Abstract
Infections with the deadliest malaria parasite, Plasmodium falciparum, are accompanied by a strong immunological response of the human host. To date, more than 30 cytokines have been detected in elevated levels in plasma of malaria patients compared to healthy controls. Endothelial cells (ECs) are a potential source of these cytokines, but so far it is not known if their cytokine secretion depends on the direct contact of the P. falciparum-infected erythrocytes (IEs) with ECs in terms of cytoadhesion. Culturing ECs with plasma from malaria patients (27 returning travellers) resulted in significantly increased secretion of IL-11, CXCL5, CXCL8, CXCL10, vascular endothelial growth factor (VEGF) and angiopoietin-like protein 4 (ANGPTL4) if compared to matching controls (22 healthy individuals). The accompanying transcriptome study of the ECs identified 43 genes that were significantly increased in expression (≥1.7 fold) after co-incubation with malaria patient plasma, including cxcl5 and angptl4. Further bioinformatic analyses revealed that biological processes such as cell migration, cell proliferation and tube development were particularly affected in these ECs. It can thus be postulated that not only the cytoadhesion of IEs, but also molecules in the plasma of malaria patients exerts an influence on ECs, and that not only the immunological response but also other processes, such as angiogenesis, are altered.
1. Introduction
Despite the advances in malaria control programs, malaria remains one of the most detrimental infectious diseases worldwide. In 2019, about 229 million cases of malaria were recorded, including 409,000 cases of deaths [1]. Within the five species that cause malaria in humans, Plasmodium falciparum is the most clinically relevant one and responsible for most deaths. The complications caused by malaria infection are multifactorial; both the parasite and the host contribute. A central part of the pathogenesis is the cytoadhesion of P. falciparum-infected erythrocytes (IEs) within the vascular bed of vitally important organs, such as the brain, heart, lung, stomach, skin and kidney [2,3,4]. Besides blockage of capillaries due to the cytoadhesion of the IEs, increased inflammatory cytokine production, endothelial dysfunction and increased vascular permeability also occur in the affected tissue [5,6,7]. As a result of the immune response induced by parasite growth and cytoadhesion to the endothelium, patients develop fever, headaches, muscle aches and rigors [8,9,10,11,12]. According to the age and immune status of the patient, severe lethal complications, such as cerebral malaria (CM), lung injury, renal failure, acidosis and severe anaemia, may develop [11,12,13]. Not only the acute complications affect the patients, but also one third of survivors (African children and adult travellers) were found to suffer from long-term health problems, such as cognitive and neurological impairments [14,15,16].
Several in vitro studies have shown that interaction of the IEs with endothelial cells (ECs) increase the expression of various genes encoding proinflammatory cytokines, such as IL-6, IL-1 and tumor necrosis factor-alpha (TNF-α), and chemokines, such as CXCL8, CCL2, CCL20, CXCL1, CXCL2 and CCL5, which are important for the recruitment of leukocytes to the endothelium during inflammation [17,18,19].
Diverse plasmodial antigens and released components, as well as endogenous metabolites associated with danger signals, have repeatedly been shown to stimulate the immune system. Glycosylphosphadidylinositol anchors of P. falciparum proteins have been the first ones described [20], leading to production of the proinflammatory cytokines in macrophages [21]. It was also shown that in monocytes fed with hemozoin, expression of genes encoding for cytokines and chemokines was increased [22]. In macrophages, incubation with hemozoin resulted in an increase in various chemokine transcripts, including CCL3, CCL4, CXCL1 and CCL2 [23], but phagocytosis of hemozoin leads to impairment of macrophage function [24]. Thus, P. falciparum malaria is accompanied by a strong immunological reaction of the host. This considerable increase in systemic and local inflammation contributes significantly to the pathogenesis of malaria. The immune response triggered by P. falciparum is a very complex event. To date, well over 30 cytokines have been described, which can be detected in serum or plasma of malaria patients in larger quantities compared to healthy controls. These include CCL2, CCL4, CXCL4, CXCL8, CXCL10, CCL3, IL-1β, IL-6, CXCL2, TNF-α, interferon-gamma (INF-γ), IL-1α, IL-12, IL-17A, IL-15, IL-10, IL1-RA, CCL20, vascular endothelial growth factor (VEGF), IL-13, IL-31, IL-33, CXCL9, IL-9, CCL28 and granulocyte-colony stimulating factor (G-CSF) [5,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Significantly increased amounts of CCL2, CCL4, CXCL4, CXCL8, CXCL10, IL-1RA, IL-6, TNF-α and G-CSF were detected in patients with CM [27,29,39,40] and the amount of some cytokines (CCL2, CCL4, CXCL4, CXCL8, CXCL10 and CCL3) directly correlates with the severity of a malaria infection [25,26,29,30,36].
Plasma levels of 29 biomarkers, including various chemokines and cytokines, were investigated in patients with CM and non-CM. However, significantly increased levels in patients with CM compared to non-CM were only found for IL-6, CXCL8 and IL-1RA [26]. A positive correlation with parasitaemia was described for CCL20 and CXCL9 [36].
To date, there are no studies comparing the levels of different cytokines in the plasma of P. falciparum-infected returning travellers and, in parallel, elucidating the influence of these plasmas on the stimulation of ECs and the associated secretion of these factors into the cell culture supernatant as well as their influence on EC gene expression.
Our studies showed that co-incubation of brain ECs with plasma from malaria patients resulted in significantly increased secretion of IL-11, CXCL5, CXCL10, VEGF and angiopoietin-like protein 4 (ANGPTL4). A comparative transcriptome analysis revealed that, in addition to an inflammatory response, biological processes such as cell migration, cell proliferation and tube development, as well as the KEGG ‘IL-17 signalling pathway’, were particularly affected.
2. Materials and Methods
2.1. Blood Plasma of Malaria Patients and Healthy Control Individuals
The study was performed on 27 EDTA-plasma samples from patients diagnosed with P. falciparum malaria, with parasitaemia between <1% and 11%. All patients were adult tropical returnees and were treated as in- or outpatients in Hamburg, Germany. Patients were either seen in the outpatient clinic of the University Medical Center Hamburg-Eppendorf (UKE) at the Bernhard Nocht Institute for Tropical Medicine, treated as inpatients at the UKE, or at the Bundeswehrkrankenhaus Hamburg. As controls, 22 plasma samples from healthy individuals were used. The study was approved by the relevant ethics committee (Ethical Review Board of the Medical Association of Hamburg, reference numbers PV3828 and PV4539) (Supplementary Table S1).
2.2. HBEC-5i Brain Endothelial Cell Line
This project was carried out using human brain endothelial cells HBEC-5i, derived from the cerebral cortex and immortalized with the SV40 large T antigen (American Type Culture Collection (ATCC), Manassas, VA, USA; no. CRL-3245). HBEC-5i cells were seeded in 0.1% gelatin-coated T25 culture flasks. For normal cell culture, DMEM/F-12 complete growth medium (Gibco, Thermo Fisher Scientific, Bremen, Germany) containing 40 µg/mL endothelial cell growth supplement (ECGS; Merck Millipore, Darmstadt, Germany), 10% heat-inactivated foetal calf serum (Capricorn Scientific, Ebsdorfergrund, Germany) and 9 µg/mL gentamycin (Sigma–Aldrich Merck, Darmstadt, Germany) was used. The endothelial cells (ECs) were cultivated at 37 °C and 5% CO2 atmosphere and split every 2–4 days when a confluence of 70–90% is reached.
2.3. Stimulation Assay of ECs with Plasma of Malaria Patients and Healthy Control Individuals
The 96-well plates were coated with 50 μL of 0.1% gelatin (Sigma–Aldrich Merck, Darmstadt, Germany) in Dulbecco’s Phosphate-Buffered Saline (DPBS; PAN, Biotech, Germany) per well and incubated at 37 °C for 30 min. After incubation, the gelatin was aspirated and 50 μL DMEM/F-12 medium was placed in each well and incubated at 37 °C for 15 min to adjust the pH value. After removal of the DMEM/F-12 medium, 1 × 104 ECs in 200 µL DMEM/F-12 medium were added to each well. The cells were cultivated for two days with a medium change after the first day.
For the stimulation assay, the cells were washed twice with 100 µL/well DMEM/F-12 medium each before addition of the human plasma. In total, 80 µL of a plasma mixture consisting of 58 µL DMEM/F-12/gentamycin medium, 2 µL heparin (10,000 units/mL; Braun, Melsungen, Germany) and 20 µL human plasma were added per well. Each plasma sample was analysed in quadruple. The 96-well plate was then incubated for 6 h at 37 °C (5% CO2). After completion of the 6 h incubation, the supernatant was removed, and the wells were washed 4 times with DMEM/F-12/gentamycin medium. Then 100 µL of DMEM/F-12 complete growth medium was added and the cells were incubated for another 42 h before the cell culture supernatant was removed; after a total amount of 48 h, the four replicates were pooled, centrifuged and the supernatant immediately frozen at −80 °C.
For the transcriptome analyses, the ECs were incubated in T25 cell culture flasks (monolayer 70–90%) containing 4.5 mL DMEM/F-12/gentamycin medium, 50 µL heparin (10,000 units/mL; Braun, Melsungen, Germany) and 500 µL plasma of malaria patients and healthy controls, respectively (plasma concentration 10%), for 7 h. Afterwards, the cells were washed and lysed with 200 µL Trizol (Invitrogen, Thermo Fisher Scientific, Bremen, Germany) and stored at −80 °C until the RNA was isolated.
2.4. LEGENDplexTM Assay
The LEGENDplex Kits used were multiplex bead-based assay panels manufactured by BioLegend, Inc. (San Diego, CA, USA). The two bead panels that were chosen for measurement of cytokine concentration in every sample included the pro-inflammatory cytokines IL-1α and IL-1β, the pro- and anti-inflammatory cytokines IL-6, IL-7, IL-12 and IFN-β, the anti-inflammatory cytokines IL-1RA, IL-10 and IL-11, and the proinflammatory chemokines CCL3, CCL20, CXCL1, CXCL5, CXCL8, CXCL10 and VEGF. The bead-assays were performed following instructions provided by the manufacturer in duplicates. After completion of the reaction, the samples were transferred to FACS tubes to be read on a flow cytometer (BD Accuri® C6 Flow Cytometer, Thermo Fisher Scientific, Bremen, Germany).
The concentration of a particular analyte was determined by the provided LEGENDplexTM Software v8 based on a known standard curve. Values with evident methodical errors were excluded. After calculating the mean of the two replicated values for each analyte, statistical analyses were performed using GraphPad Prism (version 9.02 (134) GraphPad Software Inc, San Diego, CA, USA). A Mann–Whitney U test was run to determine differences in cytokine concentration between groups. Exact p-values corrected for ties were calculated and differences considered significant for p-values ≤ 0.05. In case of normally distributed data, an independent samples t-test was performed to support the results (data not shown). Patient’s plasma samples were divided into three subgroups, based on parasitaemia. Kendall’s tau b correlation was run to determine the relationship between the analyte concentration and level of parasitaemia. The correlation between the cytokines and parasitaemia was performed by means of a correlation analysis using the nonparametric Spearman correlation (GraphPad Prism, version 9.02 (134)). For multiple testing, the Benjamini–Hochberg adjustment and conservative Bonferroni correction were applied [41].
2.5. ANGPTL4 and TNF-α ELISA
Human ANGPTL4 and TNF-α was measured using ELISA after respective dilution of the sample in a reagent dilution buffer following the instructions of the manufacturers (R&D Systems, Minneapolis, MN, USA). Significance was evaluated using the Mann–Whitney U test.
2.6. RNA Isolation
RNA was isolated using a PureLink RNA Mini Kit (Thermo Fisher Scientific, Bremen, Germany) according to the manufacturer’s instructions. Genomic DNA contamination was removed using the TURBO DNA-free Kit (Invitrogen, Thermo Fisher Scientific, Bremen, Germany) followed by a magnetic bead enzymatic wash using Agencourt RNAClean XP (Beckman Coulter, Krefeld, Germany). The concentration and quality of isolated RNA were assessed using an Agilent 2100 Bioanalyser System with the Agilent RNA 6000 Pico Kit (Agilent Technologies, Ratlingen, Germany). The RNA was sent to BGI (Shenzhen, China), where RNAseq was performed using the Illumina HiSeq 4000 PE100 platform (approximately 11 M PE reads per samples). Reads were quality and adapter trimmed using Trimmomatic [42] and aligned to the human transcriptome by RSEM [43] using Bowtie2 [44] as an aligner. Differential expression was determined using DESeq2 [45].
3. Results
3.1. Determination of Concentrations of Different Cytokines in Plasmas of Malaria Patients and Healthy Controls
In the first part of this study, the plasmas of 27 patients infected with P. falciparum with a parasitaemia between <1% and 11% and of 22 healthy individuals were analysed for the presence of 16 different cytokines. All 27 patients were adult tropical returnees with symptomatic P. falciparum malaria (Supplementary Table S1). In this study, the pro-inflammatory cytokines IL-1α and IL-1β, the pro- and anti-inflammatory cytokines IL-6, IL-7, IL-11, IL-12 and IFN-β, the anti-inflammatory cytokines IL-1RA and IL-10 and the proinflammatory chemokines CCL3, CCL20, CXCL1, CXCL5, CXCL8 and CXCL10, as well as the growth factor VEGF, were analysed using a customized LEGENDplex assay (Supplementary Table S2).
For cytokines IL-6, IL-1RA, IL-10 and IL-11 and chemokines CXCL1, CXCL8, CXCL10, CCL3 and CCL2, significantly higher concentrations were found in plasma samples of malaria patients compared to healthy controls (Figure 1A). For IL-6, IL-1RA, CCL3, CCL20 and CXCL10, the significant difference was already detected at a parasitaemia < 1.0%. For CXCL8, IL-10 and CXCL1, a significantly higher value was found at a parasitaemia ≥ 1% and for IL-11 and CXCL5 only at a parasitaemia > 2.5% (Figure 1A).
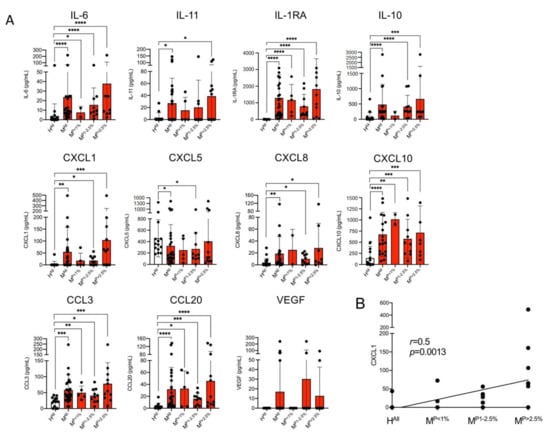
Figure 1.
Levels of cytokines in plasma derived from malaria patients (M) and healthy control individuals (H). (A) The amount of cytokines was measured with a bead-based LEGENDplex assay (n = 13–26, Supplementary Tables S2 and S3). Data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using the Mann–Whitney U test (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). (B) For CXCL1, the correlation between the amount of cytokine and parasitaemia was performed by means of a nonparametric Spearman correlation using GraphPad Prism (version 9.0.2 (134)). Abbreviations: Healthy controls (HAll); malaria patients (MAll); malaria patients with a parasitaemia < 1% (MP<1%), 1–2.5% (MP1−2.5%) and >2.5% (MP>2.5%).
The greatest increase in the amount in the plasmas of malaria patients compared to healthy controls was observed for IL-1RA (HAll: 1.8 ± 6.9 pg/m, MAll: 1296 ± 1378 pg/m; 720-fold increase), followed by CXCL1 (HAll: 3.1 ± 11.7 pg/mL, MAll: 54.4 ± 103.0 pg/mL; 17.5-fold increase), CCL20 (HAll: 3.5 ± 5.0 pg/mL, MAll: 31.8 ± 36.6 pg/mL; 9.1-fold increase) and IL-11 (HAll: 3.2 ± 8.2 pg/mL, MAll: 27.1 ± 41.5 pg/mL; 8.5-fold increase) (Figure 1A, Supplementary Table S3). For CXCL1, there is a correlation between the amount of cytokine detected and the different levels of parasitaemia (p = 0.0013, r = 0.5) (Figure 1B). For none of the other cytokines could a correlation with parasitemia be demonstrated. Interestingly, CXCL5 is the only chemokine that was detected at significantly lower levels in plasma of malaria patients than in plasma of healthy controls (Figure 1A). For VEGF, no significant difference was found between patients with malaria infection and the healthy controls, but four malaria plasma samples showed an increase of the VEGF amount, while all remaining individuals had levels beyond the detection limit of the LEGENDplex assay (Figure 1A). The amounts of IL-1 α, IL-1β, IL-7, IL-12 and IFN-β were also below the detection limit of the LEGENDplex assay.
Thus, for nine of the 16 cytokines examined, a significantly increased amount and for one (CXCL5) a lower amount was found in the plasma of infected individuals compared to healthy controls. Five cytokines were below the detection level of the assay (Figure 1A, Supplementary Table S3).
The amount of TNF-α in the plasmas of malaria patients and healthy controls was determined separately by ELISA. On average, significantly less TNF-α is present in the plasmas of malaria patients compared to controls (HAll: 3770 ± 11,925 pg/mL, MAll: 75 ± 160 pg/mL; however, this was not significant (p = 0.0531) (Figure 2, Supplementary Table S4).
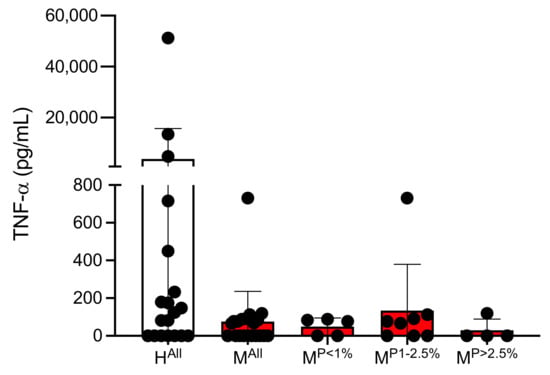
Figure 2.
Amount of TNF-α in plasmas of malaria patients and healthy individuals (Supplementary Table S4). Abbreviations: Healthy controls (HAll); malaria patients (MAll); malaria patients with a parasitaemia < 1% (MP<1%), 1–2.5% (MP1−2.5%) and >2.5% (MP>2.5%).
3.2. Determination of Concentrations of Various Cytokines in the Culture Supernatant of ECs Stimulated with Plasma from Malaria Patients and Healthy Controls
The next step was to investigate whether the plasma of malaria patients and non-infected, healthy individuals have an influence on EC cytokine secretion. It must be mentioned here that, of course, not only cytokines present in plasma, but also various plasmodial antigens and released components, as well as endogenous metabolites, can stimulate ECs. For this purpose, ECs of the brain EC line HBEC-5i were stimulated for six hours with human plasma using a concentration of 25%. Afterwards, the plasma-containing culture supernatant was removed, and the ECs were cultivated in DMEM/F-12 complete growth medium. Cell culture supernatants were collected 48 h after starting the stimulation and the level of cytokines secreted was analysed. Subsequently, the culture medium was removed, and the level of cytokines secreted in the culture supernatant was analysed (Supplementary Table S2). Preliminary studies have shown that significant effects could only be measured 48 h after stimulation.
Stimulation of ECs with plasma from malaria patients resulted in significantly increased levels of IL-11, CXCL5, CXCL8, CXCL10 and VEGF in comparison to stimulation of ECs using plasma from healthy controls (Figure 2). In all cases, the measured difference was significant; but, in contrast to the results from the plasma samples, only a 1.5–2.3-fold increase was detected (IL-11: HAll: 346.4 ± 157.3 pg/mL, MAll: 526.9 ± 219.3 pg/mL, 1.5-fold; CXCL5: HAll: 152.5 ± 128.6 pg/mL, MAll: 263.5 ± 117.8 pg/mL, 1.7-fold; CXCL8: HAll: 7085 ± 7065 pg/mL, MAll: 11,681 ± 6360 pg/mL, 1.6-fold; CXCL10: HAll: 30.4 ± 34.2 pg/mL, MAll: 69.2 ± 53.6 pg/mL, 2.3-fold; VEGF: HAll: 112.1 ± 56.2 pg/mL, MAll: 170.1 ± 59.3 pg/mL, 1.5-fold) (Figure 3, Supplementary Table S3).
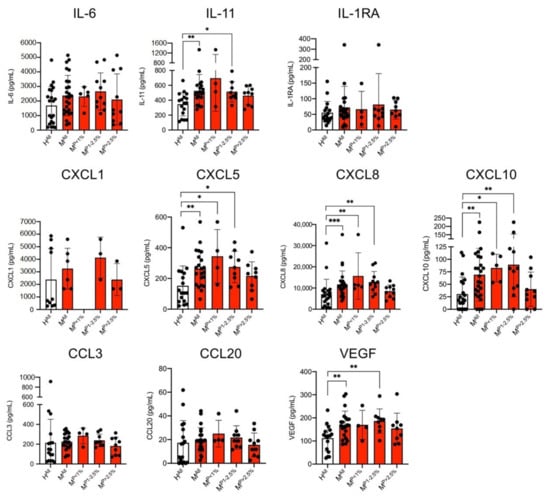
Figure 3.
Level of cytokines in the culture supernatants of endothelial cells (HBEC-5i) stimulated with plasma derived from malaria patients (M) and healthy control individuals (H) were analysed with a bead-based LEGENDplex assay (n = 6–26; Supplementary Tables S2 and S3). Data are expressed as the mean ± SD. Statistical analyses were performed using the Mann–Whitney U test (* p < 0.05; ** p < 0.01; *** p < 0.001). Abbreviations: Healthy controls (HAll); malaria patients (MAll); malaria patients with a parasitaemia < 1% (MP<1%), 1–2.5% (MP1−2.5%) and >2.5% (MP>2.5%).
Again, the amount of IL-1α, IL-1β, IL-7, IL-12 and IFN-β were below the detection limit of the LEGENDplex assay, in this case also of IL-10. In contrast to the CC levels in the plasmas, no differences were detected for the cytokines IL-6, IL-1RA and the chemokines CCL3, CCL20 and CXCL1 in the cell culture supernatants of HBEC-5i cells stimulated with plasma from malaria patients and healthy controls (Figure 3).
If we corrected for multiple testing and included all cytokines with measurable values, most of the reported differences (supernatant and plasma) are still be significant (Figure 1, Figure 3, Supplementary Table S5). For the supernatants, there is no different result with either the Benjamini–Hochberg adjustment or the conservative Bonferroni correction. For plasma, CXCL5 and IL-11 fail the Bonferroni correction, while with the Benjamini–Hochberg adjustment CXCL5 proves significant and IL-11 just misses the cut-off (p = 0.0457, cut-off = 0.0455).
No TNF-α was detected in the supernatants of endothelial cells after stimulation with plasma from the malaria patients or with plasma from the healthy controls, respectively.
3.3. Amount of Secreted Angiopoietin-like Protein 4 (ANGPTL4) in Culture Supernatant of ECs Stimulated with Plasma Derived from Malaria Patients and Healthy Individuals
Studies suggest a synergistic effect of ANGPTL4 and VEGF [46,47]. Therefore, both the plasmas as well as the culture supernatants of the ECs stimulated with plasma were examined for the presence of ANGTPL4 using an ELISA assay. On average, there was less ANGPTL4 in the plasmas of the malaria patients than in the plasmas of the controls (HAll: 656.8 ± 1108.7 ng/mL, MAll: 149.9 ± 93.4 ng/mL); however, this was not significant (Figure 4A). When the ECs were stimulated with the plasmas, the reverse was observed. Stimulation with plasma from malaria patients resulted in an increase in the measured amount of ANGPTL4 in comparison to the controls (HAll: 13.8 ± 3.4 ng/mL, MAll: 16.4 ± 5.6 ng/mL, p = 0.0691). However, the measured amounts were 10–50 times lower than in the plasmas. Considering the different parasitaemia levels separately, only plasma from patients with a parasitaemia of >2.5% has significantly higher levels of ANGPTL4 (20.7 ± 8.5 ng/mL, p = 0.0071) in the supernatant compared to the controls (Figure 4B, Supplementary Table S6).
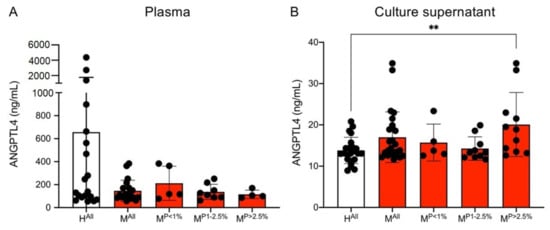
Figure 4.
Amount of ANGPTL4 in plasma (A) and culture supernatants (B) of endothelial cells (HBEC-5i) co-incubated with plasma from malaria patients and healthy individuals. Statistical analyses were performed using the Mann–Whitney U test (** p < 0.01) (Supplementary Table S6). Abbreviations: Healthy controls (HAll); malaria patients (MAll); malaria patients with a parasitaemia < 1% (MP<1%), 1–2.5% (MP1−2.5%) and >2.5% (MP>2.5%).
3.4. Comparative Transcriptome Analyses of ECs Stimulated with Plasma of Malaria Patients and Healthy Individuals
Next, we analysed whether the differences observed on the protein level in the culture supernatant after stimulation of the ECs with plasma of malaria patients could also be found on the RNA level. For this purpose, the HBEC-5i cells were stimulated with the plasma of four malaria patients and of three healthy control individuals for seven hours and subsequently their transcriptomes were analysed. The malaria patients had a parasitaemia between 2.5 and 4% (Supplementary Tables S1, S7 and S8).
After seven hours of stimulation, a significant increase was observed for il1β (p = 0.0042), cxcl1 (p = 0.0029), cxcl5 (p = 0.0059) and angptl4 (p = 0.0002) after stimulation with patient plasma. A tendency was only observed for vegf (p = 0.05). This is due to the measured expression level of the control H8. This deviates significantly from the expression level of the other two controls (expression level: 5820 vs. 860–935). A decrease in expression, albeit non-significant, after stimulation with plasma from malaria patients compared to the controls was observed for il11 expression (p = 0.05), which is in contrast to the LEGENDplex results (Figure 5, Supplementary Table S9). Using qPCR analysis for angptl4 and vegf, the difference in gene expression detected after 7 h could no longer be observed 48 h after stimulation (data not shown).
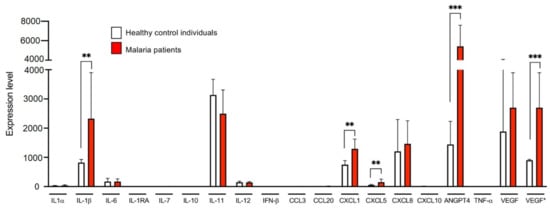
Figure 5.
Expression levels of genes coding for the examined cytokines of endothelial cells (HBEC-5i) stimulated with plasma from three healthy control individual and four malaria patients. HBEC-5i cells were incubated for 7 h in the presence of 10% plasma derived from three control individuals and four malaria patients (Supplementary Tables S1, S3, S7 and S8). Subsequently, RNA was isolated from the HBEC-5i cells and a comparative transcriptome analysis was performed (Supplementary Table S9). VEGF*: Analysis excluding sample H8. M6, M9, M10, and M11: 4 biological replicates each; H5 and H10: 2 biological replicates each; H8: 1 biological replicate. Statistical analyses were performed using the Mann–Whitney U test (** p < 0.01; *** p < 0.001).
Transcriptome analysis also provides an overall view of the changes in EC gene expression after stimulation with patient plasma. For this analysis, genes with a base mean level ≥40, a differential expression with a fold change ≤0.6/≥1.7 and a padj ≤0.05 were included. Only thirteen genes were identified that were expressed between 1.7- and 3-fold higher after stimulation with the plasma of healthy control individuals compared to stimulation with the patient plasma (Supplementary Tables S9 and S10). On the other hand, 43 genes are expressed between 1.7- and 4.5-fold higher in ECs after stimulation with plasma from malaria patients than after stimulation with plasma from healthy controls (Table 1, Supplementary Tables S9 and S11).

Table 1.
Genes differentially higher expressed (≥1.7 fold) in endothelial cells incubated with plasma from malaria patients (M6, M9, M10, and M11) compared to incubation with plasma from healthy controls (H5, H10, and H8).
To identify the biological processes in which the proteins encoded by the identified genes but also the cytokines identified by LEGENDplex and ELISA are involved, a gene set enrichment analyses (GSEA) was performed using g:Profiler analysis [48] (Table 1). The g:Profiler analyses shows that within the gene ontology term biological processes (GO:BP), ‘positive regulation of cell migration’, ‘blood vessel development’ and ‘inflammatory response’ are significantly regulated (padj = 7.602 × 10−5, 2.542 × 10−4 and 2.474 × 10−4, respectively). The KEGG pathways ‘rheumatoid arthritis (padj = 4.499 × 10−4) and ‘IL-17 signaling pathway’ (padj = 3.436 × 10−4) also were found to be upregulated (Table 1). To identify protein–protein interaction networks, a Markov Clustering (MCL) analyses was performed using the program STRING, version 11.0 [49,50]. This analysis yields four clusters, the largest comprising 20 proteins, which are involved in ‘positive regulation of cell population proliferation’ (padj 4.312 × 10−8) and ‘tube development’ (padj 1.948 × 10−5). The cluster ‘cholesterol metabolic process’ (padj 3.1 × 10−6) contains four proteins and the cluster ‘negative regulation of cell differentiation’ (padj 3.4 × 10−2) contains three proteins. In addition, an unassigned cluster (three proteins) was predicted (Figure 6, Table 1).
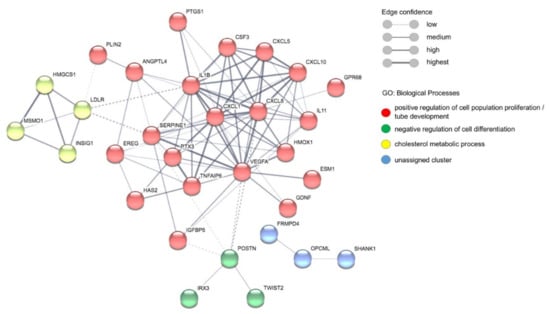
Figure 6.
Detection of protein–protein networks through Markov Clustering (MCL) using STRING: functionalprotein association networks [49,50]. Proteins that have not been assigned to a network are not included in the figure. The proteins with a red circle can be assigned to ‘positive regulation of cell population proliferation’ and ‘tube development’, proteins with a green circle can be assigned to ‘negative regulation of cell differentiation’, and proteins with a yellow circle can be assigned to ‘cholesterol metabolic process’ within the gene ontology terms biological processes (GO:BP). Proteins with a blue circle belong to an unassigned cluster.
4. Discussion
Previously, more than 30 cytokines have been identified whose production is increased due to P. falciparum infection and that, as a result, can be detected in higher amounts in plasma of malaria patients compared to healthy controls. For some of them, such as CXCL8 and CXCL10, a correlation with severity of the disease was observed [7,25,30,36,37,51]. Classical immune cells, such as macrophages/monocytes and dendritic cells, are well known for their cytokine production in malaria [22,23,52]. The role of ECs in this context is only fragmentarily understood although they are in constant contact with circulating cytokines and among the first to detect pathogens and they express receptors for pathogen and cytokine recognition (for a review, see [53]). They are the interface between the circulatory system and surrounding tissue, regulating the diapedesis of immune cells (for a review, see [54]) and transporting cytokines from the tissue to the circulatory system [55,56]. Furthermore, they were recently shown to internalize IEs, possibly leading to blood–brain barrier (BBB) breakdown [57]. However, they are also active players in innate and adaptive immune response (for a review, see [54]) and capable of cytokine secretion themselves [58,59]. It is well studied that cytoadhesion of IEs in the capillaries of various organs not only causes blockage of blood flow, which can lead to organ hypoxia and thus organ failure, but also activates ECs. This leads to increased cytokine production, which can induce endothelial dysfunction and thereby contribute to pathogenesis of CM [5,6,7]. An increase in gene expression induced by cytoadhesion has been demonstrated for a number of cytokine-encoding genes [17,18,19]. However, it is not only cytoadhesion of IEs that leads to an increase in cytokine production. This could also be demonstrated for Plasmodium antigens. It was shown that hemozoin leads to an increased secretion of CXCL8 and CCL5 from the endothelium [60]. Similarly, isolated P. falciparum histones stimulate the production of CXCL8 [61].
There is general agreement that EC activation is important in the pathogenesis of complicated forms of malaria. However, different approaches to identify the underlying mechanisms in EC activation by P. falciparum or its metabolites have so far not been able to reach a unified conclusion [62]. One explanation for the divergent results could lie in the tissue-specific variations of the endothelia, which cause different patterns of immune response [63]. However, one should also keep in mind that the immortalised cell lines used, but also the primary endothelial cells, can show different reaction types. In view of the variability in protein expression and chemokine secretion, it is of utmost importance to determine the response of ECs from human brain microvasculature to P. falciparum infection if new approaches in the treatment of cerebral malaria are to be pursued.
The aim of the study presented here was to investigate the immunostimulatory potential of plasma samples drawn from P. falciparum-infected patients on ECs in absence of adhering IEs. All 27 malaria patients included in the study were adults with symptomatic malaria. However, the severity of infection according to the WHO criteria could only be determined in a subgroup of the samples included in our study (n = 17) [64]; for the rest (n = 10), the clinical manifestation is unknown. Within these 17 patients, only three patients could be assigned to severe malaria. As a subgroup of three severe malaria patients is too small for statistical analyses with the corresponding corrections for multiple comparisons, it was decided not to distinguish between clinical manifestations in this study.
In plasmas of the 27 travel returnees infected with P. falciparum examined in this study, a LEGENDplex assay detected significantly higher concentrations for 9 of the 16 cytokines analysed compared to the corresponding healthy controls, namely, IL-6, IL-1RA, IL-10, IL-11, CCL3, CCL20, CXCL1, CXCL8 and CXCL10. Interestingly, the concentration of CXCL5 in the plasma of malaria patients was below the detected levels in controls. This is consistent with the observation made by a study conducted in Cameroon, which shows decreased serum levels of CXCL5 in P. falciparum-infected individuals. The reason for the lower amount of CXCL5 in plasma of malaria patients is not yet clear [35]. A similar observation was made for TNF-a. Again, there was a tendency for greater amounts to be present in the plasmas of the healthy controls compared to the malaria patients, which was nevertheless not significant.
The dysfunction of ECs and the associated development of vascular damage in the brain, resulting in impairment of the BBB, is one of the consequences of a malaria infection. Oggungwan and colleagues demonstrated that sera from malaria patients are able to increase cell permeability in vitro [65]. Increased endothelial permeability associated with malaria has also been shown elsewhere [66,67,68,69]. One trigger of endothelial dysfunction may be stimulation by various cytokines. They could be circulating in the blood stream or produced within the surrounding tissues or by the ECs themselves, acting in an autocrine or paracrine manner. ECs can produce pro-inflammatory and anti-inflammatory cytokines and chemokines as well as growth factors in response to various stimuli, including IL-1α, IL-1β, IL-3, IL-5, IL-6, IL-10, IL-11, CXCL8, CXCL10, IL-11 and VEGF ([70]; for a review, see [71]).
After stimulation of ECs with plasma from malaria patients, we could measure an increased amount in the culture supernatants for IL-11, CXCL5, CXCL8, CXCL10, VEGF and ANGPTL4 (only if plasma of malaria patients with a parasitaemia >2.5% were used) compared to stimulation with plasma from healthy individuals. For all other cytokines examined, the prevalence of a malaria infection in the plasma donor led to no significant differences in cytokine secretion by ECs stimulated with the plasma. This is also the case for TNF-α, which was not detectable in the supernatants of endothelial cells stimulated with both plasma from the control and malaria patients. Furthermore, no expression of the TNF-α coding gene could be detected. This is in contrast to the described increased expression of TNF-α after direct interaction of IEs with endothelial cells [17,18,19].
However, it must be emphasized here that the stimulation experiments were carried out with the immortalised brain endothelial cell line HBEC-5i. Although this exhibits essential features of cerebral ECs, there are also deviations. EC proteins, such as CD51, ICAM-1 and VCAM-1, are presented on the surface, while others, such as CD31, CD36 and CD62E, are absent [72]. In addition, HBEC-5i cells carry chondroitin sulfate A (CSA) as a dominant molecule on its surface [73]. Nevertheless, HBEC-5i exhibits essential features of cerebral EC, including tight junction structures in particular [72].
CXCL8 binds to CXCR1 and CXCR2, the most important receptor for chemotaxis and mostly expressed on neutrophils. In models of ischemic brain injury, blockage of CXCL8 shows neuroprotective effects and leads to a reduction in infarct volume. In traumatic brain injury, elevated CXCL8 levels in cerebrospinal fluids are connected to BBB damage and increased mortality (for a review, see [74]). Additionally, CXCL8 is involved in angiogenesis. It has been shown that recombinant human CXCL8 can induce EC proliferation and is also involved in capillary tube organization [75,76].
As mentioned above, stimulation with plasma from malaria patients resulted in a significantly increased concentration of CXCL8 in the culture supernatant of ECs. Thus, it can be postulated that plasmodial antigens present in plasma might stimulate this secretion, which has also been described in other studies [60,61].
CXCL5, like CXCL8, is important for neutrophil recruitment and activation. The importance of CXCL5 in malaria pathology is unknown. However, CXCL5 has been described to play a role in ischemia–reperfusion-induced injury in human brain microvascular ECs associated with BBB disruption. CXCL5 has been shown to be upregulated in ischemic stroke and this correlates positively with brain injury. In addition, CXCL5 appears to interfere with brain EC function by regulating the p38 MAP kinase signalling pathway [77]. CXCL5-induced impairment of brain endothelial barrier function has also been demonstrated in other contexts [78]. In rats, pretreatment of ECs with IL-10 inhibited CXCL5-mediated cytokine gene transcription [79]. This is consistent with IL-10 functioning as a crucial anti-inflammatory and protective cytokine in experimental cerebral malaria [80,81]. Elevated plasma concentrations of IL-10 are detected in both mild and cerebral malaria, which is compatible with our findings, but for non-survivors of cerebral malaria a decrease in IL-10 levels was shown [27,82]. An inverted ratio in cytokine concentration between the malaria and control group in plasma and supernatant, as observed in CXCL5 and ANGPTL4, does not constitute a contradiction. Instead, it highlights the need to assess cytokine profiles at the cellular level, if aiming to understand the complex interactions taking place in CM. Brain swelling due to the disruption of the BBB and (cytokine-containing) fluid influx was found to occur in 84% of children dying due to cerebral malaria, but only in 27% of the survivors [83]. However, no correlation between peripheral blood cytokine concentrations and the occurrence of brain swelling in these children could be detected, implying a more local event [84]. Cytokine concentrations measured in peripheral blood represent only the systemic effects and are affected by receptor binding, degradation and excretion. The crucial site of cytokine impact is the cell-surrounding micromilieu [85]. HBECs can secrete cytokines in an apical or basolateral direction (for review [86]). Apically released cytokines would be diluted in the circulating blood, creating a locally acting gradient.
VEGF is a key regulator of physiological angiogenesis. VEGF (i) can promote proliferation and migration of ECs; (ii) serve as a survival factor for ECs; and (iii) is known as a vascular permeability factor, based on its ability to induce vascular leakage [87,88,89,90,91]. VEGF is known to bind to vascular endothelial growth factor receptor 1 (VEGFR-1) (Flt-1) and vascular endothelial growth factor receptor 2 (VEGFR-2) (KDR/FlK-1) on ECs, resulting in a mitogen-activated protein kinase (MAPK) signalling cascade [92]. VEGF seems to play a particularly important role in the repair of brain tissue and wound healing ([93], for a review, see [94]). Increased levels of VEGF can be detected in malaria patients and an increased expression of VEGF was also observed in astrocytes of patients who died of CM [90,95]. However, the role that VEGF plays in CM in particular is still not clear. There is evidence of both a protective and a pathogenic influence for VEGF in the pathology in CM (for a review, see [94]). In our study, VEGF could not be detected in any of the 14 samples examined from the healthy individuals and in the malaria patients VEGF could only be detected in four of the 26 plasma samples analysed. This result contrasts with the findings of Furuta and colleagues mentioned above, where elevated VEGF levels were found in malaria patients compared to patients with febrile illnesses or healthy adults [90]. However, Armah and colleagues also found no difference in the VEGF levels between Ghanaian children with CM, severe malaria or not infected with Plasmodium [25]. One explanation for these divergent results in malaria research in general might lie within genetic differences. P. falciparum is the strongest known force of evolutionary selection in the recent history of humankind. Diverse adaptions led to differences in resistance, reactions and susceptibility to plasmodial infections between ethnic groups and individuals (for a review, see [96]). A different picture emerges for the amount of secreted VEGF in the culture supernatants of plasma-stimulated ECs. Here, we could detect significantly (p = 0.0044) higher concentrations in the supernatants of ECs stimulated with plasma from malaria patients compared to controls. In vitro studies also show that parasite antigens (crude extract of IEs) can induce VEGF secretion from, in this case, human mast cell lines [90].
An interplay between VEGF and ANGPTL4 has been described in different diseases, such as obesity and diabetic macular oedema [46,47]. As mentioned above, significantly lower amounts of ANGPTL4 can be detected in the plasma of malaria patients compared to the plasma of healthy individuals. This picture is reversed, however, if one considers the amounts of ANPTL4 in the culture supernatants of ECs stimulated with plasma. Here, just as for VEGF, significantly higher concentrations can be detected in the culture supernatants after simulation with plasma from the malaria patients (with a parasitaemia >2.5%) compared to plasma from the healthy individuals. Both VEGF and ANGPTL4 are proangiogenic molecules. Besides angiogenesis, ANGPTL4 is involved in several other processes, such as lipid metabolism, wound healing, inflammation, and redox regulation (for a review, see [97]). For ANGPTL4, but also VEGF, it has been shown that expression is also strongly increased by hypoxia, thereby leading to induction of angiogenesis [98,99,100].
CXCL10, like VEGF and ANGPTL4, is present in significantly higher concentrations in culture supernatants of ECs stimulated with plasma from malaria patients compared to plasma from healthy individuals. While VEGF and ANGPTL4 have angiogenic and proliferative effects, CXCL10 has angiostatic and anti-proliferative effects [101,102,103]. The important role of CXCL10 is illustrated in a study by Wilson and colleagues. Here, significantly elevated levels of CXCL10 and CXCL4 were found in patients who had died from CM compared to patients who had survived CM or patients with mild malaria [29]. CXCL10 produced by endothelial cells was shown to play a key role in inducing firm adhesion of T cells and preventing cell detachment from the brain vasculature. The induction of CXCL10 was completely dependent on IFN-γ receptor signalling and played a crucial role in mediating the T-cell–endothelial cell adhesion events that initiate the inflammatory processes that damage the endothelium and promote the development of CM [104]. Bodnar and colleagues showed that incubation of ECs with CXCL10 also significantly reduced tube formation [105].
That the angiogenesis of ECs is strongly influenced by the plasma of malaria patients also becomes clear when looking at the differential gene expression after stimulation of ECs with plasma from malaria patients in comparison to healthy individuals (Table 1). In particular, GO terms such as ‘positive regulation of cell migration’, ‘blood vessel/tube development’, ‘negative regulation of cell differentiation’ and ‘inflammatory response’ were significantly upregulated in ECs stimulated with patients’ plasma in comparison to the controls. Based on these results, it can be postulated that there must be a very delicate balance between these molecules to stimulate proliferation of ECs on the one hand and to limit angiogenesis as well as endothelial dysfunction.
5. Conclusions
Our results clearly show that not only cytoadhesion of IEs can lead to stimulation of ECs, inducing the production of various cytokines, but also the plasma of malaria patients, specifically, the parasite and host molecules contained therein, which trigger these processes and thus cause a different cytokine profile than the plasma of healthy controls. IL-11, CXCL5, CXCL8, CXCL10, VEGF and ANGPTL4 have been secreted in significantly higher amounts. This is consistent with the pre-existing finding that plasma from malaria patients impairs endothelial barrier integrity in human umbilical vein ECs [65]. We were able to demonstrate the activation of ECs derived from the microvasculature of the human brain and specify their response. However, we did not identify the plasma factors responsible for this effect and thus cannot say whether they are of parasitic or host-specific origin.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cells10071656/s1. Table S1—List of plasmas examined, indicating the donor’s parasitaemia; Table S2—Number of plasmas analysed from malaria patients and healthy individuals and number of culture supernatants analysed from HBEC-5i cells stimulated with individual plasma samples from malaria patients and healthy individuals. Table S3—Levels of various cytokines determined using a LEGENDplex assay in plasma from malaria patients and control individuals and in culture supernatant of endothelial cells (HBEC-5i) stimulated with these plasmas. Table S4—Levels of TNF-α in plasma from malaria patients and control individuals. Table S5—Adjustment for multiple comparison (cut-offs that are met for the corresponding analyte are shown in bolt). Table S6—Levels of ANGPTL4 in plasma from malaria patients and control individuals and in culture supernatant of endothelial cells (HEBEC-5i) stimulated with these plasmas. Table S7—Levels of cytokines in the plasma of three control individuals (H5, H8, H10) and of four malaria patients (M6, M9, M10, M11), which were used to stimulated endothelial cells (HBEC-5i) for transcriptome analysis. Table S8—Levels of cytokines in the culture supernatant of endothelial cells (HBEC-5i), stimulated with plasma of three control individuals (H5, H8, H10) and of four malaria patients (M6, M9, M10, M11). Table S9—Transcriptome analyses of endothelial cells (HBEC-5i) stimulated with plasma from three healthy control individuals (H5, H10, H8) and from four malaria patients (M6, M9, M10, M11). Table S10—Genes whose expression is significantly decreased after co-incubation of endothelial cells (HBEC-5i) with plasma from malaria patients (M) compared to the healthy controls (H). Table S11—Genes whose expression is significantly increased after co-incubation of endothelial cells (HBEC-5i) with plasma from malaria patients (M) compared to the healthy controls (H).
Author Contributions
Conceptualization, M.R., M.D. and I.B.; methodology, M.R., A.K., M.D., C.F. and T.J.; software, S.L. and I.B.; validation, M.R. and I.B.; formal analysis, M.R., A.K. and I.B.; investigation, M.R., A.K., M.D., J.B., Y.W. and C.F.; writing—original draft preparation, M.R. and I.B.; writing—review and editing, M.R., J.S., T.J., A.B., T.R., N.G.M. and I.B.; supervision, I.B., funding acquisition, M.D. and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jürgen Manchot Stiftung (M.D.), German Center for Infec tion Research (DZIF) (M.R.), Leibniz Center Infection (J.B.) and Chinese Scholarship Council (Y.W.). The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the relevant ethics committee: Ethical Review Board of the Medical Association of Hamburg, Germany; reference numbers PV3828 and PV4539.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within this article and corresponding supplementary material.
Acknowledgments
We thank Ulricke Richardt and Susann Ofori for excellent technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. World Malaria Report 2020; WHO Team, Global Malaria Programme: Geneva, Switzerland, 2020; ISBN 978-92-4-001579-1. [Google Scholar]
- Milner, D.A., Jr.; Lee, J.J.; Frantzreb, C.; Whitten, R.O.; Kamiza, S.; Carr, R.A.; Pradham, A.; Factor, R.E.; Playforth, K.; Liomba, G.; et al. Quantitative assessment of multiorgan sequestration of parasites in fatal pediatric cerebral malaria. J. Infect. Dis. 2015, 212, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Milner, D., Jr.; Factor, R.; Whitten, R.; Carr, R.A.; Kamiza, S.; Pinkus, G.; Molyneux, M.; Taylor, T. Pulmonary pathology in pediatric cerebral malaria. Hum. Pathol. 2013, 44, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.E.; Fu, W.J.; Carr, R.A.; Whitten, R.O.; Mueller, J.S.; Fosiko, N.G.; Lewallen, S.; Liomba, N.G.; Molyneux, M.E. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Lyke, K.E.; Burges, R.; Cissoko, Y.; Sangare, L.; Dao, M.; Diarra, I.; Kone, A.; Harley, R.; Plowe, C.V.; Doumbo, O.K.; et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 2004, 72, 5630–5637. [Google Scholar] [CrossRef]
- Nishanth, G.; Schluter, D. Blood-brain barrier in cerebral malaria: Pathogenesis and therapeutic intervention. Trends Parasitol. 2019, 35, 516–528. [Google Scholar] [CrossRef]
- Dunst, J.; Kamena, F.; Matuschewski, K. Cytokines and chemokines in cerebral malaria pathogenesis. Front. Cell. Infect. Microbiol. 2017, 7, 324. [Google Scholar] [CrossRef]
- Hasday, J.D.; Bannerman, D.; Sakarya, S.; Cross, A.S.; Singh, I.S.; Howard, D.; Drysdale, B.E.; Goldblum, S.E. Exposure to febrile temperature modifies endothelial cell response to tumor necrosis factor-alpha. J. Appl. Physiol. 2001, 90, 90–98. [Google Scholar] [CrossRef]
- Oakley, M.S.; Gerald, N.; McCutchan, T.F.; Aravind, L.; Kumar, S. Clinical and molecular aspects of malaria fever. Trends Parasitol. 2011, 27, 442–449. [Google Scholar] [CrossRef]
- Oakley, M.S.; Kumar, S.; Anantharaman, V.; Zheng, H.; Mahajan, B.; Haynes, J.D.; Moch, J.K.; Fairhurst, R.; McCutchan, T.F.; Aravind, L. Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites. Infect. Immun. 2007, 75, 2012–2025. [Google Scholar] [CrossRef]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef]
- Cunnington, A.J.; Riley, E.M.; Walther, M. Microvascular dysfunction in severe Plasmodium falciparum malaria. J. Infect. Dis. 2013, 207, 369–370. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Kalantari, P.; Fitzgerald, K.A.; Golenbock, D.T. Innate sensing of malaria parasites. Nat. Rev. Immunol. 2014, 14, 744–757. [Google Scholar] [CrossRef]
- Boivin, M.J.; Bangirana, P.; Byarugaba, J.; Opoka, R.O.; Idro, R.; Jurek, A.M.; John, C.C. Cognitive impairment after cerebral malaria in children: A prospective study. Pediatrics 2007, 119, e360–e366. [Google Scholar] [CrossRef]
- Idro, R.; Marsh, K.; John, C.C.; Newton, C.R. Cerebral malaria: Mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatric Res. 2010, 68, 267–274. [Google Scholar] [CrossRef]
- Roze, E.; Thiebaut, M.M.; Mazevet, D.; Bricaire, F.; Danis, M.; Deseilligny, C.P.; Caumes, E. Neurologic sequelae after severe falciparum malaria in adult travelers. Eur. Neurol. 2001, 46, 192–197. [Google Scholar] [CrossRef]
- Chakravorty, S.J.; Carret, C.; Nash, G.B.; Ivens, A.; Szestak, T.; Craig, A.G. Altered phenotype and gene transcription in endothelial cells, induced by Plasmodium falciparum-infected red blood cells: Pathogenic or protective? Int. J. Parasitol. 2007, 37, 975–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tripathi, A.K.; Sha, W.; Shulaev, V.; Stins, M.F.; Sullivan, D.J., Jr. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood 2009, 114, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Viebig, N.K.; Wulbrand, U.; Forster, R.; Andrews, K.T.; Lanzer, M.; Knolle, P.A. Direct activation of human endothelial cells by Plasmodium falciparum-infected erythrocytes. Infect. Immun. 2005, 73, 3271–3277. [Google Scholar] [CrossRef]
- Schofield, L.; Hackett, F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J. Exp. Med. 1993, 177, 145–153. [Google Scholar] [CrossRef]
- Zhu, J.; Krishnegowda, G.; Gowda, D.C. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: The requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J. Biol. Chem. 2005, 280, 8617–8627. [Google Scholar] [CrossRef] [PubMed]
- Giribaldi, G.; Prato, M.; Ulliers, D.; Gallo, V.; Schwarzer, E.; Akide-Ndunge, O.B.; Valente, E.; Saviozzi, S.; Calogero, R.A.; Arese, P. Involvement of inflammatory chemokines in survival of human monocytes fed with malarial pigment. Infect. Immun. 2010, 78, 4912–4921. [Google Scholar] [CrossRef]
- Jaramillo, M.; Godbout, M.; Olivier, M. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J. Immunol. 2005, 174, 475–484. [Google Scholar] [CrossRef]
- Schwarzer, E.; Bellomo, G.; Giribaldi, G.; Ulliers, D.; Arese, P. Phagocytosis of malarial pigment haemozoin by human monocytes: A confocal microscopy study. Parasitology 2001, 123, 125–131. [Google Scholar] [CrossRef]
- Armah, H.B.; Wilson, N.O.; Sarfo, B.Y.; Powell, M.D.; Bond, V.C.; Anderson, W.; Adjei, A.A.; Gyasi, R.K.; Tettey, Y.; Wiredu, E.K.; et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar. J. 2007, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Dieye, Y.; Mbengue, B.; Dagamajalu, S.; Fall, M.M.; Loke, M.F.; Nguer, C.M.; Thiam, A.; Vadivelu, J.; Dieye, A. Cytokine response during non-cerebral and cerebral malaria: Evidence of a failure to control inflammation as a cause of death in African adults. PeerJ 2016, 4, e1965. [Google Scholar] [CrossRef]
- Jain, V.; Armah, H.B.; Tongren, J.E.; Ned, R.M.; Wilson, N.O.; Crawford, S.; Joel, P.K.; Singh, M.P.; Nagpal, A.C.; Dash, A.P.; et al. Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar. J. 2008, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Herbert, F.; Tchitchek, N.; Bansal, D.; Jacques, J.; Pathak, S.; Becavin, C.; Fesel, C.; Dalko, E.; Cazenave, P.A.; Preda, C.; et al. Evidence of IL-17, IP-10, and IL-10 involvement in multiple-organ dysfunction and IL-17 pathway in acute renal failure associated to Plasmodium falciparum malaria. J. Transl. Med. 2015, 13, 369. [Google Scholar] [CrossRef]
- Wilson, N.O.; Jain, V.; Roberts, C.E.; Lucchi, N.; Joel, P.K.; Singh, M.P.; Nagpal, A.C.; Dash, A.P.; Udhayakumar, V.; Singh, N.; et al. CXCL4 and CXCL10 predict risk of fatal cerebral malaria. Dis. Markers 2011, 30, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Patel, S.; Gonca, M.; David, C.; Otterdal, K.; Ueland, T.; Dalen, I.; Kvaloy, J.T.; Mollnes, T.E.; Aukrust, P.; et al. Cytokine network in adults with falciparum malaria and HIV-1: Increased IL-8 and IP-10 levels are associated with disease severity. PLoS ONE 2014, 9, e114480. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Fesel, C.; Jain, R.; Cazenave, P.A.; Mishra, G.C.; Pied, S. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J. Infect. Dis. 2006, 194, 198–207. [Google Scholar] [CrossRef]
- Colborn, J.M.; Ylostalo, J.H.; Koita, O.A.; Cisse, O.H.; Krogstad, D.J. Human gene expression in uncomplicated Plasmodium falciparum malaria. J. Immunol. Res. 2015, 2015, 162639. [Google Scholar] [CrossRef]
- Bwanika, R.; Kato, C.D.; Welishe, J.; Mwandah, D.C. Cytokine profiles among patients co-infected with Plasmodium falciparum malaria and soil borne helminths attending Kampala International University Teaching Hospital, in Uganda. Allergy Asthma Clin. Immunol. 2018, 14, 10. [Google Scholar] [CrossRef]
- Thuma, P.E.; van Dijk, J.; Bucala, R.; Debebe, Z.; Nekhai, S.; Kuddo, T.; Nouraie, M.; Weiss, G.; Gordeuk, V.R. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J. Infect. Dis. 2011, 203, 211–219. [Google Scholar] [CrossRef]
- Che, J.N.; Nmorsi, O.P.; Nkot, B.P.; Isaac, C.; Okonkwo, B.C. Chemokines responses to Plasmodium falciparum malaria and co-infections among rural Cameroonians. Parasitol. Int. 2015, 64, 139–144. [Google Scholar] [CrossRef]
- Ayimba, E.; Hegewald, J.; Segbena, A.Y.; Gantin, R.G.; Lechner, C.J.; Agosssou, A.; Banla, M.; Soboslay, P.T. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin. Exp. Immunol. 2011, 166, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Burgmann, H.; Hollenstein, U.; Wenisch, C.; Thalhammer, F.; Looareesuwan, S.; Graninger, W. Serum concentrations of MIP-1 alpha and interleukin-8 in patients suffering from acute Plasmodium falciparum malaria. Clin. Immunol. Immunopathol. 1995, 76, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Otterdal, K.; Berg, A.; Michelsen, A.E.; Patel, S.; Gregersen, I.; Sagen, E.L.; Halvorsen, B.; Yndestad, A.; Ueland, T.; Langeland, N.; et al. Plasma levels of interleukin 27 in falciparum malaria is increased independently of co-infection with HIV: Potential immune-regulatory role during malaria. BMC Infect. Dis. 2020, 20, 65. [Google Scholar] [CrossRef]
- John, C.C.; Panoskaltsis-Mortari, A.; Opoka, R.O.; Park, G.S.; Orchard, P.J.; Jurek, A.M.; Idro, R.; Byarugaba, J.; Boivin, M.J. Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am. J. Trop. Med. Hyg. 2008, 78, 198–205. [Google Scholar] [CrossRef] [PubMed]
- John, C.C.; Park, G.S.; Sam-Agudu, N.; Opoka, R.O.; Boivin, M.J. Elevated serum levels of IL-1ra in children with Plasmodium falciparum malaria are associated with increased severity of disease. Cytokine 2008, 41, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, A.; Ma, T.; Menon, D.; Deshpande, M.; Jee, K.; Dinabandhu, A.; Vancel, J.; Lu, D.; Montaner, S. Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema. J. Clin. Investig. 2019, 129, 4593–4608. [Google Scholar] [CrossRef]
- Gealekman, O.; Burkart, A.; Chouinard, M.; Nicoloro, S.M.; Straubhaar, J.; Corvera, S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1056–E1064. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Snel, B.; Lehmann, G.; Bork, P.; Huynen, M.A. STRING: A web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000, 28, 3442–3444. [Google Scholar] [CrossRef]
- Ioannidis, L.J.; Nie, C.Q.; Hansen, D.S. The role of chemokines in severe malaria: More than meets the eye. Parasitology 2014, 141, 602–613. [Google Scholar] [CrossRef]
- Wu, X.; Gowda, N.M.; Kumar, S.; Gowda, D.C. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J. Immunol. 2010, 184, 4338–4348. [Google Scholar] [CrossRef]
- Opitz, B.; Hippenstiel, S.; Eitel, J.; Suttorp, N. Extra- and intracellular innate immune recognition in endothelial cells. Thromb. Haemost. 2007, 98, 319–326. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Middleton, J.; Patterson, A.M.; Gardner, L.; Schmutz, C.; Ashton, B.A. Leukocyte extravasation: Chemokine transport and presentation by the endothelium. Blood 2002, 100, 3853–3860. [Google Scholar] [CrossRef]
- Mordelet, E.; Davies, H.A.; Hillyer, P.; Romero, I.A.; Male, D. Chemokine transport across human vascular endothelial cells. Endothelium 2007, 14, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Adams, Y.; Olsen, R.W.; Bengtsson, A.; Dalgaard, N.; Zdioruk, M.; Satpathi, S.; Behera, P.K.; Sahu, P.K.; Lawler, S.E.; Qvortrup, K.; et al. Plasmodium falciparum erythrocyte membrane protein 1 variants induce cell swelling and disrupt the blood-brain barrier in cerebral malaria. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Nilsen, E.M.; Johansen, F.E.; Jahnsen, F.L.; Lundin, K.E.; Scholz, T.; Brandtzaeg, P.; Haraldsen, G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut 1998, 42, 635–642. [Google Scholar] [CrossRef]
- Briones, M.A.; Phillips, D.J.; Renshaw, M.A.; Hooper, W.C. Expression of chemokine by human coronary-artery and umbilical-vein endothelial cells and its regulation by inflammatory cytokines. Coron. Artery Dis. 2001, 12, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Basilico, N.; Corbett, Y.; D’Alessandro, S.; Parapini, S.; Prato, M.; Girelli, D.; Misiano, P.; Olliaro, P.; Taramelli, D. Malaria pigment stimulates chemokine production by human microvascular endothelium. Acta Trop. 2017, 172, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Gillrie, M.R.; Lee, K.; Gowda, D.C.; Davis, S.P.; Monestier, M.; Cui, L.; Hien, T.T.; Day, N.P.J.; Ho, M. Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction. Am. J. Pathol. 2012, 180, 1028–1039. [Google Scholar] [CrossRef]
- Chakravorty, S.J.; Hughes, K.R.; Craig, A.G. Host response to cytoadherence in Plasmodium falciparum. Biochem. Soc. Trans. 2008, 36, 221–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hillyer, P.; Mordelet, E.; Flynn, G.; Male, D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: Discriminating the tissue-specific functions that affect leucocyte migration. Clin. Exp. Immunol. 2003, 134, 431–441. [Google Scholar] [CrossRef]
- Wichers, J.S.; Tonkin-Hill, G.; Thye, T.; Krumkamp, R.; Kreuels, B.; Strauss, J.; von Thien, H.; Scholz, J.A.; Smedegaard Hansson, H.; Weisel Jensen, R.; et al. Common virulence gene expression in adult first-time infected malaria patients and severe cases. Elife 2021, 10. [Google Scholar] [CrossRef]
- Oggungwan, K.; Glaharn, S.; Ampawong, S.; Krudsood, S.; Viriyavejakul, P. FTY720 restores endothelial cell permeability induced by malaria sera. Sci. Rep. 2018, 8, 10959. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Sullivan, D.J.; Stins, M.F. Plasmodium falciparum-infected erythrocytes increase intercellular adhesion molecule 1 expression on brain endothelium through NF-kappaB. Infect. Immun. 2006, 74, 3262–3270. [Google Scholar] [CrossRef]
- Mohan, A.; Sharma, S.K.; Bollineni, S. Acute lung injury and acute respiratory distress syndrome in malaria. J. Vector Borne Dis. 2008, 45, 179–193. [Google Scholar] [PubMed]
- Pal, P.; Daniels, B.P.; Oskman, A.; Diamond, M.S.; Klein, R.S.; Goldberg, D.E. Plasmodium falciparum histidine-rich protein II compromises brain endothelial barriers and may promote cerebral malaria pathogenesis. mBio 2016, 7. [Google Scholar] [CrossRef]
- Gallego-Delgado, J.; Basu-Roy, U.; Ty, M.; Alique, M.; Fernandez-Arias, C.; Movila, A.; Gomes, P.; Weinstock, A.; Xu, W.; Edagha, I.; et al. Angiotensin receptors and beta-catenin regulate brain endothelial integrity in malaria. J. Clin. Investig. 2016, 126, 4016–4029. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Unkeless, J.C.; Ravetch, J.V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 1985, 315, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Virtue, A.; Shen, J.; Wang, H.; Yang, X.F. An evolving new paradigm: Endothelial cells—Conditional innate immune cells. J. Hematol. Oncol. 2013, 6, 61. [Google Scholar] [CrossRef]
- Wassmer, S.C.; Combes, V.; Candal, F.J.; Juhan-Vague, I.; Grau, G.E. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect. Immun. 2006, 74, 645–653. [Google Scholar] [CrossRef]
- Dorpinghaus, M.; Furstenwerth, F.; Roth, L.K.; Bouws, P.; Rakotonirinalalao, M.; Jordan, V.; Sauer, M.; Rehn, T.; Pansegrau, E.; Hohn, K.; et al. Stringent selection of knobby Plasmodium falciparum-infected erythrocytes during cytoadhesion at febrile temperature. Microorganisms 2020, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Kossmann, T.; Morganti-Kossmann, M.C. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010, 30, 459–473. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Heidemann, J.; Ogawa, H.; Dwinell, M.B.; Rafiee, P.; Maaser, C.; Gockel, H.R.; Otterson, M.F.; Ota, D.M.; Lugering, N.; Domschke, W.; et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J. Biol. Chem. 2003, 278, 8508–8515. [Google Scholar] [CrossRef]
- Yu, M.; Ma, X.; Jiang, D.; Wang, L.; Zhan, Q.; Zhao, J. CXC chemokine ligand 5 (CXCL5) disrupted the permeability of human brain microvascular endothelial cells via regulating p38 signal. Microbiol. Immunol. 2021, 65, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, A.; Schuhmann, M.K.; Silwedel, C.; Monoranu, C.M.; Stoll, G.; Buttmann, M. Human brain endothelial CXCR2 is inflammation-inducible and mediates CXCL5- and CXCL8-triggered paraendothelial barrier breakdown. Int. J. Mol. Sci. 2019, 20, 602. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Melby, P.C.; Sarau, H.M.; Raveendran, M.; Perla, R.P.; Marelli-Berg, F.M.; Dulin, N.O.; Singh, I.S. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway. J. Biol. Chem. 2003, 278, 4675–4686. [Google Scholar] [CrossRef]
- Kossodo, S.; Monso, C.; Juillard, P.; Velu, T.; Goldman, M.; Grau, G.E. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 1997, 91, 536–540. [Google Scholar] [CrossRef]
- Sanni, L.A.; Jarra, W.; Li, C.; Langhorne, J. Cerebral edema and cerebral hemorrhages in interleukin-10-deficient mice infected with Plasmodium chabaudi. Infect. Immun. 2004, 72, 3054–3058. [Google Scholar] [CrossRef] [PubMed]
- Mandala, W.L.; Msefula, C.L.; Gondwe, E.N.; Drayson, M.T.; Molyneux, M.E.; MacLennan, C.A. Cytokine profiles in Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef]
- Seydel, K.B.; Kampondeni, S.D.; Valim, C.; Potchen, M.J.; Milner, D.A.; Muwalo, F.W.; Birbeck, G.L.; Bradley, W.G.; Fox, L.L.; Glover, S.J.; et al. Brain swelling and death in children with cerebral malaria. N. Engl. J. Med. 2015, 372, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Harawa, V.; Njie, M.; Kessler, A.; Choko, A.; Kumwenda, B.; Kampondeni, S.; Potchen, M.; Kim, K.; Jaworowski, A.; Taylor, T.; et al. Brain swelling is independent of peripheral plasma cytokine levels in Malawian children with cerebral malaria. Malar. J. 2018, 17, 435. [Google Scholar] [CrossRef]
- Jason, J.; Archibald, L.K.; Nwanyanwu, O.C.; Byrd, M.G.; Kazembe, P.N.; Dobbie, H.; Jarvis, W.R. Comparison of serum and cell-specific cytokines in humans. Clin. Diagn. Lab. Immunol. 2001, 8, 1097–1103. [Google Scholar] [CrossRef]
- Worzfeld, T.; Schwaninger, M. Apicobasal polarity of brain endothelial cells. J. Cereb. Blood Flow Metab. 2016, 36, 340–362. [Google Scholar] [CrossRef]
- Ferrara, N.; Chen, H.; Davis-Smyth, T.; Gerber, H.P.; Nguyen, T.N.; Peers, D.; Chisholm, V.; Hillan, K.J.; Schwall, R.H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998, 4, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 1998, 273, 13313–13316. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, Y.; Dellian, M.; Safabakhsh, N.; Ferrara, N.; Jain, R.K. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc. Natl. Acad. Sci. USA 1996, 93, 14765–14770. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Kimura, M.; Watanabe, N. Elevated levels of vascular endothelial growth factor (VEGF) and soluble vascular endothelial growth factor receptor (VEGFR)-2 in human malaria. Am. J. Trop. Med. Hyg. 2010, 82, 136–139. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, M.; Ralph, G.S.; Storkebaum, E.; Walmsley, L.E.; Mitrophanous, K.A.; Kingsman, S.M.; Carmeliet, P.; Mazarakis, N.D. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 2004, 429, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Krum, J.M.; Khaibullina, A. Inhibition of endogenous VEGF impedes revascularization and astroglial proliferation: Roles for VEGF in brain repair. Exp. Neurol. 2003, 181, 241–257. [Google Scholar] [CrossRef]
- Canavese, M.; Spaccapelo, R. Protective or pathogenic effects of vascular endothelial growth factor (VEGF) as potential biomarker in cerebral malaria. Pathog. Glob. Health 2014, 108, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.H.; Winkler, S.; Kremsner, P.G.; Meyermann, R.; Schluesener, H.J. Angiogenic proteins in brains of patients who died with cerebral malaria. J. Neuroimmunol. 2003, 142, 101–111. [Google Scholar] [CrossRef]
- Kwiatkowski, D.P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 2005, 77, 171–192. [Google Scholar] [CrossRef]
- La Paglia, L.; Listi, A.; Caruso, S.; Amodeo, V.; Passiglia, F.; Bazan, V.; Fanale, D. Potential role of ANGPTL4 in the cross talk between metabolism and cancer through PPAR signaling pathway. PPAR Res. 2017, 2017, 8187235. [Google Scholar] [CrossRef] [PubMed]
- Le Jan, S.; Amy, C.; Cazes, A.; Monnot, C.; Lamande, N.; Favier, J.; Philippe, J.; Sibony, M.; Gasc, J.M.; Corvol, P.; et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am. J. Pathol. 2003, 162, 1521–1528. [Google Scholar] [CrossRef]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Ko, E.J.; Jeon, K.Y.; Kim, H.; Jo, J.O.; Baek, K.W.; Kang, Y.J.; Choi, Y.H.; Hong, Y.; Ock, M.S.; et al. Induction of angiogenesis by malarial infection through hypoxia dependent manner. Korean J. Parasitol. 2019, 57, 117–125. [Google Scholar] [CrossRef]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Polverini, P.J. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem. Biophys. Res. Commun. 1995, 210, 51–57. [Google Scholar] [CrossRef]
- Campanella, G.S.; Colvin, R.A.; Luster, A.D. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS ONE 2010, 5, e12700. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, E.W.; Lian, J.; Ozga, A.J.; Miyabe, Y.; Ji, S.W.; Bromley, S.K.; Mempel, T.R.; Luster, A.D. CXCL10 stabilizes T cell-brain endothelial cell adhesion leading to the induction of cerebral malaria. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J.; Yates, C.C.; Wells, A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ. Res. 2006, 98, 617–625. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).