Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection
Abstract
1. Introduction
2. Materials and Methods
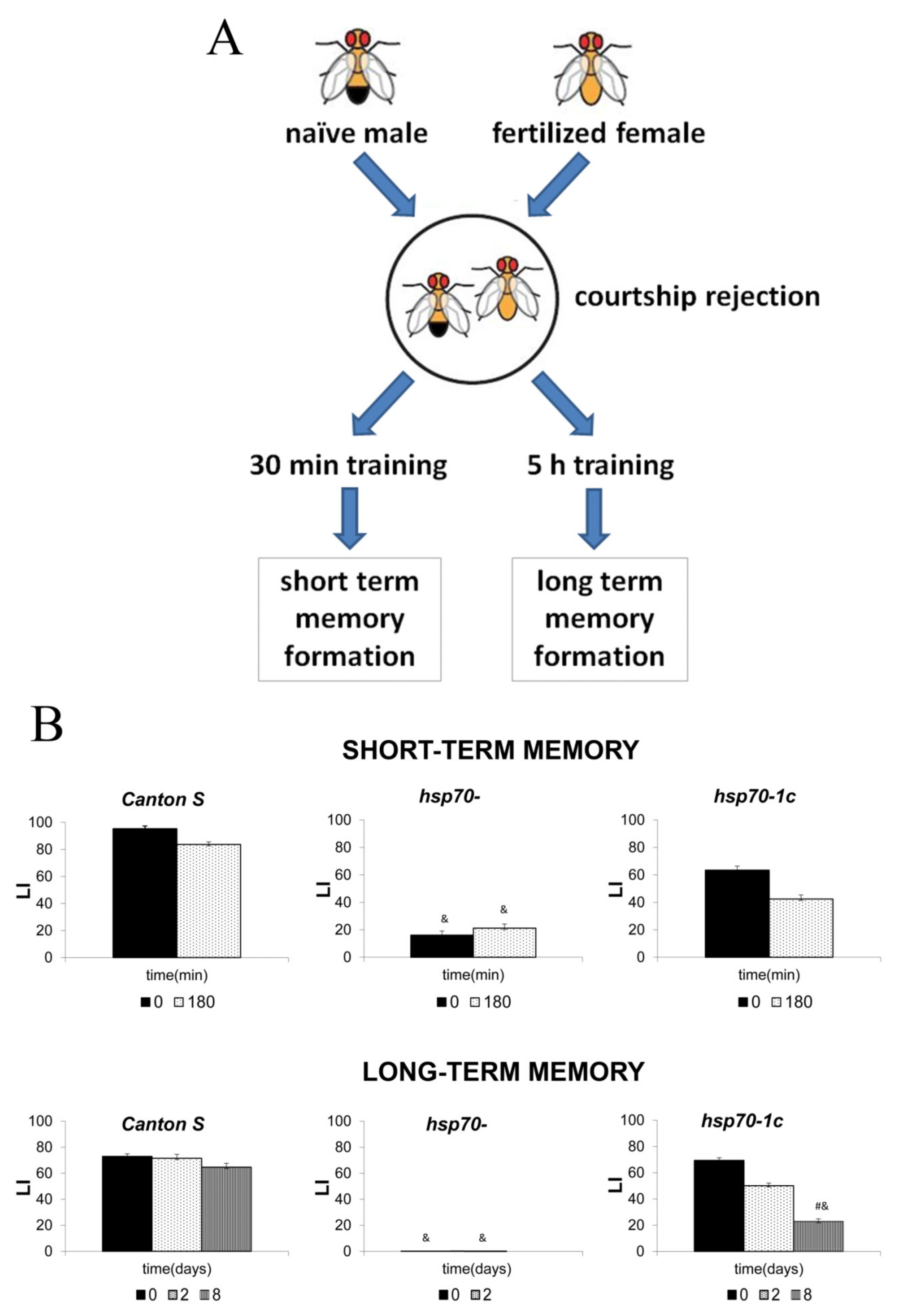
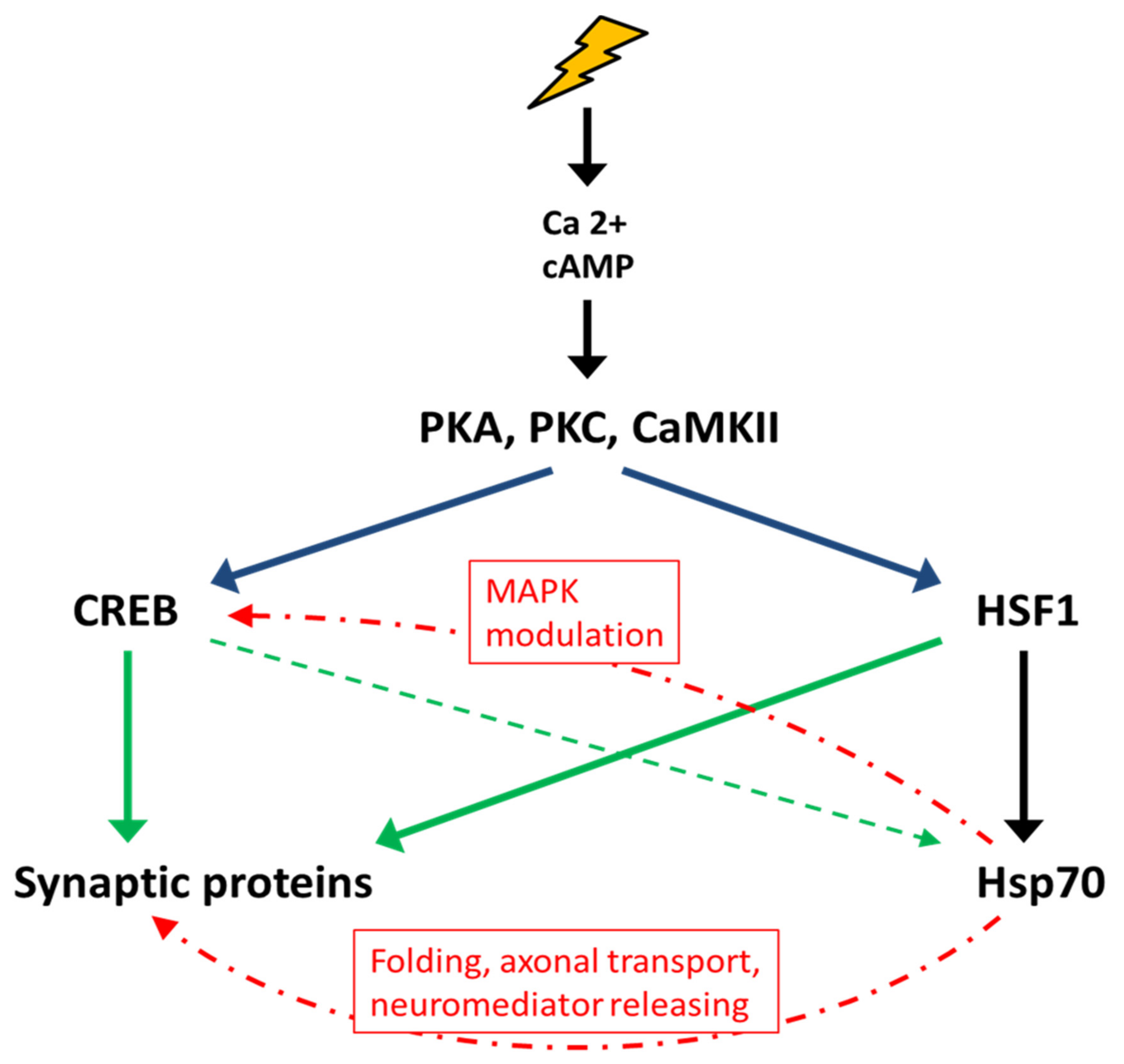
3. Mechanisms of Hsp70 Regulation and Memory Formation
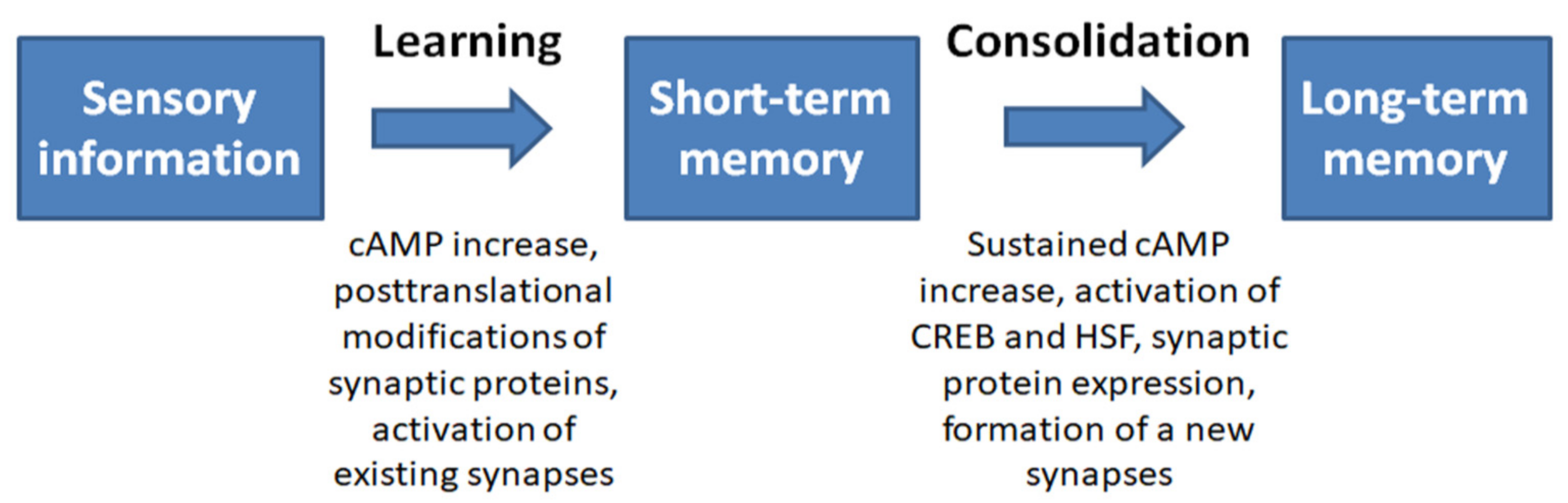
4. Relationship between the Stress Response System and Memory
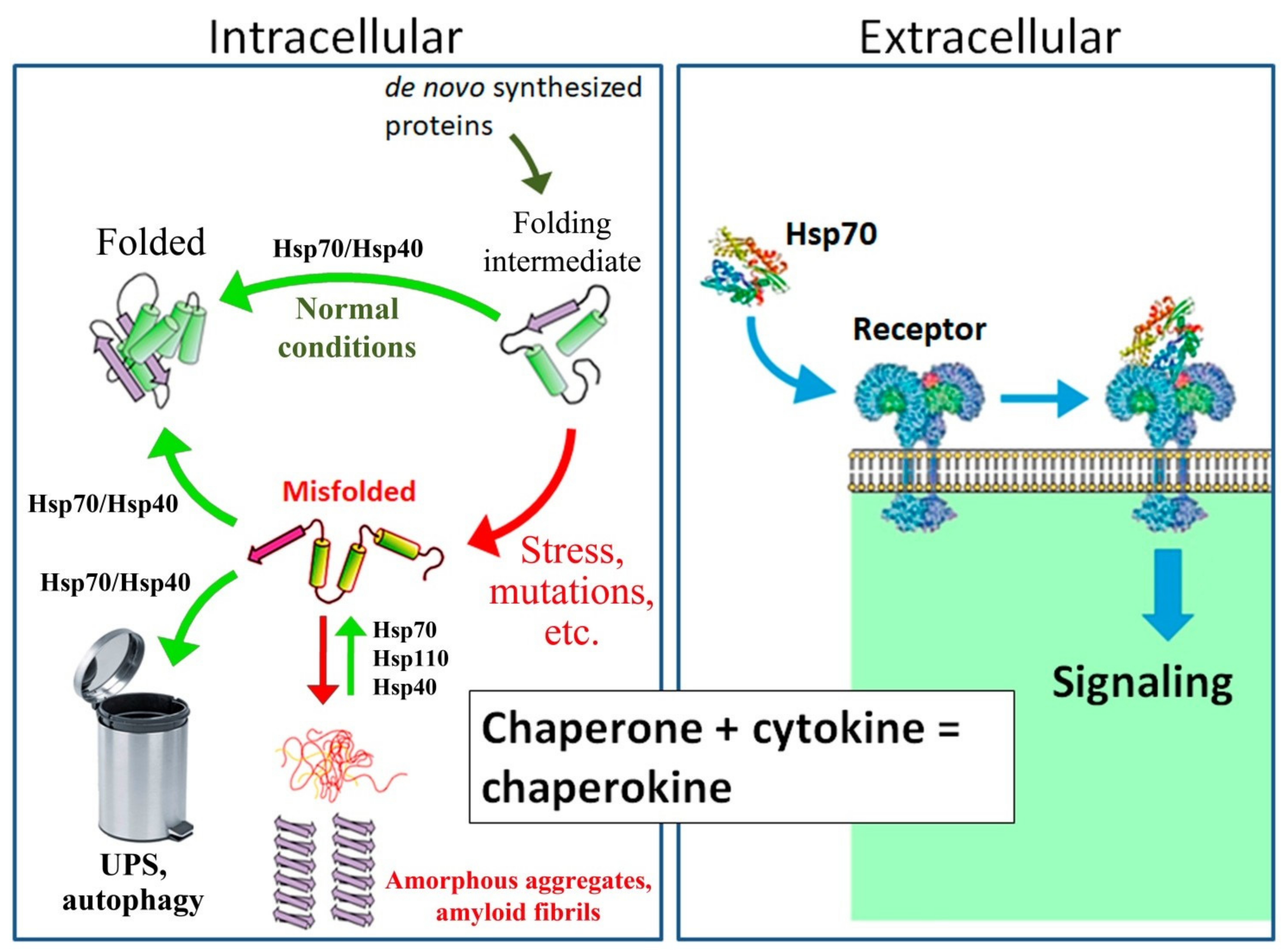
5. Hsp70 is Involved in the Functioning of Synapses and Protects the Synaptic Network from Stress-Induced Damage
6. Hsp70 Is an Effective Neuroprotector for Brain Ischemia
7. Hsp70 Prevents Neurodegeneration and Promotes Memory Recovery in Alzheimer’s Disease Models
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease: |
| APP | amyloid precursor protein; |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II; |
| cAMP | cyclic adenosine monophosphate; |
| CRE | cAMP response elements; |
| CNS | central nervous system; |
| CREB | CRE-binding protein; |
| ERK | extracellular signal-regulated kinases; |
| HS | heat shock; |
| HSE | heat shock element; |
| HSF | heat shock factor; |
| Hsps | heat shock proteins; |
| JNK | c-Jun N-terminal kinases; |
| LTM | long-term memory; |
| LTP | long-term potentiation; |
| MAPK | mitogen-activated protein kinase; |
| PKA | cAMP-dependent protein kinase; |
| PKC | protein kinase C; |
| STM | short-term memory; |
| STP | short-term potentiation |
References
- Robertson, R.; Kuhnert, C.; Dawson, J. Thermal avoidance during flight in the locust Locusta migratoria. J. Exp. Biol. 1996, 199, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.R.; Bennett, E.L. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behav. Brain Res. 1996, 78, 57–65. [Google Scholar] [CrossRef]
- Sandry, J.; Trafimow, D.; Marks, M.J.; Rice, S. Adaptive Memory: Evaluating Alternative Forms of Fitness-Relevant Processing in the Survival Processing Paradigm. PLoS ONE 2013, 8, e60868. [Google Scholar] [CrossRef]
- Forest, J.; Sunada, H.; Dodd, S.; Lukowiak, K. Training Lymnaea in the presence of a predator scent results in a long-lasting ability to form enhanced long-term memory. J. Comp. Physiol. A 2016, 202, 399–409. [Google Scholar] [CrossRef]
- Hawkins, R.D.; Byrne, J.H. Associative Learning in Invertebrates. Cold Spring Harb. Perspect. Biol. 2015, 7, a021709. [Google Scholar] [CrossRef]
- Lukowiak, K.; Orr, M.; De Caigny, P.; Lukowiak, K.S.; Rosenegger, D.; Han, J.I.; Dalesman, S. Ecologically relevant stressors modify long-term memory formation in a model system. Behav. Brain Res. 2010, 214, 18–24. [Google Scholar] [CrossRef]
- Zhao, H.; Bucci, D.; Weltzin, M.; Drew, K. Effects of aversive stimuli on learning and memory in Arctic ground squirrels. Behav. Brain Res. 2004, 151, 219–224. [Google Scholar] [CrossRef]
- Ooi, F.K.; Prahlad, V. Olfactory experience primes the heat shock transcription factor HSF-1 to enhance the expression of molecular chaperones inC. elegans. Sci. Signal. 2017, 10, eaan4893. [Google Scholar] [CrossRef]
- Cohen, A.O.; Matese, N.G.; Filimontseva, A.; Shen, X.; Shi, T.; Livne, E.; Hartley, C.A. Aversive learning strengthens episodic memory in both adolescents and adults. Learn. Mem. 2019, 26, 272–279. [Google Scholar] [CrossRef]
- Sitaraman, D.; Zars, M.; LaFerriere, H.; Chen, Y.-C.; Sable-Smith, A.; Kitamoto, T.; Rottinghaus, G.E.; Zars, T. Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 5579–5584. [Google Scholar] [CrossRef]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nat. Cell Biol. 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Morimoto, R. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Yura, T.; Nagai, H.; Mori, H. Regulation of the Heat-Shock Response in Bacteria. Annu. Rev. Microbiol. 1993, 47, 321–350. [Google Scholar] [CrossRef]
- Rubin, D.M.; Mehta, A.D.; Zhu, J.; Shoham, S.; Chen, X.; Wells, Q.R.; Palter, K.B. Genomic structure and sequence analysis of Drosophila melanogaster HSC70 genes. Gene 1993, 128, 155–163. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, Y.; Lee, J.E.; Yenari, M.A. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin. Ther. Targets 2018, 22, 191–199. [Google Scholar] [CrossRef]
- Finka, A.; Sharma, S.K.; Goloubinoff, P. Multi-layered molecular mechanisms of polypeptide holding, unfolding and disaggregation by HSP70/HSP110 chaperones. Front. Mol. Biosci. 2015, 2, 29. [Google Scholar] [CrossRef]
- Gao, X.; Carroni, M.; Nussbaum-Krammer, C.; Mogk, A.; Nillegoda, N.B.; Szlachcic, A.; Guilbride, D.L.; Saibil, H.R.; Mayer, M.P.; Bukau, B. Human Hsp70 Disaggregase Reverses Parkinson’s-Linked α-Synuclein Amyloid Fibrils. Mol. Cell 2015, 59, 781–793. [Google Scholar] [CrossRef]
- Wentink, A.S.; Nillegoda, N.B.; Feufel, J.; Ubartaitė, G.; Schneider, C.P.; Rios, P.D.L.; Hennig, J.; Barducci, A.; Bukau, B. Molecular dissection of amyloid disaggregation by human HSP70. Nat. Cell Biol. 2020, 587, 483–488. [Google Scholar] [CrossRef]
- Witt, S.N. Hsp70 molecular chaperones and Parkinson’s disease. Biopolymers 2010, 93, 218–228. [Google Scholar] [CrossRef]
- Aneja, R.; Odoms, K.; Dunsmore, K.; Shanley, T.P.; Wong, H. Extracellular Heat Shock Protein-70 Induces Endotoxin Tolerance in THP-1 Cells. J. Immunol. 2006, 177, 7184–7192. [Google Scholar] [CrossRef]
- Kustanova, G.A.; Murashev, A.N.; Karpov, V.L.; Margulis, B.A.; Guzhova, I.V.; Prokhorenko, I.R.; Grachev, S.V.; Evgen’Ev, M.B. Exogenous heat shock protein 70 mediates sepsis manifestations and decreases the mortality rate in rats. Cell Stress Chaperones 2006, 11, 276–286. [Google Scholar] [CrossRef]
- Rozhkova, E.; Yurinskaya, M.; Zatsepina, O.; Garbuz, D.; Karpov, V.; Surkov, S.; Murashev, A.; Ostrov, V.; Margulis, B.; Evgen’Ev, M.; et al. Exogenous mammalian extracellular HSP70 reduces endotoxin manifestations at the cellular and organism levels. Ann. N. Y. Acad. Sci. 2010, 1197, 94–107. [Google Scholar] [CrossRef]
- Vinokurov, M.; Ostrov, V.; Yurinskaya, M.; Garbuz, D.; Murashev, A.; Antonova, O.; Evgen’Ev, M. Recombinant human Hsp70 protects against lipoteichoic acid-induced inflammation manifestations at the cellular and organismal levels. Cell Stress Chaperones 2011, 17, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Borges, T.J.; Lopes, R.L.; Pinho, N.G.; Machado, F.D.; Souza, A.P.D.; Bonorino, C. Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBPβ and C/EBPδ. Int. J. Hyperth. 2013, 29, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-H.; Yang, R.-C.; Lin, S.-J.; Liou, S.-F.; Dai, Z.-K.; Yeh, J.-L.; Wu, J.-R. Exogenous Heat Shock Cognate Protein 70 Pretreatment Attenuates Cardiac and Hepatic Dysfunction with Associated Anti-inflammatory Responses in Experimental Septic Shock. Shock 2014, 42, 540–547. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Sinha, D.; Mukherjee, S.; Biswas, R.; Biswas, T. LPS stimulates and Hsp70 down-regulates TLR4 to orchestrate differential cytokine response of culture-differentiated innate memory CD8+ T cells. Cytokine 2015, 73, 44–52. [Google Scholar] [CrossRef]
- Asea, A. Stress proteins and initiation of immune response: Chaperokine activity of hsp72. Exerc. Immunol. Rev. 2005, 11, 34–45. [Google Scholar] [PubMed]
- Morimoto, R.I.; Kline, M.P.; Bimston, D.N.; Cotto, J.J. The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 1997, 32, 17–29. [Google Scholar] [PubMed]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef]
- Garbuz, D.G. Regulation of heat shock gene expression in response to stress. Mol. Biol. 2017, 51, 352–367. [Google Scholar] [CrossRef]
- Morimoto, R.I.; Santoro, M. Stress—Inducible responses and heat shock proteins: New pharmacologic targets for cytoprotection. Nat. Biotechnol. 1998, 16, 833–838. [Google Scholar] [CrossRef]
- Voellmy, R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones 2004, 9, 122–133. [Google Scholar] [CrossRef]
- Morange, M. HSFs in Development. Mol. Chaperones Health Dis. 2006, 172, 153–169. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Bausinger, H.; Lipsker, D.; Ziylan, U.; Manié, S.; Briand, J.; Cazenave, J.; Muller, S.; Haeuw, J.; Ravanat, C.; De La Salle, H.; et al. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur. J. Immunol. 2002, 32, 3708–3713. [Google Scholar] [CrossRef]
- Kandel, E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 2012, 5, 14. [Google Scholar] [CrossRef]
- Mayford, M.; Siegelbaum, S.A.; Kandel, E.R. Synapses and Memory Storage. Cold Spring Harb. Perspect. Biol. 2012, 4, a005751. [Google Scholar] [CrossRef]
- Flexner, J.B.; Flexner, L.B.; Stellar, E. Memory in Mice as Affected by Intracerebral Puromycin. Science 1963, 141, 57–59. [Google Scholar] [CrossRef]
- Kandel, E.R. The Molecular Biology of Memory Storage: A Dialogue Between Genes and Synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef]
- Giese, K.P.; Mizuno, K. The roles of protein kinases in learning and memory. Learn. Mem. 2013, 20, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nat. Cell Biol. 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Lynch, M.A. Long-Term Potentiation and Memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.; Sassone-Corsi, P.; Verma, I.M. Two adjacent promoter elements mediate nerve growth factor activation of the c-fos gene and bind distinct nuclear complexes. Proc. Natl. Acad. Sci. USA 1988, 85, 9474–9478. [Google Scholar] [CrossRef] [PubMed]
- Thekkuveettil, A.; Lakhotia, S.C. Regulation of HSP70 in excitatory neurons: Possible implications for neuronal functioning. J. Biosci. 1996, 21, 631–639. [Google Scholar] [CrossRef]
- Robertson, M. Interpersonal Therapy: An Introduction for Clinicians. Australas. Psychiatry 1999, 7, 25–27. [Google Scholar] [CrossRef]
- Alberini, C.M. Transcription Factors in Long-Term Memory and Synaptic Plasticity. Physiol. Rev. 2009, 89, 121–145. [Google Scholar] [CrossRef]
- Choi, H.S.; Li, B.; Lin, Z.; Huang, E.; Liu, A.Y. cAMP and cAMP-dependent protein kinase regulate the human heat shock protein 70 gene promoter activity. J. Biol. Chem. 1991, 266, 11858–11865. [Google Scholar] [CrossRef]
- Han, S.I.; Oh, S.Y.; Woo, S.H.; Kim, K.H.; Kim, J.H.; Kim, H.D.; Kang, H.S. Implication of a small GTPase Rac1 in the activation of c-Jun N-terminal kinase and heat shock factor in response to heat shock. J. Biol. Chem. 2001, 276, 1889–1895. [Google Scholar] [CrossRef]
- Bijur, G.N.; Jope, R.S. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J. Neurochem. 2000, 75, 2401–2408. [Google Scholar] [CrossRef]
- Murshid, A.; Chou, S.D.; Prince, T.; Zhang, Y.; Bharti, A.; Calderwood, S.K. Protein kinase A binds and activates heat shock factor 1. PLoS ONE 2010, 5, e13830. [Google Scholar] [CrossRef]
- Gungor, B.; Gombos, I.; Crul, T.; Ayaydin, F.; Szabó, L.; Török, Z.; Mátés, L.; Vígh, L.; Horváth, I. Rac1 Participates in Thermally Induced Alterations of the Cytoskeleton, Cell Morphology and Lipid Rafts, and Regulates the Expression of Heat Shock Proteins in B16F10 Melanoma Cells. PLoS ONE 2014, 9, e89136. [Google Scholar] [CrossRef]
- Török, Z.; Crul, T.; Maresca, B.; Schütz, G.J.; Viana, F.; Dindia, L.; Piotto, S.; Brameshuber, M.; Balogh, G.; Péter, M.; et al. Plasma membranes as heat stress sensors: From lipid-controlled molecular switches to therapeutic applications. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1594–1618. [Google Scholar] [CrossRef] [PubMed]
- Price, B.D.; Calderwood, S.K. Ca2+ is essential for multistep activation of the heat shock factor in permeabilized cells. Mol. Cell. Biol. 1991, 11, 3365–3368. [Google Scholar] [CrossRef]
- Porto, R.R.; Alvares, L.D.O. Role of HSP70 in Plasticity and Memory. In Heat Shock Proteins in Human Diseases; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 53–67. [Google Scholar]
- Donovan, M.R.; Marr, M.T. dFOXO Activates Large and Small Heat Shock Protein Genes in Response to Oxidative Stress to Maintain Proteostasis in Drosophila. J. Biol. Chem. 2016, 291, 19042–19050. [Google Scholar] [CrossRef]
- Kaletsky, R.; Lakhina, V.; Arey, R.N.; Williams, A.; Landis, J.N.; Ashraf, J.; Murphy, C.T. The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nat. Cell Biol. 2016, 529, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Webb, A.E. Neuronal functions of FOXO/DAF-16. Nutr. Health Aging 2017, 4, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, J.M.; Haro, L.S.; Barea-Rodriguez, E.J. Learning associated increase in heat shock cognate 70 mRNA and protein expression. Neurobiol. Learn. Mem. 2003, 79, 142–151. [Google Scholar] [CrossRef]
- Igaz, L.M.; Bekinschtein, P.; Izquierdo, I.; Medina, J.H. One-trial aversive learning induces late changes in hippocampal CaMKIIalpha, Homer 1a, Syntaxin 1a and ERK2 protein levels. Mol. Brain Res. 2004, 132, 1–12. [Google Scholar] [CrossRef]
- Porto, R.R.; Dutra, F.D.; Crestani, A.P.; Holsinger, R.D.; Quillfeldt, J.A.; De Bittencourt, P.I.H.; Alvares, L.D.O. HSP70 Facilitates Memory Consolidation of Fear Conditioning through MAPK Pathway in the Hippocampus. Neuroscience 2018, 375, 108–118. [Google Scholar] [CrossRef] [PubMed]
- King, M.K.; Pardo, M.; Cheng, Y.; Downey, K.; Jope, R.S.; Beurel, E. Glycogen synthase kinase-3 inhibitors: Rescuers of cognitive impairments. Pharmacol. Ther. 2014, 141, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Q.; Wang, X.-L.; Cao, X.-H.; Ye, Z.; Li, L.; Cai, W.-Q. Increased heat shock transcription factor 1 in the cerebellum reverses the deficiency of Purkinje cells in Alzheimer’s disease. Brain Res. 2013, 1519, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Katsuno, M.; Adachi, H.; Minamiyama, M.; Doi, H.; Matsumoto, S.; Miyazaki, Y.; Iida, M.; Tohnai, G.; Nakatsuji, H.; et al. Heat shock factor-1 influences pathological lesion distribution of polyglutamine-induced neurodegeneration. Nat. Commun. 2013, 4, 1405. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Liu, D.; Li, J.J.; Xue, Y.; Sakata, K.; Zhu, L.Q.; Heldt, S.A.; Xu, H.; Liao, F.F. Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 2014, 34, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.-H.; Reuter, S.; Schmucker, S.; Dicato, M.; Diederich, M. Induction of heat shock response by curcumin in human leukemia cells. Cancer Lett. 2009, 279, 145–154. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin Improves Amyloid β-Peptide (1–42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS ONE 2015, 10, e0131525. [Google Scholar] [CrossRef]
- Rao, A.; Steward, O. Evidence that protein constituents of postsynaptic membrane specializations are locally synthesized: Analysis of proteins synthesized within synaptosomes. J. Neurosci. 1991, 11, 2881–2895. [Google Scholar] [CrossRef]
- Hooper, P.L.; Durham, H.D.; Török, Z.; Hooper, P.L.; Crul, T.; Vígh, L. The central role of heat shock factor 1 in synaptic fidelity and memory consolidation. Cell Stress Chaperones 2016, 21, 745–753. [Google Scholar] [CrossRef]
- Suzuki, T.; Usuda, N.; Murata, S.; Nakazawa, A.; Ohtsuka, K.; Takagi, H. Presence of molecular chaperones, heat shock cognate (Hsc) 70 and heat shock proteins (Hsp) 40, in the postsynaptic structures of rat brain. Brain Res. 1999, 816, 99–110. [Google Scholar] [CrossRef]
- Dewji, N.N.; Do, C. Heat shock factor-1 mediates the transcriptional activation of Alzheimer’s beta-amyloid precursor protein gene in response to stress. Mol. Brain Res. 1996, 35, 325–328. [Google Scholar] [CrossRef]
- Dewji, N.N.; Do, C.; Bayney, R.M. Transcriptional activation of Alzheimer’s beta-amyloid precursor protein gene by stress. Mol. Brain Res. 1995, 33, 245–253. [Google Scholar] [CrossRef]
- Chaouloff, F.; Berton, O.; Mormède, P. Serotonin and stress. Neuropsychopharmacology 1999, 21, 28S–32S. [Google Scholar] [CrossRef]
- Joëls, M.; Baram, T.Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tatum, M.C.; Ooi, F.K.; Chikka, M.R.; Chauve, L.; Martinez-Velazquez, L.A.; Steinbusch, H.W.; Morimoto, R.I.; Prahlad, V. Neuronal Serotonin Release Triggers the Heat Shock Response in C. elegans in the Absence of Temperature Increase. Curr. Biol. 2015, 25, 163–174. [Google Scholar] [CrossRef]
- Enver, T.; Soneji, S.; Joshi, C.; Brown, J.; Iborra, F.; Orntoft, T.; Thykjaer, T.; Maltby, E.; Smith, K.; Abu Dawud, R.; et al. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum. Mol. Genet. 2005, 14, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Wingen, M.; Ramaekers, J.; Evers, E.; Riedel, W. Serotonin and Human Cognitive Performance. Curr. Pharm. Des. 2006, 12, 2473–2486. [Google Scholar] [CrossRef]
- Pijanowska, J.; Kloc, M. Daphnia response to predation threat involves heat-shock proteins and the actin and tubulin cytoskeleton. Genesis 2004, 38, 81–86. [Google Scholar] [CrossRef]
- Kagawa, N.; Mugiya, Y. Brain HSP70 mRNA Expression is Linked with Plasma Cortisol Levels in Goldfish (Carassius auratus) Exposed to a Potential Predator. Zool. Sci. 2002, 19, 735–740. [Google Scholar] [CrossRef][Green Version]
- Frenkel, L.; Dimant, B.; Suarez, L.; Portiansky, E.; Delorenzi, A. Food odor, visual danger stimulus, and retrieval of an aversive memory trigger heat shock protein HSP70 expression in the olfactory lobe of the crab Chasmagnathus granulatus. Neuroscience 2012, 201, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Abe, K.; Hongo, M.; Utsumi, A.; Itoyama, Y. Brain-gut induction of heat shock protein (HSP) 70 mRNA by psychophysiological stress in rats. Brain Res. 1997, 757, 146–148. [Google Scholar] [CrossRef]
- Fukudo, S.; Abe, K.; Itoyama, Y.; Mochizuki, S.; Sawai, T.; Hongo, M. Psychophysiological stress induces heat shock cognate protein 70 messenger RNA in the hippocampus of rats. Neuroscience 1999, 91, 1205–1208. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, W.; Jung, H.Y.; Kang, M.S.; Kim, J.W.; Hahn, K.R.; Yoo, D.Y.; Yoon, Y.S.; Hwang, I.K.; Kim, D.W. Heat shock protein 70 increases cell proliferation, neuroblast differentiation, and the phosphorylation of CREB in the hippocampus. Lab. Anim. Res. 2019, 35, 21. [Google Scholar] [CrossRef]
- Ambrosini, M.V.; Mariucci, G.; Tantucci, M.; Van Hooijdonk, L.; Ammassari-Teule, M. Hippocampal 72-kDa heat shock protein expression varies according to mice learning performance independently from chronic exposure to stress. Hippocampus 2005, 15, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Bevilaqua, L.R.; Rossato, J.I.; Bonini, J.S.; Medina, J.H.; Cammarota, M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006, 29, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K. Heat shock proteins in extracellular signaling. Methods 2007, 43, 167. [Google Scholar] [CrossRef][Green Version]
- De Maio, A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: A form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 2011, 16, 235–249. [Google Scholar] [CrossRef]
- Ammon-Treiber, S.; Grecksch, G.; Angelidis, C.; Vezyraki, P.; Höllt, V.; Becker, A. Emotional and learning behaviour in mice overexpressing heat shock protein 70. Neurobiol. Learn. Mem. 2008, 90, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.L.; Lukowiak, K.; Henry, T.B. Time-related expression profiles for heat shock protein gene transcripts (HSP40, HSP70) in the central nervous system of Lymnaea stagnalis exposed to thermal stress. Commun. Integr. Biol. 2015, 8, e1040954. [Google Scholar] [CrossRef]
- Grotewiel, M.S.; Beck, C.D.O.; Wu, K.H.; Zhu, X.-R.; Davis, R.L. Integrin-mediated short-term memory in Drosophila. Nat. Cell Biol. 1998, 391, 455–460. [Google Scholar] [CrossRef]
- Nikitina, E.A.; Kaminskaya, A.N.; Molotkov, D.A.; Popov, A.V.; Savvateeva-Popova, E.V. Effect of heat shock on courtship behavior, sound production, and learning in comparison with the brain content of LIMK1 in Drosophila melanogaster males with altered structure of the LIMK1 gene. J. Evol. Biochem. Physiol. 2014, 50, 154–166. [Google Scholar] [CrossRef]
- Teigen, K.H. Yerkes-Dodson: A Law for all Seasons. Theory Psychol. 1994, 4, 525–547. [Google Scholar] [CrossRef]
- Chu, B.; Soncin, F.; Price, B.D.; Stevenson, M.A.; Calderwood, S.K. Sequential Phosphorylation by Mitogen-activated Protein Kinase and Glycogen Synthase Kinase 3 Represses Transcriptional Activation by Heat Shock Factor-1. J. Biol. Chem. 1996, 271, 30847–30857. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Heat Shock Factor 1 as a Coordinator of Stress and Developmental Pathways. Chem. Biol. Pteridines Folates 2007, 594, 78–88. [Google Scholar] [CrossRef]
- Gabai, V.L.; Meriin, A.B.; Yaglom, J.A.; Volloch, V.Z.; Sherman, M.Y. Role of Hsp70 in regulation of stress-kinase JNK: Implications in apoptosis and aging. FEBS Lett. 1998, 438, 1–4. [Google Scholar] [CrossRef]
- Kumar, Y.; Tatu, U.; Budigi, Y. Stress protein flux during recovery from simulated ischemia: Induced heat shock protein 70 confers cytoprotection by suppressing JNK activation and inhibiting apoptotic cell death. Proteomics 2003, 3, 513–526. [Google Scholar] [CrossRef]
- Thomas, G.M.; Huganir, R.L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 2004, 5, 173–183. [Google Scholar] [CrossRef]
- Giachello, C.N.G.; Fiumara, F.; Giacomini, C.; Corradi, A.; Milanese, C.; Ghirardi, M.; Benfenati, F.; Montarolo, P.G. MAPK/Erk-dependent phosphorylation of synapsin mediates formation of functional synapses and short-term homosynaptic plasticity. J. Cell Sci. 2010, 123, 881–893. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, M.; Yoon, B.-W.; Kim, Y.-J.; Ma, S.-J.; Roh, J.-K.; Lee, J.-S.; Seo, J.-S. Targeted hsp70.1 Disruption Increases Infarction Volume After Focal Cerebral Ischemia in Mice. Stroke 2001, 32, 2905–2912. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwon, H.-M.; Kim, Y.-J.; Lee, K.-M.; Kim, M.; Yoon, B.-W. Effects of Hsp70.1 Gene Knockout on the Mitochondrial Apoptotic Pathway After Focal Cerebral Ischemia. Stroke 2004, 35, 2195–2199. [Google Scholar] [CrossRef]
- Yurinskaya, M.; Zatsepina, O.; Vinokurov, M.; Bobkova, N.; Garbuz, D.; Morozov, A.; Kulikova, D.; Mitkevich, V.; Makarov, A.; Funikov, S.; et al. The Fate of Exogenous Human HSP70 Introduced into Animal Cells by Different Means. Curr. Drug Deliv. 2015, 12, 524–532. [Google Scholar] [CrossRef]
- Choudhury, S.; Bae, S.; Ke, Q.; Lee, J.Y.; Kim, J.; Kang, P.M. Mitochondria to nucleus translocation of AIF in mice lacking Hsp70 during ischemia/reperfusion. Basic Res. Cardiol. 2011, 106, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zatsepina, O.G.; Nikitina, E.A.; Shilova, V.Y.; Chuvakova, L.N.; Sorokina, S.; Vorontsova, J.E.; Tokmacheva, E.V.; Funikov, S.Y.; Rezvykh, A.P.; Evgen’Ev, M.B. Hsp70 affects memory formation and behaviorally relevant gene expression in Drosophila melanogaster. Cell Stress Chaperones 2021, 26, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Manzerra, P.; Rush, S.J.; Brown, I.R. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J. Cell Physiol. 1997, 170, 130–137. [Google Scholar] [CrossRef]
- Foster, J.; Brown, I. Basal expression of stress-inducible hsp70 mRNA detected in hippocampal and cortical neurons of normal rabbit brain. Brain Res. 1996, 724, 73–83. [Google Scholar] [CrossRef][Green Version]
- Bodega, G.; Hernández, C.; Suárez, I.; Martín, M.; Fernández, B. HSP70 constitutive expression in rat central nervous system from postnatal development to maturity. J. Histochem. Cytochem. 2002, 50, 1161–1168. [Google Scholar] [CrossRef]
- Loones, M.-T.; Rallu, M.; Mezger, V.; Morange, M. HSP gene expression and HSF2 in mouse development. Cell. Mol. Life Sci. 1997, 53, 179–190. [Google Scholar] [CrossRef]
- Loones, M.-T.; Chang, Y.; Morange, M. The distribution of heat shock proteins in the nervous system of the unstressed mouse embryo suggests a role in neuronal and non-neuronal differentiation. Cell Stress Chaperones 2000, 5, 291–305. [Google Scholar] [CrossRef]
- D’Souza, S.M.; Brown, I.R. Constitutive expression of heat shock proteins Hsp90, Hsc70, Hsp70 and Hsp60 in neural and non-neural tissues of the rat during postnatal development. Cell Stress Chaperones 1998, 3, 188–199. [Google Scholar] [CrossRef]
- Aquino, D.A.; Klipfel, A.A.; Brosnan, C.F.; Norton, W.T. The 70-kDa heat shock cognate protein (HSC70) is a major constituent of the central nervous system and is up-regulated only at the mRNA level in acute experimental autoimmune encephalomyelitis. J. Neurochem. 1993, 61, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.R.; Rush, S.J. Expression of heat shock genes (hsp70) in the mammalian brain: Distinguishing constitutively expressed and hyperthermia-inducible mRNA species. J. Neurosci. Res. 1990, 25, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Izumoto, S.; Herbert, J. Widespread constitutive expression of HSP90 messenger RNA in rat brain. J. Neurosci. Res. 1993, 35, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Mambula, S.S.; Gray, P.J.; Theriault, J.R. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007, 581, 3689–3694. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, R.M.; Wu, Y.; Khandjian, E.W. Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev. Genet. 1993, 14, 112–118. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Rezzani, R.; Rodella, L.; Tiberio, L.; Schiaffonati, L.; Bianchi, R. Cell-specific expression of heat shock transcription factors 1 and 2 in unstressed rat spinal cord. Neurosci. Lett. 1999, 268, 73–76. [Google Scholar] [CrossRef]
- Bechtold, D.A.; Rush, S.J.; Brown, I.R. Localization of the Heat-Shock Protein Hsp70 to the Synapse Following Hyperthermic Stress in the Brain. J. Neurochem. 2001, 74, 641–646. [Google Scholar] [CrossRef]
- Morgan, J.R.; Prasad, K.; Jin, S.; Augustine, G.J.; Lafer, E. Uncoating of Clathrin-Coated Vesicles in Presynaptic Terminals: Roles for Hsc70 and Auxilin. Neuron 2001, 32, 289–300. [Google Scholar] [CrossRef]
- Leshchyns’Ka, I.; Sytnyk, V.; Richter, M.; Andreyeva, A.; Puchkov, D.; Schachner, M. The Adhesion Molecule CHL1 Regulates Uncoating of Clathrin-Coated Synaptic Vesicles. Neuron 2006, 52, 1011–1025. [Google Scholar] [CrossRef]
- Brown, I.R. Heat Shock Proteins at the Synapse: Implications for Functional Protection of the Nervous System. In Heat Shock Proteins and the Brain: Implications for Neurodegenerative Diseases and Neuroprotection; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; pp. 239–254. [Google Scholar]
- Klose, M.K.; Robertson, R.M. Stress-induced thermoprotection of neuromuscular transmission. Integr. Comp. Biol. 2004, 44, 14–20. [Google Scholar] [CrossRef]
- Manzerra, P.; Brown, I.R. Expression of heat shock genes (hsp70) in the rabbit spinal cord: Localization of constitutive and hyperthermia-inducible mRNA species. J. Neurosci. Res. 1992, 31, 606–615. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, And The Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Evgen’Ev, M.B.; Garbuz, D.G.; Zatsepina, O.G. Heat Shock Proteins and Whole Body Adaptation to Extreme Environments; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Fink, S.L.; Chang, L.K.; Ho, D.Y.; Sapolsky, R.M. Defective Herpes Simplex Virus Vectors Expressing the Rat Brain Stress-Inducible Heat Shock Protein 72 Protect Cultured Neurons from Severe Heat Shock. J. Neurochem. 2002, 68, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Plumier, J.; Krueger, A.; Currie, R.; Kontoyiannis, D.; Kollias, G.; Pagpulatos, G. Response of transgenic mice expressing the human 70-kDa heat shock protein to cerebral ischemia. Cell Stress Chaperones 1997, 2, 162–167. [Google Scholar] [CrossRef]
- Yenari, M.A.; Fink, S.L.; Sun, G.H.; Chang, L.K.; Patel, M.; Kunis, D.M.; Onley, D.; Ho, D.Y.; Sapolsky, R.M.; Steinbrg, G.K. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann. Neurol. 1998, 44, 584–591. [Google Scholar] [CrossRef]
- Chen, S.; Brown, I.R. Translocation of constitutively expressed heat shock protein Hsc70 to synapse-enriched areas of the cerebral cortex after hyperthermic stress. J. Neurosci. Res. 2007, 85, 402–409. [Google Scholar] [CrossRef]
- Fei, G.-H.; Guo, C.; Sun, H.-S.; Feng, Z.-P. HSP70 Reduces Chronic Hypoxia-Induced Neural Suppression via Regulating Expression of Syntaxin. Chem. Biol. Pteridines Folates 2008, 605, 35–40. [Google Scholar] [CrossRef]
- Xiao, C.; Mileva-Seitz, V.; Seroude, L.; Robertson, R.M. Targeting HSP70 to motoneurons protects locomotor activity from hyperthermia inDrosophila. Dev. Neurobiol. 2007, 67, 438–455. [Google Scholar] [CrossRef]
- Giffard, R.G.; Yenari, M.A. Many Mechanisms for Hsp70 Protection from Cerebral Ischemia. J. Neurosurg. Anesthesiol. 2004, 16, 53–61. [Google Scholar] [CrossRef]
- Kelly, S.; McCulloch, J.; Horsburgh, K. Minimal ischaemic neuronal damage and HSP70 expression in MF1 strain mice following bilateral common carotid artery occlusion. Brain Res. 2001, 914, 185–195. [Google Scholar] [CrossRef]
- Sharp, F.R.; Lu, A.; Tang, Y.; Millhorn, D.E. Multiple Molecular Penumbras after Focal Cerebral Ischemia. Br. J. Pharmacol. 2000, 20, 1011–1032. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.F.; Shiraishi, K.; Hisanaga, K.; Sagar, S.M.; Mandabach, M.; Sharp, F.R. Heat shock proteins as markers of neural injury. Mol. Brain Res. 1989, 6, 93–100. [Google Scholar] [CrossRef]
- Kelly, S.; Bieneman, A.; Horsburgh, K.; Hughes, D.; Sofroniew, M.V.; McCulloch, J.; Uney, J.B. Targeting Expression of hsp70i to Discrete Neuronal Populations Using the Lmo-1 Promoter: Assessment of the Neuroprotective Effects of hsp70i In vivo and In vitro. Br. J. Pharmacol. 2001, 21, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, D.; Hong, S.; Matsumori, Y.; Kayama, T.; Swanson, R.; Dillman, W.H.; Liu, J.; Panter, S.S.; Weinstein, P.R. Overexpression of Rat Heat Shock Protein 70 Reduces Neuronal Injury after Transient Focal Ischemia, Transient Global Ischemia, or Kainic Acid-induced Seizures. Neurosurgery 2003, 53, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Shichinohe, H.; Kuroda, S.; Ishikawa, T.; Iwasaki, Y. Neuroprotective effect of a heat shock protein inducer, geranylgeranylacetone in permanent focal cerebral ischemia. Brain Res. 2005, 1032, 176–182. [Google Scholar] [CrossRef]
- Zhao, Z.; Faden, A.I.; Loane, D.; Lipinski, M.M.; Sabirzhanov, B.; Stoica, B. Neuroprotective Effects of Geranylgeranylacetone in Experimental Traumatic Brain Injury. Br. J. Pharmacol. 2013, 33, 1897–1908. [Google Scholar] [CrossRef]
- Matsumori, Y.; Hong, S.M.; Aoyama, K.; Fan, Y.; Kayama, T.; Sheldon, R.A.; Vexler, Z.S.; Ferriero, D.M.; Weinstein, P.R.; Liu, J. Hsp70 Overexpression Sequesters AIF and Reduces Neonatal Hypoxic/Ischemic Brain Injury. Br. J. Pharmacol. 2005, 25, 899–910. [Google Scholar] [CrossRef]
- Feinstein, D.L.; Galea, E.; Aquino, D.A.; Li, G.C.; Xu, H.; Reis, D.J. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J. Biol. Chem. 1996, 271, 17724–17732. [Google Scholar] [CrossRef]
- Bobkova, N.V.; Garbuz, D.G.; Nesterova, I.; Medvinskaya, N.; Samokhin, A.; Alexandrova, I.; Yashin, V.; Karpov, V.; Kukharsky, M.; Ninkina, N.N.; et al. Therapeutic Effect of Exogenous Hsp70 in Mouse Models of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 38, 425–435. [Google Scholar] [CrossRef]
- Evgen’Ev, M.; Bobkova, N.; Krasnov, G.; Garbuz, D.; Funikov, S.; Kudryavtseva, A.; Kulikov, A.; Samokhin, A.; Maltsev, A.; Nesterova, I. The Effect of Human HSP70 Administration on a Mouse Model of Alzheimer’s Disease Strongly Depends on Transgenicity and Age. J. Alzheimer’s Dis. 2019, 67, 1391–1404. [Google Scholar] [CrossRef]
- Demyanenko, S.; Nikul, V.; Rodkin, S.; Davletshin, A.; Evgen’Ev, M.B.; Garbuz, D.G. Exogenous recombinant Hsp70 mediates neuroprotection after photothrombotic stroke. Cell Stress Chaperones 2021, 26, 103–114. [Google Scholar] [CrossRef]
- Zhan, X.; Ander, B.P.; Liao, I.H.; Hansen, J.E.; Kim, C.; Clements, D.; Weisbart, R.H.; Nishimura, R.N.; Sharp, F.R. Recombinant Fv-Hsp70 Protein Mediates Neuroprotection After Focal Cerebral Ischemia in Rats. Stroke 2010, 41, 538–543. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Kaltwasser, B.; Fengyan, J.; Hermann, D.M.; Bähr, M. TAT-Hsp70 Induces Neuroprotection Against Stroke Via Anti-Inflammatory Actions Providing Appropriate Cellular Microenvironment for Transplantation of Neural Precursor Cells. Br. J. Pharmacol. 2013, 33, 1778–1788. [Google Scholar] [CrossRef]
- Cappelletti, P.; Binda, E.; Tunesi, M.; Albani, D.; Giordano, C.; Molla, G.; Pollegioni, L. Recombinant human Tat-Hsp70-2: A tool for neuroprotection. Protein Expr. Purif. 2017, 138, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Kochanek, K.D.; Murphy, S.L.; Xu, J.; Arias, E. Deaths: Final Data for 2017. Natl. Vital Stat. Rep. 2019, 68, 1–77. [Google Scholar]
- Bayer, T.A. Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur. Neuropsychopharmacol. 2015, 25, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Briston, T.; Hicks, A.R. Mitochondrial dysfunction and neurodegenerative proteinopathies: Mechanisms and prospects for therapeutic intervention. Biochem. Soc. Trans. 2018, 46, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Ciccocioppo, F.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Simeone, P.; Pieragostino, D.; DEL Boccio, P.; Marchisio, M.; Miscia, S. Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen. Res. 2020, 15, 850–856. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef]
- Müller, U.C.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Saeed, M.U. Alzheimer’s disease. In Comprehensive Textbook of Geriatric Psychiatry, 3rd ed.; Sadavoy, J., Jarvik, L.F., Grossberg, G.T., Meyers, B.S., Eds.; WW Norton: New York, NY, USA, 2004; pp. 449–509. [Google Scholar]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis. J. Alzheimer’s Dis. 2010, 20, S265–S279. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Nazem, A.; Sankowski, R.; Bacher, M.; Al-Abed, Y. Rodent models of neuroinflammation for Alzheimer’s disease. J. Neuroinflamm. 2015, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Gulisano, W.; Palmeri, A.; Arancio, O. Rodent models for Alzheimer’s disease drug discovery. Expert Opin. Drug Discov. 2015, 10, 703–711. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef]
- Bobkova, N.V.; Nesterova, I.V.; Nesterov, V.V. The state of cholinergic structures in forebrain of bulbectomized mice. Bull. Exp. Biol. Med. 2001, 131, 427–431. [Google Scholar] [CrossRef]
- Holland, D.; Brewer, J.B.; Hagler, D.J.; Fennema-Notestine, C.; Dale, A.M.; Initiative, T.A.D.N. Subregional neuroanatomical change as a biomarker for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 20954–20959. [Google Scholar] [CrossRef]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris Water Maze Test for Learning and Memory Deficits in Alzheimer’s Disease Model Mice. J. Vis. Exp. 2011, 53, e2920. [Google Scholar] [CrossRef]
- Leak, R.K. Heat shock proteins in neurodegenerative disorders and aging. J. Cell Commun. Signal. 2014, 8, 293–310. [Google Scholar] [CrossRef]
- Manavalan, A.; Mishra, M.; Feng, L.; Sze, S.K.; Akatsu, H.; Heese, K. Brain site-specific proteome changes in aging-related dementia. Exp. Mol. Med. 2013, 45, e39. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Schwartz, D.; Gygi, S.P.; Kosik, K.S. CHIP-Hsc70 Complex Ubiquitinates Phosphorylated Tau and Enhances Cell Survival. J. Biol. Chem. 2004, 279, 4869–4876. [Google Scholar] [CrossRef]
- Petrucelli, L.; Dickson, D.; Kehoe, K.; Taylor, J.; Snyder, H.; Grover, A.; De Lucia, M.; McGowan, E.; Lewis, J.; Prihar, G.; et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004, 13, 703–714. [Google Scholar] [CrossRef]
- Kumar, P.; Jha, N.K.; Jha, S.K.; Ramani, K.; Ambasta, R.K. Tau Phosphorylation, Molecular Chaperones, and Ubiquitin E3 Ligase: Clinical Relevance in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 43, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.G.; Wisén, S.; Gestwicki, J.E. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J. Biol. Chem. 2006, 281, 33182–33191. [Google Scholar] [CrossRef] [PubMed]
- Rivera, I.; Capone, R.; Cauvi, D.M.; Arispe, N.; De Maio, A. Modulation of Alzheimer’s amyloid β peptide oligomerization and toxicity by extracellular Hsp70. Cell Stress Chaperones 2018, 23, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Magrané, J.; Smith, R.C.; Walsh, K.; Querfurth, H.W. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J. Neurosci. 2004, 24, 1700–1706. [Google Scholar] [CrossRef]
- Hoshino, T.; Murao, N.; Namba, T.; Takehara, M.; Adachi, H.; Katsuno, M.; Sobue, G.; Matsushima, T.; Suzuki, T.; Mizushima, T. Suppression of Alzheimer’s Disease-Related Phenotypes by Expression of Heat Shock Protein 70 in Mice. J. Neurosci. 2011, 31, 5225–5234. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Suzuki, K.; Matsushima, T.; Yamakawa, N.; Suzuki, T.; Mizushima, T. Suppression of Alzheimer’s Disease-Related Phenotypes by Geranylgeranylacetone in Mice. PLoS ONE 2013, 8, e76306. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.R.; Chen, S. Suppression of Alzheimer’s disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp. Ther. Med. 2017, 14, 5267–5274. [Google Scholar] [CrossRef]
- Verma, P.; Pfister, J.A.; Mallick, S.; D’Mello, S.R. HSF1 protects neurons through a novel trimerization- and HSP-independent mechanism. J. Neurosci. 2014, 34, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Bobkova, N.; Guzhova, I.; Margulis, B.; Nesterova, I.; Medvinskaya, N.; Medvedinskaya, N.; Samokhin, A.; Alexandrova, I.; Garbuz, D.; Nudler, E.; et al. Dynamics of endogenous Hsp70 synthesis in the brain of olfactory bulbectomized mice. Cell Stress Chaperones 2013, 18, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sulistio, Y.A.; Heese, K. The Ubiquitin-Proteasome System and Molecular Chaperone Deregulation in Alzheimer’s Disease. Mol. Neurobiol. 2015, 53, 905–931. [Google Scholar] [CrossRef] [PubMed]
- Auluck, P.K.; Bonini, N.M. Pharmacological prevention of Parkinson disease in Drosophila. Nat. Med. 2002, 8, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Klucken, J.; Shin, Y.; Masliah, E.; Hyman, B.T.; McLean, P.J. Hsp70 Reduces α-Synuclein Aggregation and Toxicity. J. Biol. Chem. 2004, 279, 25497–25502. [Google Scholar] [CrossRef]
- Flower, T.R.; Chesnokova, L.S.; Froelich, C.A.; Dixon, C.; Witt, S.N. Heat Shock Prevents Alpha-synuclein-induced Apoptosis in a Yeast Model of Parkinson’s Disease. J. Mol. Biol. 2005, 351, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Outeiro, T.F.; Putcha, P.; Tetzlaff, J.E.; Spoelgen, R.; Koker, M.; Carvalho, F.; Hyman, B.T.; McLean, P.J. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS ONE 2008, 3, e1867. [Google Scholar] [CrossRef]
- Tao, J.; Berthet, A.; Citron, Y.R.; Tsiolaki, P.L.; Stanley, R.; Gestwicki, J.E.; Agard, D.A.; McConlogue, L. Hsp70 chaperone blocks α-synuclein oligomer formation via a novel engagement mechanism. J. Biol. Chem. 2021, 296, 100613. [Google Scholar] [CrossRef]
- Davis, A.; Pratt, W.B.; Lieberman, A.P.; Osawa, Y. Targeting Hsp70 facilitated protein quality control for treatment of polyglutamine diseases. Cell. Mol. Life Sci. 2020, 77, 977–996. [Google Scholar] [CrossRef]
- Mays, C.E.; Armijo, E.; Morales, R.; Kramm, C.; Flores, A.; Tiwari, A.; Bian, J.; Telling, G.C.; Pandita, T.K.; Hunt, C.R.; et al. Prion disease is accelerated in mice lacking stress-induced heat shock protein 70 (HSP70). J. Biol. Chem. 2019, 294, 13619–13628. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Saidi, L.-J.; Wahlster, L. Molecular chaperones and protein folding as therapeutic targets in Parkinson’s disease and other synucleinopathies. Acta Neuropathol. Commun. 2013, 1, 79. [Google Scholar] [CrossRef]
- Kakimura, J.; Kitamura, Y.; Takata, K.; Umeki, M.; Suzuki, S.; Shibagaki, K.; Taniguchi, T.; Nomura, Y.; Gebicke-Haerter, P.J.; Smith, M.A.; et al. Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J. 2002, 16, 601–603. [Google Scholar] [CrossRef]
- De Mena, L.; Chhangani, D.; Fernandez-Funez, P.; Rincon-Limas, D.E. secHsp70 as a tool to approach amyloid-β42 and other extracellular amyloids. Fly 2017, 11, 179–184. [Google Scholar] [CrossRef]
- Evgen’Ev, M.B.; Krasnov, G.S.; Nesterova, I.V.; Garbuz, D.G.; Karpov, V.L.; Morozov, A.V.; Snezhkina, A.V.; Samokhin, A.N.; Sergeev, A.; Kulikov, A.M.; et al. Molecular Mechanisms Underlying Neuroprotective Effect of Intranasal Administration of Human Hsp70 in Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 59, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
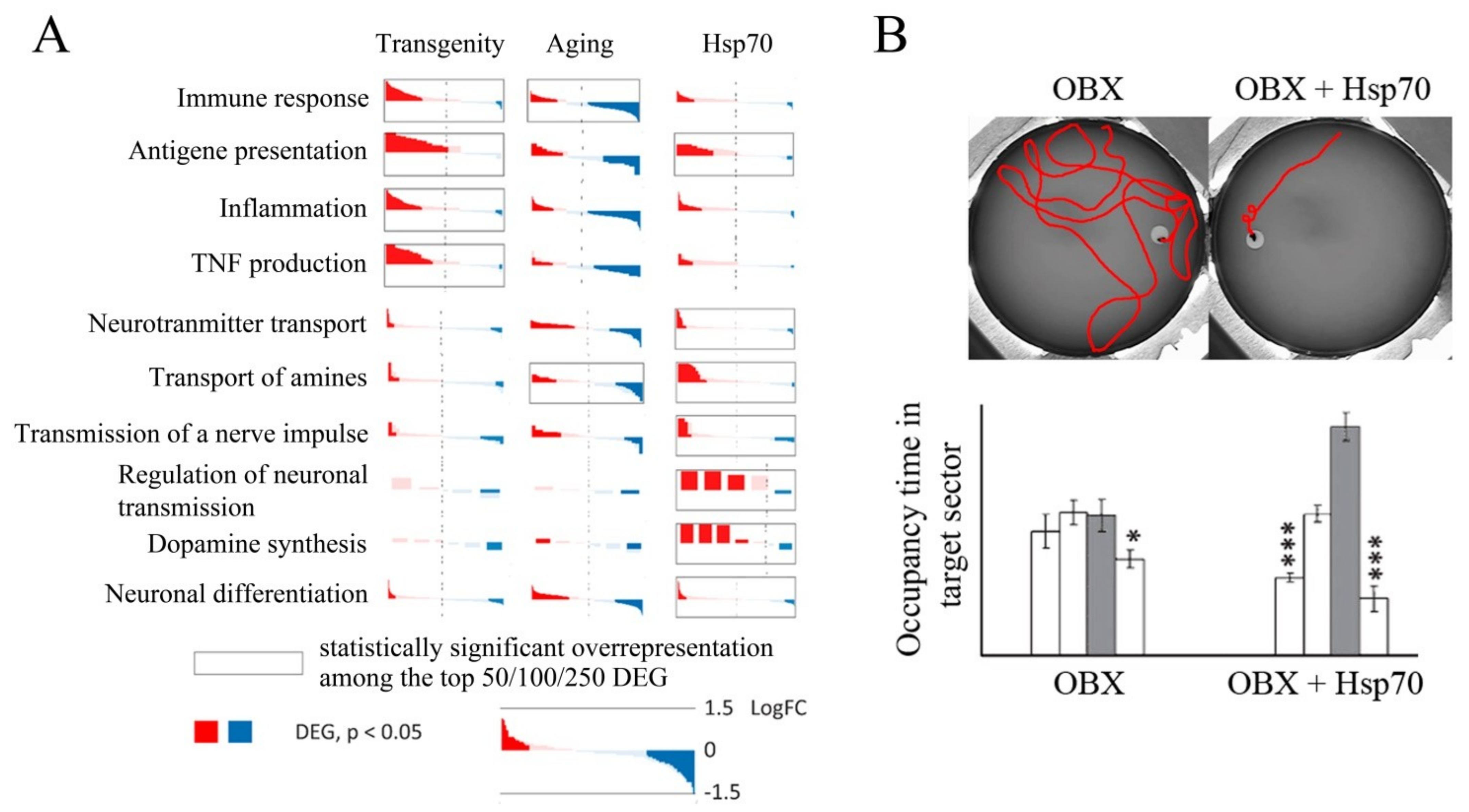
- Bobkova, N.V.; Evgen’Ev, M.; Garbuz, D.G.; Kulikov, A.M.; Morozov, A.; Samokhin, A.; Velmeshev, D.; Medvinskaya, N.; Nesterova, I.; Pollock, A.; et al. Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc. Natl. Acad. Sci. USA 2015, 112, 16006–16011. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, T.; Gray, J.; Priestman, D.A.; Wallom, K.-L.; Atkins, J.; Olsen, O.D.; Klein, A.; Drndarski, S.; Petersen, N.H.T.; Ingemann, L.; et al. Heat shock protein–based therapy as a potential candidate for treating the sphingolipidoses. Sci. Transl. Med. 2016, 8, 355ra118. [Google Scholar] [CrossRef]
- Kalmar, B.; Lu, C.-H.; Greensmith, L. The role of heat shock proteins in Amyotrophic Lateral Sclerosis: The therapeutic potential of Arimoclomol. Pharmacol. Ther. 2014, 141, 40–54. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zatsepina, O.G.; Evgen’ev, M.B.; Garbuz, D.G. Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection. Cells 2021, 10, 1638. https://doi.org/10.3390/cells10071638
Zatsepina OG, Evgen’ev MB, Garbuz DG. Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection. Cells. 2021; 10(7):1638. https://doi.org/10.3390/cells10071638
Chicago/Turabian StyleZatsepina, Olga G., Michael B. Evgen’ev, and David G. Garbuz. 2021. "Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection" Cells 10, no. 7: 1638. https://doi.org/10.3390/cells10071638
APA StyleZatsepina, O. G., Evgen’ev, M. B., & Garbuz, D. G. (2021). Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection. Cells, 10(7), 1638. https://doi.org/10.3390/cells10071638