Development of Optimized Vitrification Procedures Using Closed Carrier System to Improve the Survival and Developmental Competence of Vitrified Mouse Oocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Oocyte Collection
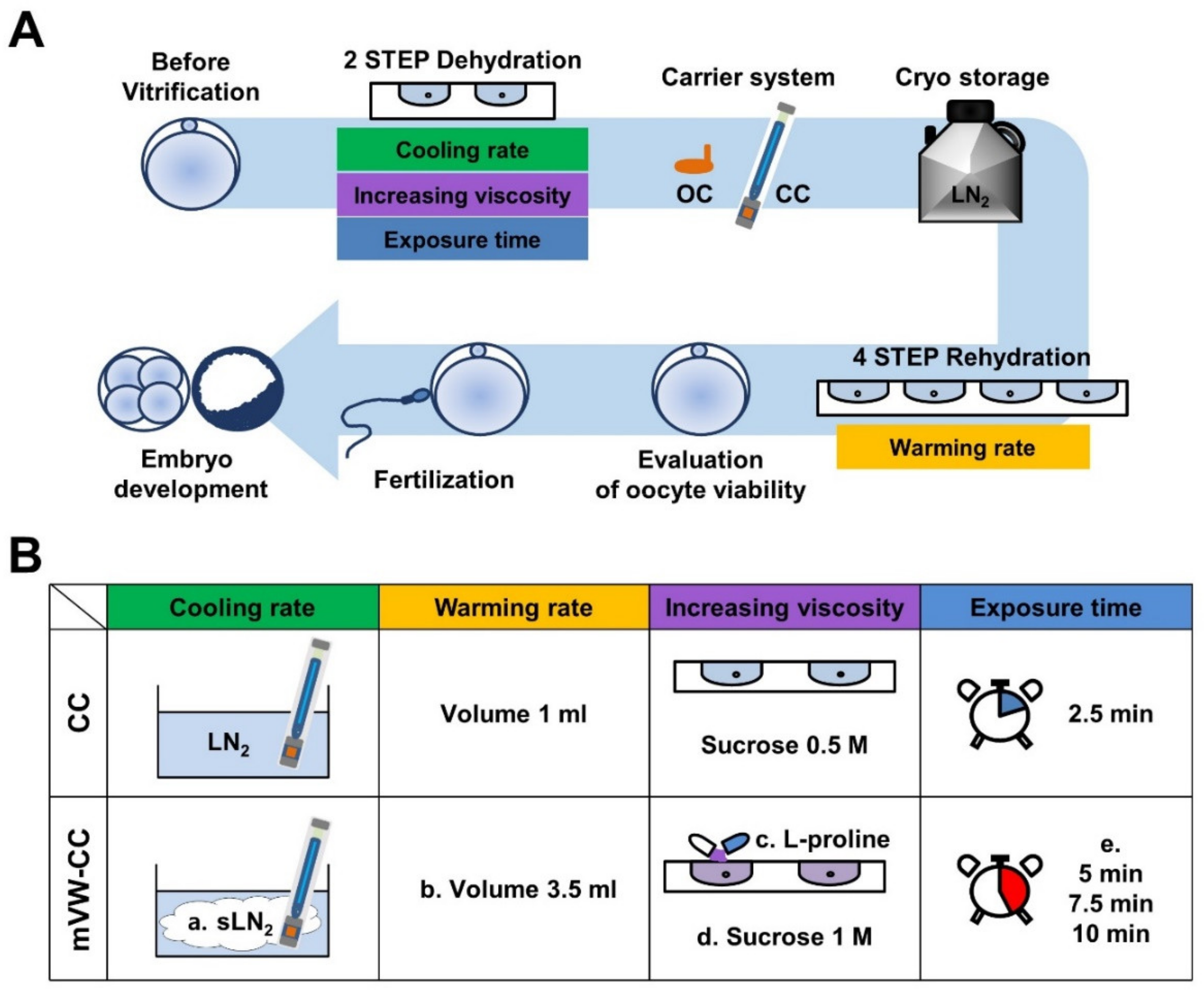
2.2. Vitrification of Oocytes Using Different Carrier Systems
2.3. Vitrification and Warming of Mouse Oocytes
2.4. Evaluation of Oocyte Viability Following Vitrification and Warming
2.5. In Vitro Fertilization and Embryo Culture
2.6. Immunofluorescence Staining of Oocytes and Blastocysts
2.7. Data Analysis
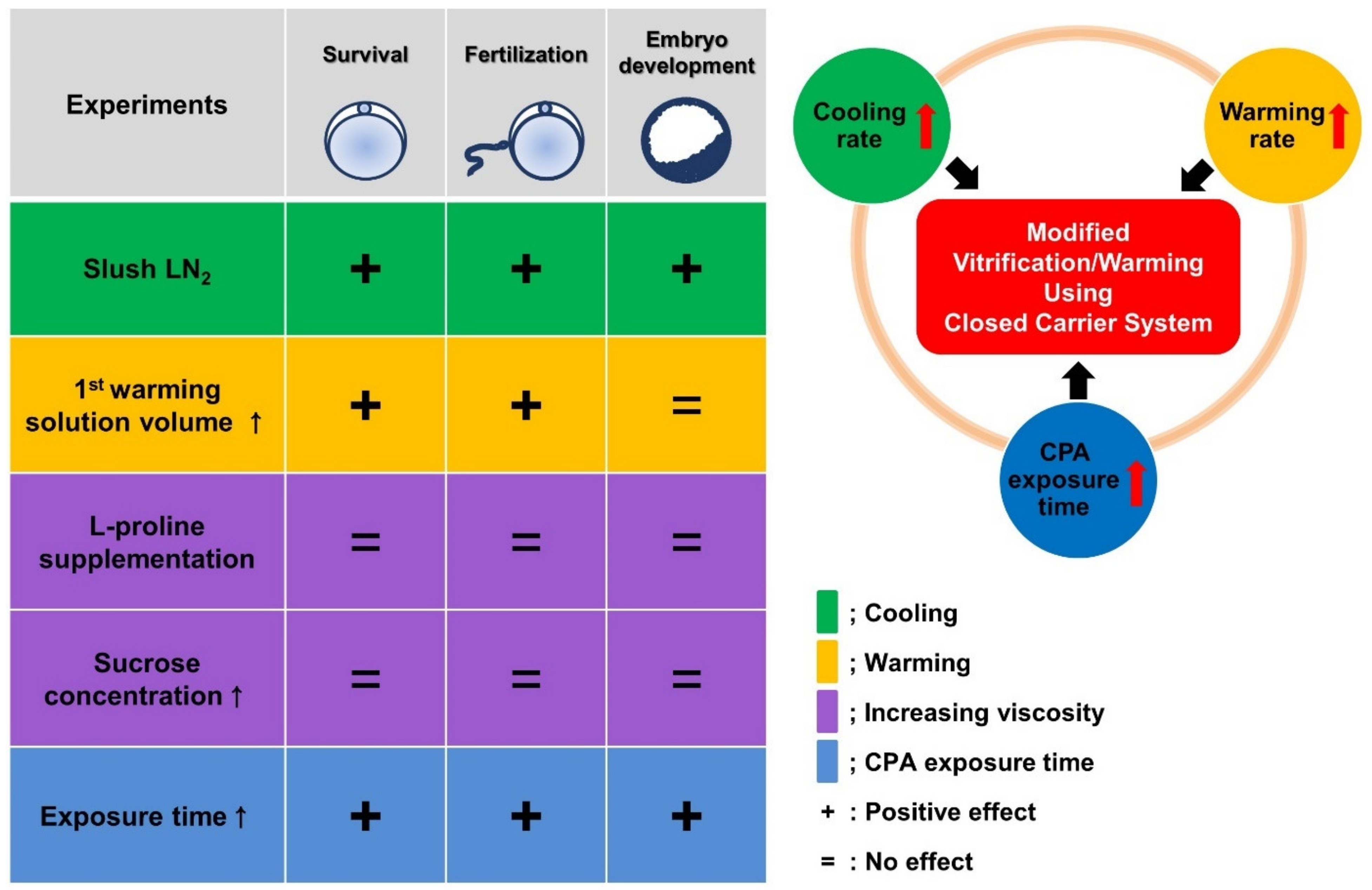
3. Results
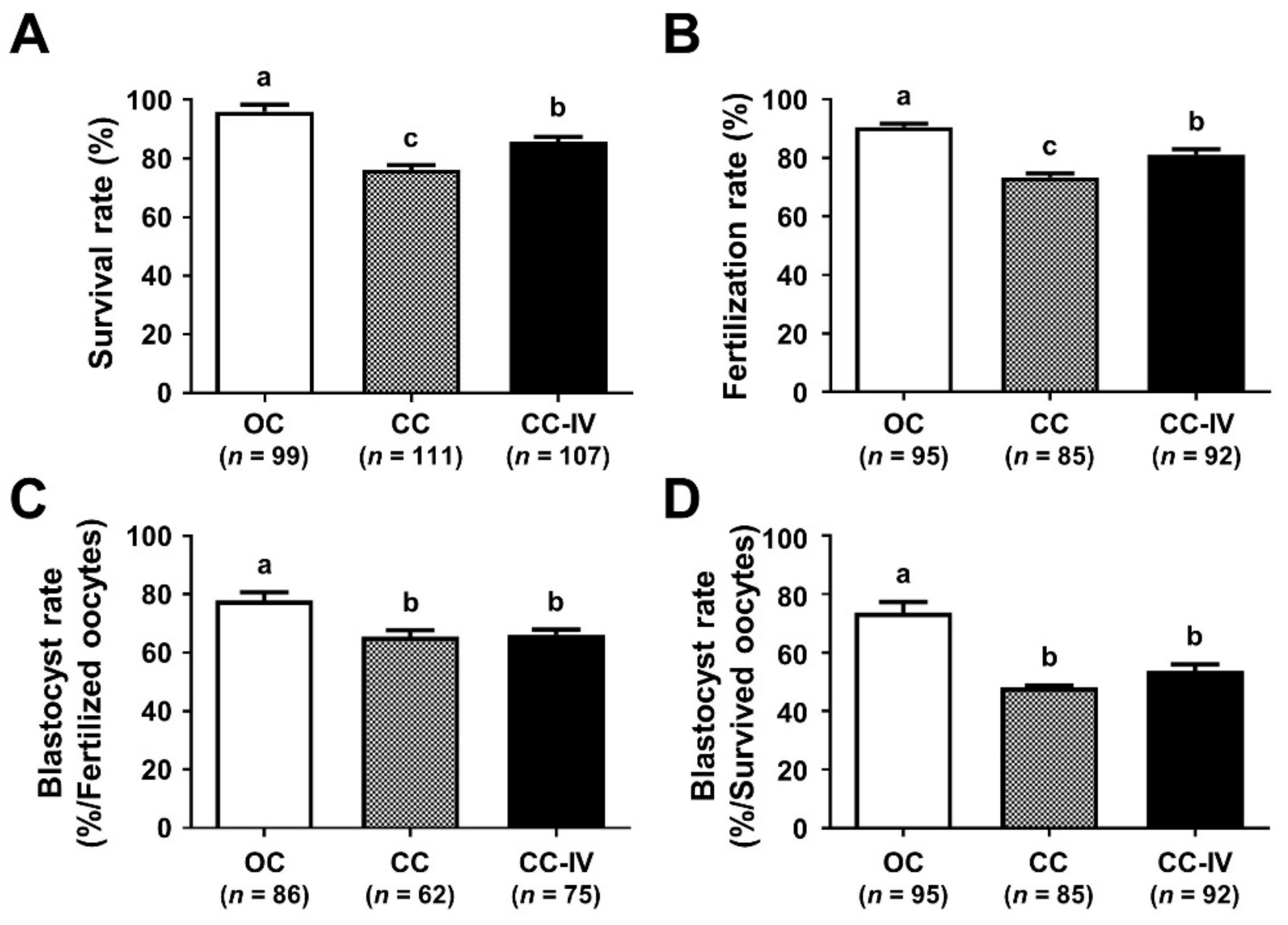
3.1. Comparison of the Survival and Developmental Competence of VW Mouse Oocytes in CC and OC
3.2. Increased Cooling Rate Significantly Improves the Survival and Developmental Competence of VW Oocytes Using the CC
3.3. Increase in the Volume of First Warming Solution Improved the Survival and Developmental Competence of VW Oocytes
3.4. Increased Sucrose Concentration and l-Proline Supplementation Did Not Provide any Positive Effects on the Reproductive Outcomes of VW Oocytes
3.5. Optimization of CPA Exposure Time Improved the Reproductive Outcomes of VW Oocytes
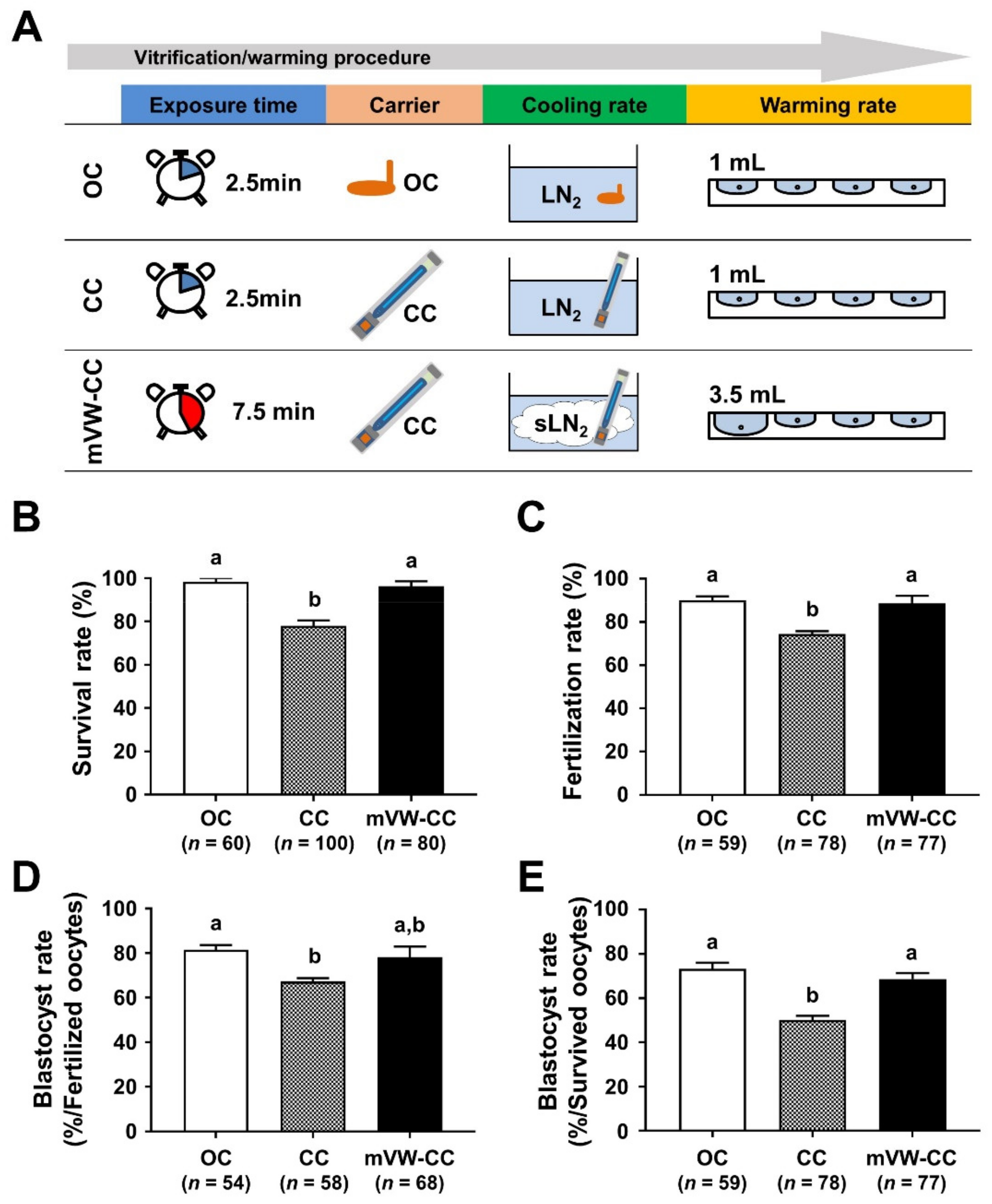
3.6. Modified Vitrification/Warming Using CC Significantly Improved the Reproductive Outcomes of the VW Oocytes
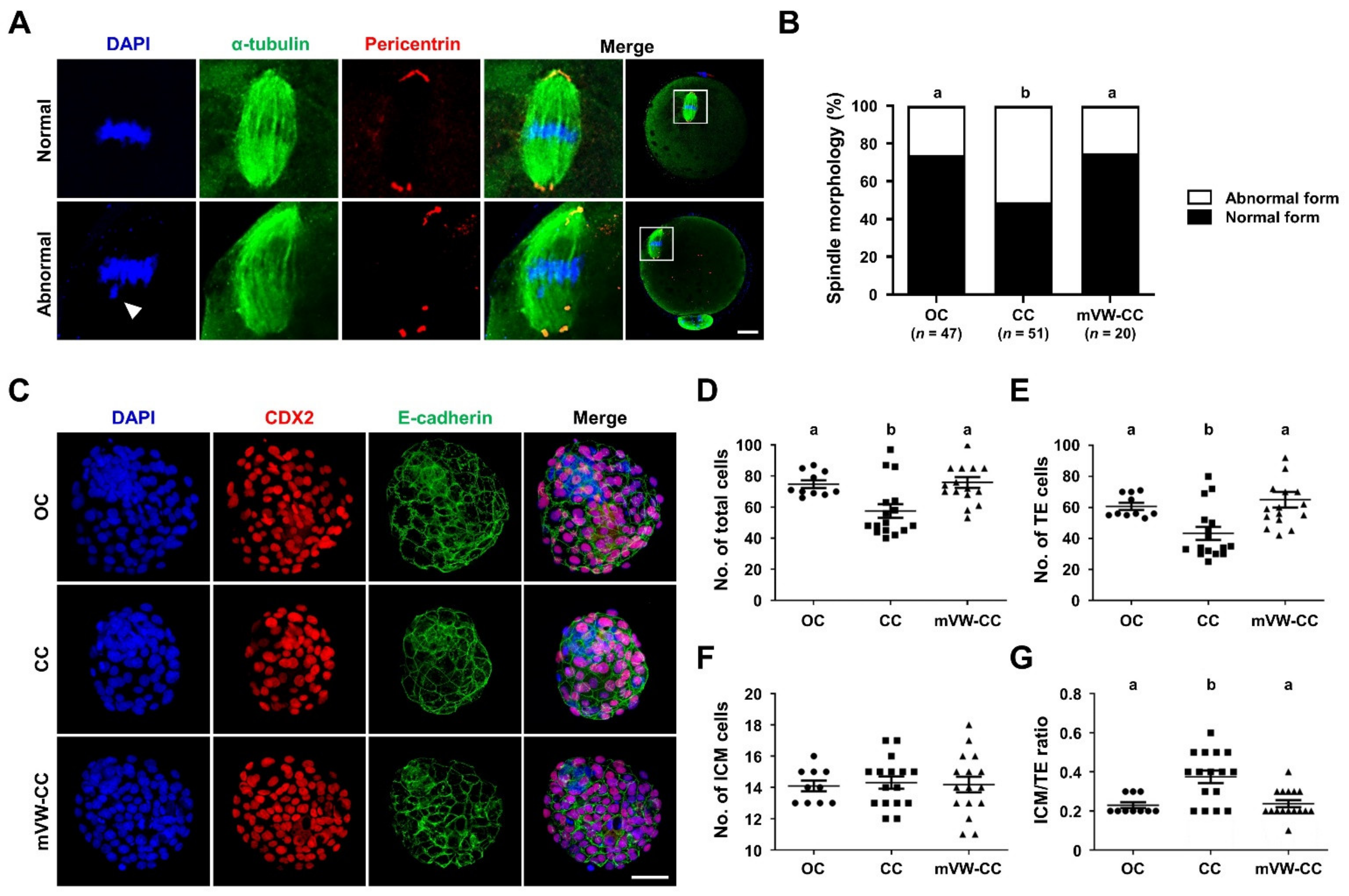
3.7. The mVW-CC Is as Efficient as the OC in Maintaining the Structural Integrity and Developmental Competence of Oocytes after Vitrification
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: A committee opinion. Fertil. Steril. 2013, 100, 1224–1231. [Google Scholar] [CrossRef]
- Fleischer, R.T.; Vollenhoven, B.J.; Weston, G.C. The effects of chemotherapy and radiotherapy on fertility in premenopausal women. Obstet. Gynecol. Surv. 2011, 66, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Noyes, N.; Knopman, J.M.; Melzer, K.; Fino, M.E.; Friedman, B.; Westphal, L.M. Oocyte cryopreservation as a fertility preservation measure for cancer patients. Reprod. Biomed. Online 2011, 23, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Rabadan, S.; Domingo, J.; Cobo, A.; Pellicer, A.; Garcia-Velasco, J.A. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod. Biomed. Online 2014, 29, 722–728. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, D.R.; Han, J.E.; Kim, Y.S.; Lee, W.S.; Won, H.J.; Kim, J.W.; Yoon, T.K. Live birth with vitrified-warmed oocytes of a chronic myeloid leukemia patient nine years after allogenic bone marrow transplantation. J. Assist. Reprod. Genet. 2011, 28, 1167–1170. [Google Scholar] [CrossRef]
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef]
- Mesen, T.B.; Mersereau, J.E.; Kane, J.B.; Steiner, A.Z. Optimal timing for elective egg freezing. Fertil. Steril. 2015, 103, 1551–1556.e1554. [Google Scholar] [CrossRef]
- Jain, J.K.; Paulson, R.J. Oocyte cryopreservation. Fertil. Steril. 2006, 86, 1037–1046. [Google Scholar] [CrossRef]
- Koutlaki, N.; Schoepper, B.; Maroulis, G.; Diedrich, K.; Al-Hasani, S. Human oocyte cryopreservation: Past, present and future. Reprod. Biomed. Online 2006, 13, 427–436. [Google Scholar] [CrossRef]
- Clark, N.A.; Swain, J.E. Oocyte cryopreservation: Searching for novel improvement strategies. J. Assist. Reprod. Genet. 2013, 30, 865–875. [Google Scholar] [CrossRef]
- Bromfield, J.; Coticchio, G.; Hutt, K.; Sciajno, R.; Borini, A.; Albertini, D.F. Meiotic spindle dynamics in human oocytes following slow-cooling cryopreservation. Hum. Reprod. 2009, 24, 2114–2123. [Google Scholar] [CrossRef]
- Tamura, A.N.; Huang, T.T.; Marikawa, Y. Impact of vitrification on the meiotic spindle and components of the microtubule-organizing center in mouse mature oocytes. Biol. Reprod. 2013, 89, 112. [Google Scholar] [CrossRef]
- Smith, G.D.; Motta, E.E.; Serafini, P. Theoretical and experimental basis of oocyte vitrification. Reprod. Biomed. Online 2011, 23, 298–306. [Google Scholar] [CrossRef]
- De Munck, N.; Vajta, G. Safety and efficiency of oocyte vitrification. Cryobiology 2017, 78, 119–127. [Google Scholar] [CrossRef]
- Chian, R.-C.; Wang, Y.; Li, Y.-R. Oocyte vitrification: Advances, progress and future goals. J. Assist. Reprod. Genet. 2014, 31, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Zamora, M.; Obradors, A.; Garcia, D.; Rodriguez, A.; Vassena, R. Comparison of two different oocyte vitrification methods: A prospective, paired study on the same genetic background and stimulation protocol. Hum. Reprod. 2019, 34, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef]
- Nagy, Z.P.; Anderson, R.E.; Feinberg, E.C.; Hayward, B.; Mahony, M.C. The Human Oocyte Preservation Experience (HOPE) Registry: Evaluation of cryopreservation techniques and oocyte source on outcomes. Reprod. Biol. Endocrinol. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Cai, H.; Niringiyumukiza, J.D.; Li, Y.; Lai, Q.; Jia, Y.; Su, P.; Xiang, W. Open versus closed vitrification system of human oocytes and embryos: A systematic review and meta-analysis of embryologic and clinical outcomes. Reprod. Biol. Endocrinol. 2018, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bielanski, A.; Nadin-Davis, S.; Sapp, T.; Lutze-Wallace, C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology 2000, 40, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; Bellver, J.; de los Santos, M.J.; Remohí, J. Viral screening of spent culture media and liquid nitrogen samples of oocytes and embryos from hepatitis B, hepatitis C, and human immunodeficiency virus chronically infected women undergoing in vitro fertilization cycles. Fertil. Steril. 2012, 97, 74–78. [Google Scholar] [CrossRef]
- Alteri, A.; Pisaturo, V.; Somigliana, E.; Viganò, P. Cryopreservation in reproductive medicine during the COVID-19 pandemic: Rethinking policies and European safety regulations. Hum. Reprod. 2020, 35, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; García-Velasco, J.A.; Coello, A.; Domingo, J.; Pellicer, A.; Remohí, J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil. Steril. 2016, 105, 755–764. [Google Scholar] [CrossRef]
- A review of best practices of rapid-cooling vitrification for oocytes and embryos: A committee opinion. Fertil. Steril. 2021, 115, 305–310. [CrossRef] [PubMed]
- Youm, H.S.; Choi, J.-R.; Oh, D.; Rho, Y.H. Closed versus open vitrification for human blastocyst cryopreservation: A meta-analysis. Cryobiology 2017, 77, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Youm, H.S.; Choi, J.-R.; Oh, D.; Rho, Y.H. Survival rates in closed and open Vitrification for human mature oocyte cryopreservation: A meta-analysis. Gynecol. Obstet. Investig. 2018, 83, 268–274. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.K.; Eum, J.H.; Song, H.; Lee, W.S.; Lyu, S.W. Embryo Selection Based on Morphological Parameters in a Single Vitrified-Warmed Blastocyst Transfer Cycle. Reprod. Sci. 2021, 28, 1060–1068. [Google Scholar] [CrossRef]
- Guerrero, J.; Gallardo, M.; Rodríguez-Arnedo, A.; Ten, J.; Bernabeu, R. Comparison of two closed carriers for vitrification of human blastocysts in a donor program. Cryobiology 2018, 81, 12–16. [Google Scholar] [CrossRef]
- Bonetti, A.; Cervi, M.; Tomei, F.; Marchini, M.; Ortolani, F.; Manno, M. Ultrastructural evaluation of human metaphase II oocytes after vitrification: Closed versus open devices. Fertil. Steril. 2011, 95, 928–935. [Google Scholar] [CrossRef]
- Yoon, T.K.; Lee, D.R.; Cha, S.K.; Chung, H.M.; Lee, W.S.; Cha, K.Y. Survival rate of human oocytes and pregnancy outcome after vitrification using slush nitrogen in assisted reproductive technologies. Fertil. Steril. 2007, 88, 952–956. [Google Scholar] [CrossRef]
- Minasi, M.G.; Fabozzi, G.; Casciani, V.; Ferrero, S.; Litwicka, K.; Greco, E. Efficiency of slush nitrogen vitrification of human oocytes vitrified with or without cumulus cells in relation to survival rate and meiotic spindle competence. Fertil. Steril. 2012, 97, 1220–1225. [Google Scholar] [CrossRef]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; De Stefano, C.; Ferraro, R.; Sudhakaran, S.; Gualtieri, R. Successful slush nitrogen vitrification of human ovarian tissue. Fertil. Steril. 2016, 105, 1523–1531. [Google Scholar] [CrossRef]
- Barbato, V.; Gualtieri, R.; Capriglione, T.; Pallotta, M.M.; Braun, S.; Di Nardo, M.; Costanzo, V.; Ferraro, R.; Catapano, G.; Talevi, R. Slush nitrogen vitrification of human ovarian tissue does not alter gene expression and improves follicle health and progression in long-term in vitro culture. Fertil. Steril. 2018, 110, 1356–1366. [Google Scholar] [CrossRef]
- Lee, H.-J.; Elmoazzen, H.; Wright, D.; Biggers, J.; Rueda, B.R.; Heo, Y.S.; Toner, M.; Toth, T.L. Ultra-rapid vitrification of mouse oocytes in low cryoprotectant concentrations. Reprod. Biomed. Online 2010, 20, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yavin, S.; Aroyo, A.; Roth, Z.; Arav, A. Embryo cryopreservation in the presence of low concentration of vitrification solution with sealed pulled straws in liquid nitrogen slush. Hum. Reprod. 2009, 24, 797–804. [Google Scholar] [CrossRef]
- Jin, B.; Mazur, P. High survival of mouse oocytes/embryos after vitrification without permeating cryoprotectants followed by ultra-rapid warming with an IR laser pulse. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, G.; Zhang, Z.; Xu, X.; He, X. Magnetic induction heating of superparamagnetic nanoparticles during rewarming augments the recovery of hUCM-MSCs cryopreserved by vitrification. Acta Biomater. 2016, 33, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Mazur, P. Ultra-rapid warming yields high survival of mouse oocytes cooled to −196 °C in dilutions of a standard vitrification solution. PLoS ONE 2012, 7, e36058. [Google Scholar] [CrossRef] [PubMed]
- Canesin, H.S.; Brom-de-Luna, J.G.; Choi, Y.-H.; Pereira, A.M.; Macedo, G.G.; Hinrichs, K. Vitrification of germinal-vesicle stage equine oocytes: Effect of cryoprotectant exposure time on in vitro embryo production. Cryobiology 2018, 81, 185–191. [Google Scholar] [CrossRef]
- Yong, K.W.; Laouar, L.; Elliott, J.A.; Jomha, N.M. Review of non-permeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology 2020, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R.; Porcu, E.; Marsella, T.; Rocchetta, G.; Venturoli, S.; Flamigni, C. Human oocyte cryopreservation: New perspectives regarding oocyte survival. Hum. Reprod. 2001, 16, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Alfoteisy, B.; Singh, J.; Anzar, M. Natural honey acts as a nonpermeating cryoprotectant for promoting bovine oocyte vitrification. PLoS ONE 2020, 15, e0238573. [Google Scholar] [CrossRef]
- Kornienko, E.V.; Romanova, A.B.; Ikonopistseva, M.V.; Malenko, G.P. Optimization of triacetate cellulose hollow fiber vitrification (HFV) method for cryopreservation of in vitro matured bovine oocytes. Cryobiology 2020, 97, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Li, L.-X.; Liu, X.-L.; Yang, Y.-X.; Wan, X.-P. Sucrose affecting successful transplantation of vitrified-thawed mouse ovarian tissues. J. Assist. Reprod. Genet. 2009, 26, 137–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Xue, X.; Yan, J.; Yan, L.-Y.; Jin, X.-H.; Zhu, X.-H.; He, Z.-Z.; Liu, J.; Li, R.; Qiao, J. L-proline: A highly effective cryoprotectant for mouse oocyte vitrification. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.D.; Kearsley, A.J.; Higgins, A.Z. Mathematical optimization of procedures for cryoprotectant equilibration using a toxicity cost function. Cryobiology 2012, 64, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhata, S.; Hayashi, M.; Fujii, Y.; Motoyama, H.; Endo, Y. Effect of equilibration time on clinical and neonatal outcomes in human blastocysts vitrification. Reprod. Med. Biol. 2020, 19, 270–276. [Google Scholar] [CrossRef]
- Kader, A.; Choi, A.; Sharma, R.K.; Falcone, T.; Agarwal, A. Effect of varying equilibration time in a two-step vitrification method on the post-warming DNA integrity of mouse blastocysts. Fertil. Steril. 2010, 93, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.; Catt, S.; Taylor-Robinson, A.W. A comparative analysis of the efficacy of three cryopreservation protocols on the survival of in vitro-derived cattle embryos at pronuclear and blastocyst stages. Cryobiology 2017, 77, 58–63. [Google Scholar]
- Martínez-Rodero, I.; García-Martínez, T.; Ordóñez-León, E.A.; Vendrell-Flotats, M.; Olegario Hidalgo, C.; Esmoris, J.; Mendibil, X.; Azcarate, S.; López-Béjar, M.; Yeste, M. A Shorter Equilibration Period Improves Post-Warming Outcomes after Vitrification and in Straw Dilution of In Vitro-Produced Bovine Embryos. Biology 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.; Saenz, J.; Risco, R. Human oocytes and zygotes are ready for ultra-fast vitrification after 2 minutes of exposure to standard CPA solutions. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Szurek, E.A.; Higgins, A.Z.; Lee, S.R.; Eroglu, A. Optimization of cryoprotectant loading into murine and human oocytes. Cryobiology 2014, 68, 18–28. [Google Scholar] [CrossRef]
- Cobo, A.; Garrido, N.; Pellicer, A.; Remohí, J. Six year’s experience in ovum donation using vitrified oocytes: Report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil. Steril. 2015, 104, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Domingo, J.; Cobo, A.; Martínez, M.; Carmona, L.; Pellicer, A. Five years’ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil. Steril. 2013, 99, 1994–1999. [Google Scholar] [CrossRef]
- Kuwayama, M.; Vajta, G.; Kato, O.; Leibo, S.P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. Biomed. Online 2005, 11, 300–308. [Google Scholar] [CrossRef]
- Gardner, D.K.; Weissman, A.; Howles, C.M.; Shoham, Z. Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wang, L.; Liu, J.; Zhou, G.-B.; Hou, Y.-P.; Li, J.-J.; Zhu, S.-E. Quantitative investigations on the effects of exposure durations to the combined cryoprotective agents on mouse oocyte vitrification procedures. Biol. Reprod. 2011, 85, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Bernard, A.; Fuller, B.; Hunter, J.; Shaw, R. Vitrification of mouse oocytes using short cryoprotectant exposure: Effects of varying exposure times on survival. Mol. Reprod. Dev. 1992, 33, 210–214. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.K.; Lee, J.H.; Park, E.A.; Lim, H.J.; Lyu, S.W.; Lee, W.S.; Kim, J.; Song, H. Development of Optimized Vitrification Procedures Using Closed Carrier System to Improve the Survival and Developmental Competence of Vitrified Mouse Oocytes. Cells 2021, 10, 1670. https://doi.org/10.3390/cells10071670
Park JK, Lee JH, Park EA, Lim HJ, Lyu SW, Lee WS, Kim J, Song H. Development of Optimized Vitrification Procedures Using Closed Carrier System to Improve the Survival and Developmental Competence of Vitrified Mouse Oocytes. Cells. 2021; 10(7):1670. https://doi.org/10.3390/cells10071670
Chicago/Turabian StylePark, Jae Kyun, Ju Hee Lee, Eun A Park, Hyunjung J. Lim, Sang Woo Lyu, Woo Sik Lee, Jayeon Kim, and Haengseok Song. 2021. "Development of Optimized Vitrification Procedures Using Closed Carrier System to Improve the Survival and Developmental Competence of Vitrified Mouse Oocytes" Cells 10, no. 7: 1670. https://doi.org/10.3390/cells10071670
APA StylePark, J. K., Lee, J. H., Park, E. A., Lim, H. J., Lyu, S. W., Lee, W. S., Kim, J., & Song, H. (2021). Development of Optimized Vitrification Procedures Using Closed Carrier System to Improve the Survival and Developmental Competence of Vitrified Mouse Oocytes. Cells, 10(7), 1670. https://doi.org/10.3390/cells10071670







