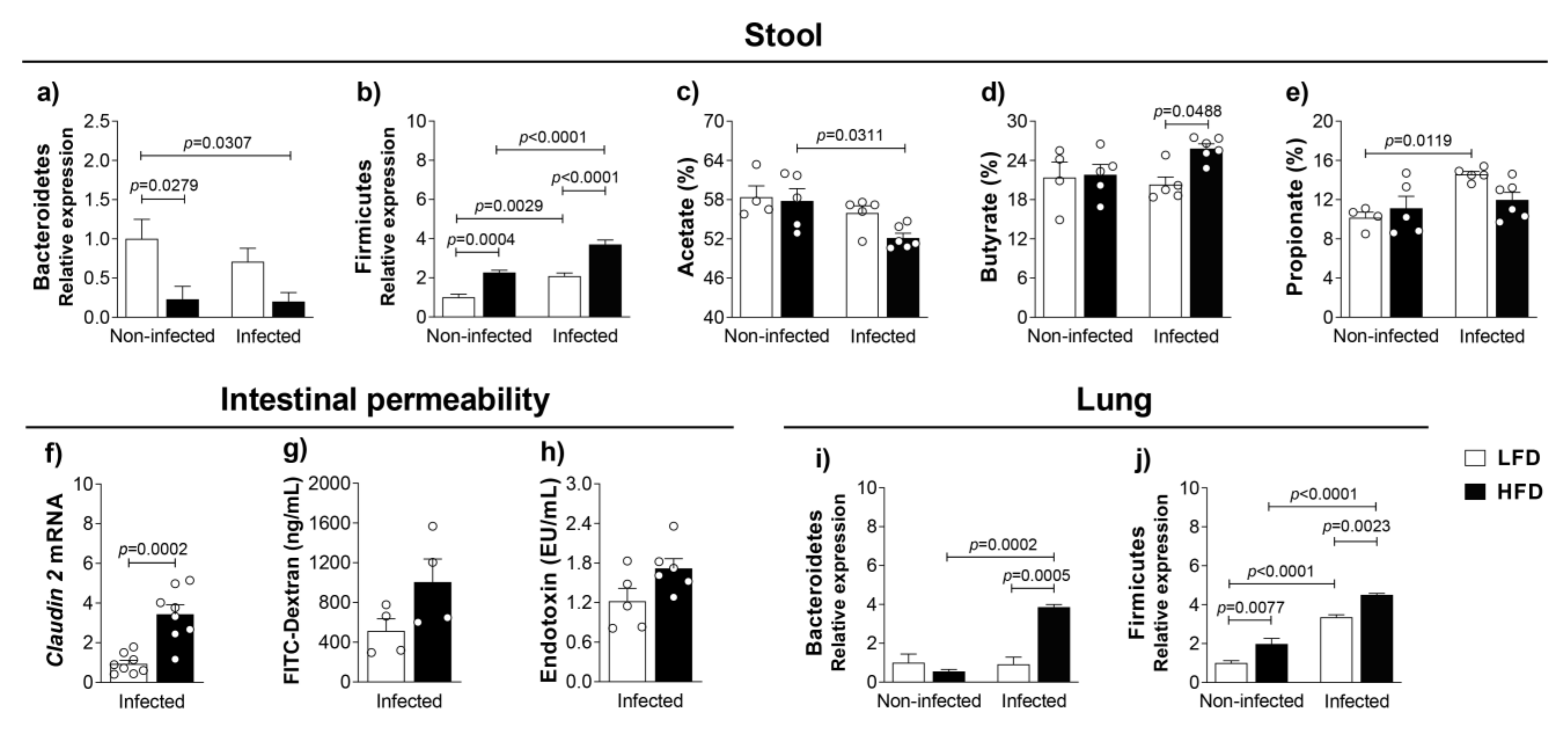
3.3. Obesity-Induced Dysbiosis Is Characterized by an Accentuated Increase of Firmicutes and Bacteroidetes in the Lungs and Firmicutes in the Stool of Infected Mice
3.4. Fecal Transfer Increases Bacterial Load and Restores IFN-γ Levels in the Lungs of Obese and Infected Mice Previously Treated With Antibiotics
3.5. Fecal Transplantation from Obese Mice Restores Obesity-Induced Dysbiosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef] [Green Version]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Bingula, R.; Filaire, M.; Radosevic-Robin, N.; Bey, M.; Berthon, J.-Y.; Bernalier-Donadille, A.; Vasson, M.-P.; Filaire, E. Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer. J. Oncol. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- McAleer, J.P.; Kolls, J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018, 48, 39–49. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-156571-4. [Google Scholar]
- Winglee, K.; Eloe-Fadrosh, E.; Gupta, S.; Guo, H.; Fraser, C.; Bishai, W. Aerosol Mycobacterium tuberculosis Infection Causes Rapid Loss of Diversity in Gut Microbiota. PLoS ONE 2014, 9, e97048. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.; Maiga, M.; Yuan, W.; Thovarai, V.; Costa, D.L.; Mittereder, L.R.; Wipperman, M.F.; Glickman, M.S.; Dzutsev, A.; Trinchieri, G.; et al. Longitudinal profiling reveals a persistent intestinal dysbiosis triggered by conventional anti-tuberculosis therapy. Microbiome 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mendonca, L.; Dhariwal, A.; Fontes, G.; Menzies, D.; Xia, J.; Divangahi, M.; King, I.L. Intestinal dysbiosis compromises alveolar macrophage immunity to Mycobacterium tuberculosis. Mucosal Immunol. 2019, 12, 772–783. [Google Scholar] [CrossRef]
- Khan, N.; Vidyarthi, A.; Nadeem, S.; Negi, S.; Nair, G.; Agrewala, J.N. Alteration in the Gut Microbiota Provokes Susceptibility to Tuberculosis. Front. Immunol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Corral, D.; Colom, A.; Levillain, F.; Peixoto, A.; Hudrisier, D.; Poquet, Y.; Neyrolles, O. The Host Microbiota Contributes to Early Protection Against Lung Colonization by Mycobacterium tuberculosis. Front. Immunol. 2018, 9, 2656. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; Naya, I.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.A.Á.; De La Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Nathella, P.K.; Babu, S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 2017, 152, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N. Innate immune activation in obesity. Mol. Asp. Med. 2013, 34, 12–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.P.; Sridhar, R.; Banurekha, V.V.; Jawahar, M.S.; Nutman, T.B.; Babu, S. Expansion of Pathogen-Specific T-Helper 1 and T-Helper 17 Cells in Pulmonary Tuberculosis With Coincident Type 2 Diabetes Mellitus. J. Infect. Dis. 2013, 208, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.; Orme, I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P. IFNγ-producing CD4 + T lymphocytes: The double-edged swords in tuberculosis. Clin. Transl. Med. 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, T.B.; De Souza, A.I.; Gembre, A.F.; Piñeros, A.R.; Prado, R.D.Q.; Silva, J.S.; Ramalho, L.; Bonato, V.L.D. Genetic background affects the expansion of macrophage subsets in the lungs ofMycobacterium tuberculosis-infected hosts. Immunology 2016, 148, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Piñeros, A.R.; Campos, L.W.; da Fonseca, D.M.; Bertolini, T.B.; Gembre, A.F.; Prado, R.Q.; Alves-Filho, J.C.; Ramos, S.G.; Russo, M.; Bonato, V.L.D. M2 macrophages or IL-33 treatment attenuate ongoing Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, srep41240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Nyman, M.; Jönsson, J. Åke Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2005, 20, 674–682. [Google Scholar] [CrossRef]
- Ahmad, R.; Sorrell, M.F.; Batra, S.K.; Dhawan, P.; Singh, A.B. Gut permeability and mucosal inflammation: Bad, good or context dependent. Mucosal Immunol. 2017, 10, 307–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef]
- Cooke, G.S.; Campbell, S.J.; Sillah, J.; Gustafson, P.; Bah, B.; Sirugo, G.; Bennett, S.; McAdam, K.P.W.J.; Sow, O.; Lienhardt, C.; et al. Polymorphism within the Interferon-γ/Receptor Complex Is Associated with Pulmonary Tuberculosis. Am. J. Respir. Crit. Care Med. 2006, 174, 339–343. [Google Scholar] [CrossRef]
- Elkington, P.T.; Ugarte-Gil, C.; Friedland, J.S. Matrix metalloproteinases in tuberculosis. Eur. Respir. J. 2011, 38, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect. Dis. 2006, 6, 438–446. [Google Scholar] [CrossRef]
- Turnbaugh, P.; Ley, R.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Reese, A.T.; Dunn, R. Drivers of Microbiome Biodiversity: A Review of General Rules, Feces, and Ignorance. mBio 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Mande, S.S. Diet, Microbiota and Gut-Lung Connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Meng, Z.; Kuerban, K.; Qi, F.; Liu, J.; Wei, Y.; Wang, Q.; Jiang, S.; Feng, M.; Ye, L. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, A.Z.; Rodrigues, N.D.C.; Gonzaga, M.I.; Paiolo, J.C.C.; De Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Junior, E.M.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella copri and Bacteroides vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sekyere, J.O.; Maningi, N.; Fourie, P.B. Mycobacterium tuberculosis, antimicrobials, immunity, and lung–gut microbiota crosstalk: Current updates and emerging advances. Ann. N. Y. Acad. Sci. 2020, 1467, 21–47. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.; Fava, F.; Tuohy, K.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang-Sun, W.; Augusto, L.A.; Zhao, L.; Caroff, M. Desulfovibrio desulfuricansisolates from the gut of a single individual: Structural and biological lipid A characterization. FEBS Lett. 2014, 589, 165–171. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Tian, X.; Taylor, C.; Blanchard, E.; Luo, M.; Bhattacharjee, M.B.; Freeborn, J.; Park, S.; Couturier, J.; et al. Lactobacillus reuteri Reduces the Severity of Experimental Autoimmune Encephalomyelitis in Mice by Modulating Gut Microbiota. Front. Immunol. 2019, 10, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Hoang, T.K.; Tran, D.Q.; Rhoads, J.M.; Liu, Y. Adenosine A2A Receptor Deletion Blocks the Beneficial Effects of Lactobacillus reuteri in Regulatory T-Deficient Scurfy Mice. Front. Immunol. 2017, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, M.M.; Björkander, S.; Pang, Y.; Lundqvist, L.; Ndi, M.; Ott, M.; Escribá, I.B.; Jaeger, M.-C.; Roos, S.; Sverremark-Ekström, E. Extracellular Membrane Vesicles from Lactobacilli Dampen IFN-γ Responses in a Monocyte-Dependent Manner. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, T.B.; Piñeros, A.R.; Prado, R.Q.; Gembre, A.F.; Ramalho, L.N.Z.; Alves-Filho, J.C.; Bonato, V.L.D. CCR4-dependent reduction in the number and suppressor function of CD4+Foxp3+ cells augments IFN-γ-mediated pulmonary inflammation and aggravates tuberculosis pathogenesis. Cell Death Dis. 2018, 10, 11. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma Albornoz, S.P.; Fraga-Silva, T.F.d.C.; Gembre, A.F.; de Oliveira, R.S.; de Souza, F.M.; Rodrigues, T.S.; Kettelhut, I.d.C.; Manca, C.S.; Jordao, A.A.; Ramalho, L.N.Z.; et al. Obesity-Induced Dysbiosis Exacerbates IFN-γ Production and Pulmonary Inflammation in the Mycobacterium tuberculosis Infection. Cells 2021, 10, 1732. https://doi.org/10.3390/cells10071732
Palma Albornoz SP, Fraga-Silva TFdC, Gembre AF, de Oliveira RS, de Souza FM, Rodrigues TS, Kettelhut IdC, Manca CS, Jordao AA, Ramalho LNZ, et al. Obesity-Induced Dysbiosis Exacerbates IFN-γ Production and Pulmonary Inflammation in the Mycobacterium tuberculosis Infection. Cells. 2021; 10(7):1732. https://doi.org/10.3390/cells10071732
Chicago/Turabian StylePalma Albornoz, Sandra Patricia, Thais Fernanda de Campos Fraga-Silva, Ana Flávia Gembre, Rômulo Silva de Oliveira, Fernanda Mesquita de Souza, Tamara Silva Rodrigues, Isis do Carmo Kettelhut, Camila Sanches Manca, Alceu Afonso Jordao, Leandra Naira Zambelli Ramalho, and et al. 2021. "Obesity-Induced Dysbiosis Exacerbates IFN-γ Production and Pulmonary Inflammation in the Mycobacterium tuberculosis Infection" Cells 10, no. 7: 1732. https://doi.org/10.3390/cells10071732
APA StylePalma Albornoz, S. P., Fraga-Silva, T. F. d. C., Gembre, A. F., de Oliveira, R. S., de Souza, F. M., Rodrigues, T. S., Kettelhut, I. d. C., Manca, C. S., Jordao, A. A., Ramalho, L. N. Z., Ribolla, P. E. M., Carlos, D., & Bonato, V. L. D. (2021). Obesity-Induced Dysbiosis Exacerbates IFN-γ Production and Pulmonary Inflammation in the Mycobacterium tuberculosis Infection. Cells, 10(7), 1732. https://doi.org/10.3390/cells10071732







