The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers
Abstract
1. Introduction
1.1. Membrane Trafficking
1.2. Multiple Export Pathways from the TGN
2. PKD Is a Key Regulator of TGN Export and Golgi Lipid Homeostasis
2.1. Discovery of PKD as a Central Regulator of TGN Export
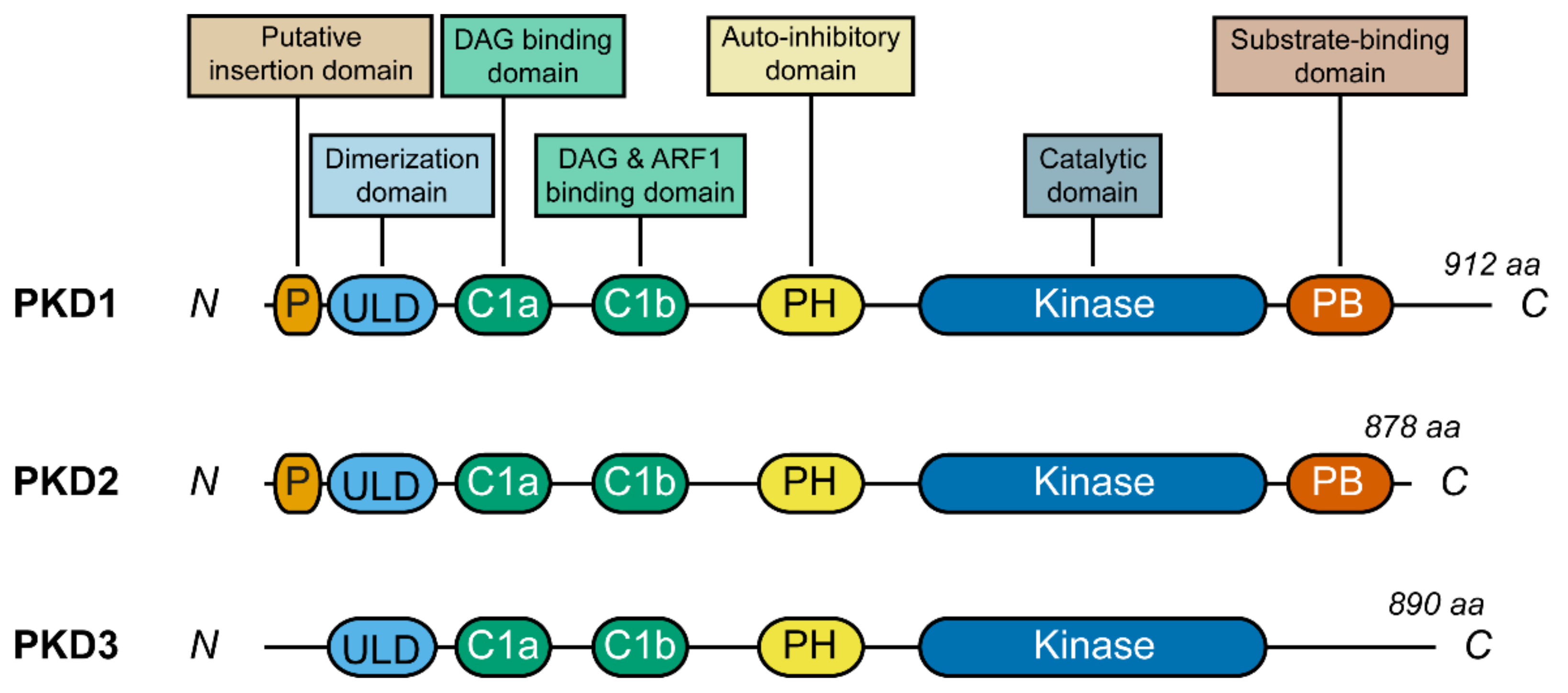
2.2. PKD Structure
2.3. PKD Roles in Constitutive and Regulated Protein Secretion
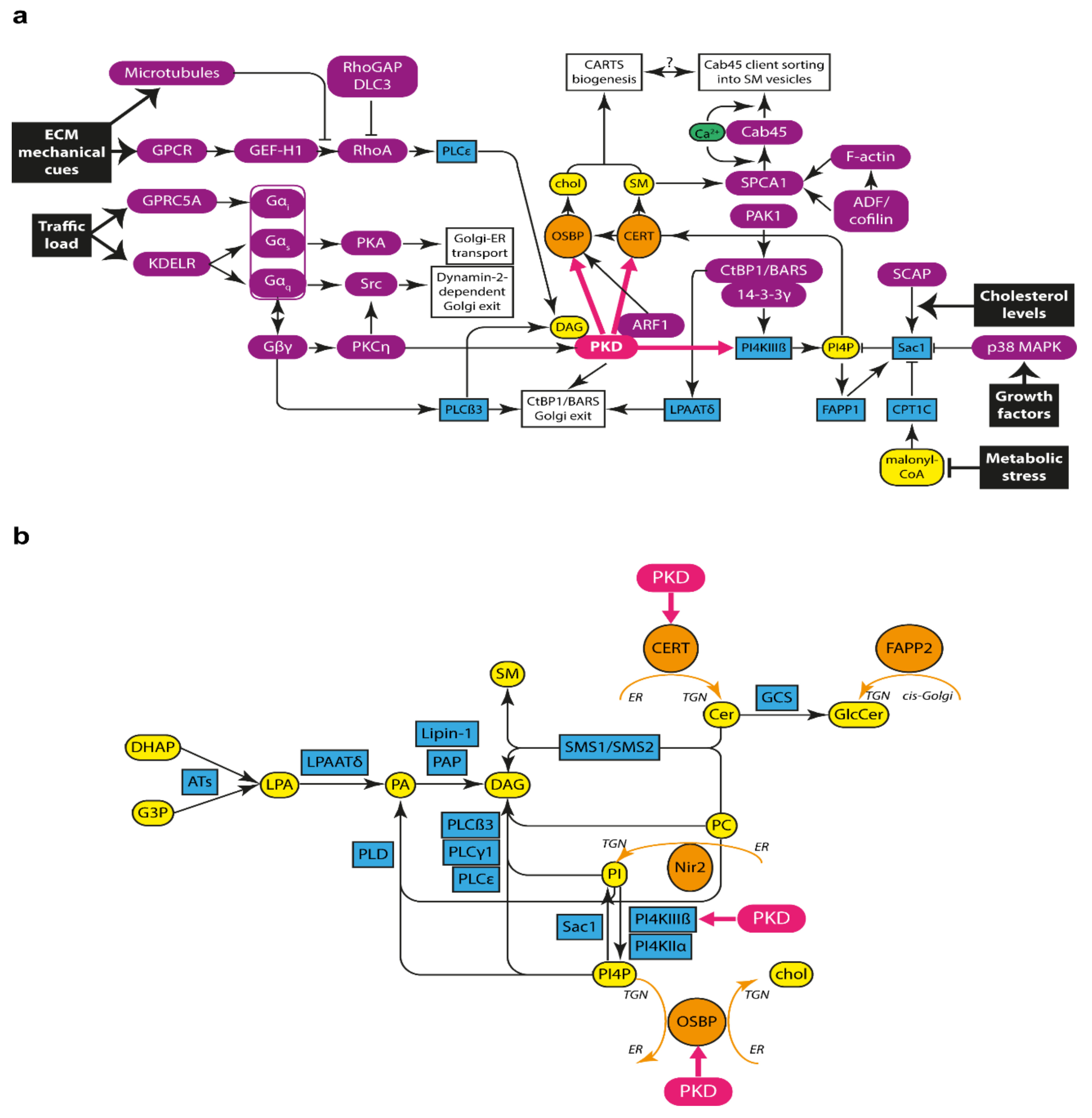
2.4. PKD Recruitment to the TGN and Activation
2.5. PKD Phosphorylates Substrates at the TGN
- PI4KIIIß: PKD phosphorylates PI4KIIIß, triggering its activation [83]. Active PI4KIIIß phosphorylates the lipid phosphatidylinositol (PI) in the 4-position to yield PI 4-phosphate (PI4P). Interestingly, it has been suggested that ARF1 plays a determinant role in the localization and activation of PI4KIIIß, hence underscoring the interconnection amongst all these components associated to PKD-mediated transport carrier formation [17].
- CERT: PKD phosphorylates the ceramide transport protein (CERT) [94]. CERT localizes to ER-Golgi membrane contact sites (MCSs), and it was the first lipid transfer protein (LTP) found to shuttle lipids between two distinct membranes without a vesicular intermediate [95]. CERT contains a canonical PH domain, which has specificity for binding to PI4P and hence targets this protein to the trans-Golgi/TGN membranes; an FFAT (two phenylalanines in an acidic tract) motif for interaction with the ER-localized vesicle-associated membrane protein-associated proteins (VAPs); and a steroidogenic acute regulatory protein-related lipid transfer (START) domain, which is responsible for the directional transfer of ceramide from the ER to the Golgi membranes [95]. Upon phosphorylation by PKD, CERT does not bind PI4P and is therefore released from the TGN, which supposedly improves the efficiency of non-vesicular lipid transfer by enhancing its dynamics at ER-Golgi MCSs.
- OSBP: Another substrate of PKD at ER-Golgi MCSs is the oxysterol-binding protein (OSBP) [96]. OSBP is a member of the OSBP-related protein (ORP)/oxysterol-binding homology (Osh) family of sterol sensor proteins. Similar to CERT, OSBP contains a PH domain that binds PI4P and also to ARF1-GTP, thus targeting OSBP to the late Golgi membranes; as well as an FFAT motif for trans ER anchoring through VAPs. In addition, OSBP contains an OSBP-related domain (ORD), which acts as a bidirectional lipid transfer domain that catalyzes the transport of cholesterol from the ER to the trans-Golgi membranes, and the reciprocal transport of PI4P from the Golgi to the ER membrane [97]. Mechanistically, there is experimental evidence suggesting that OSBP can function as a ferry-bridge protein, meaning that it combines its tethering function—by linking the ER and Golgi membranes together—with its lipid transfer function—by shuttling lipids between these two membranes [98]. Importantly, this ferry-bridge mode of action has also been proposed for other proteins with a similar domain structure, such as CERT [98]. Likewise to CERT, PKD-mediated phosphorylation of OSBP releases it from PI4P at the TGN for efficient lipid transfer [17,96].
3. CARTS Are PKD-Dependent Transport Carriers from the TGN to the Cell Surface
3.1. Isolation and Identification of CARTS
3.2. Features of CARTS Pathway
4. Lipid Requirements for PKD-Mediated CARTS Formation
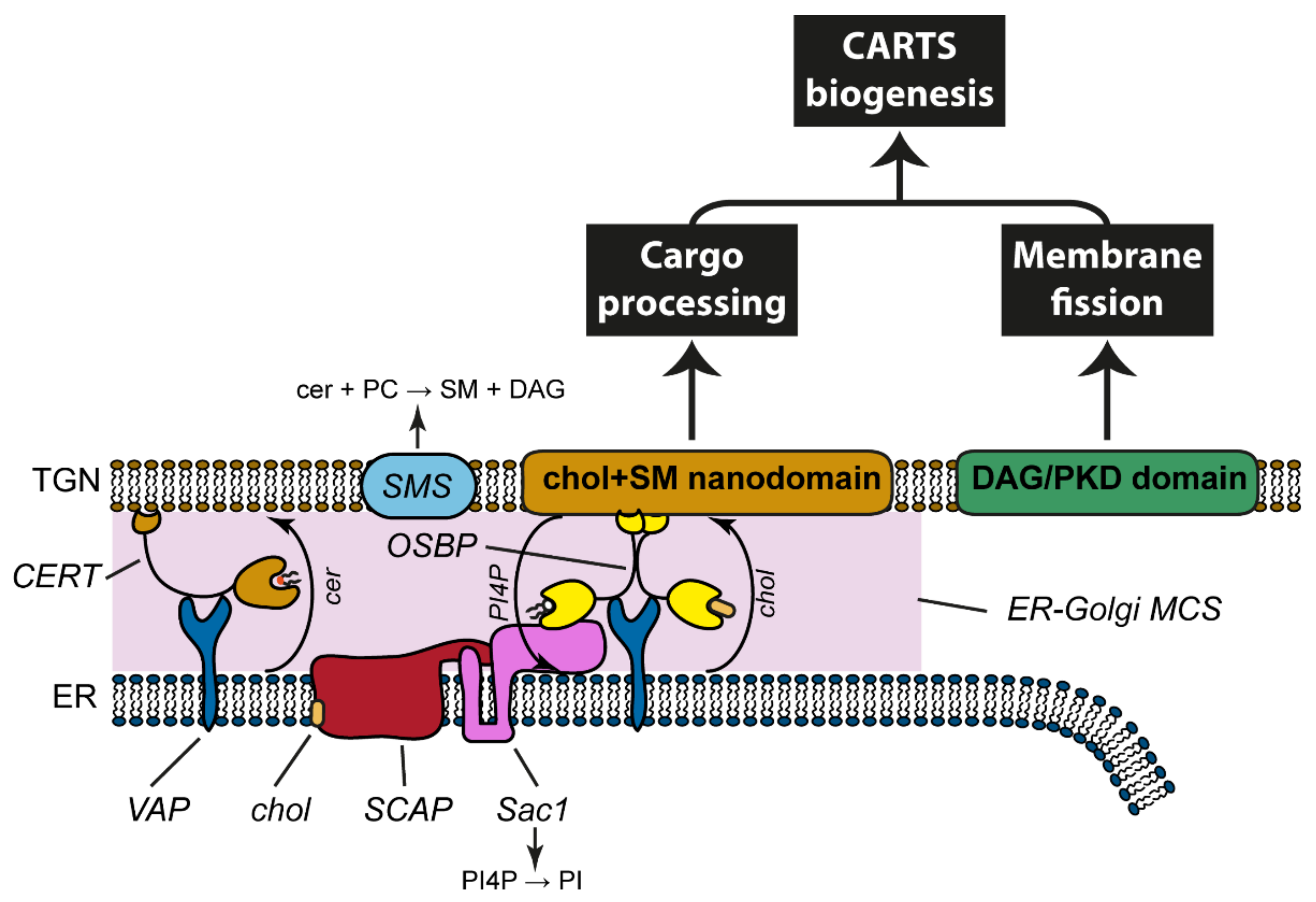
4.1. ER-Golgi MCSs Are a Platform for Non-Vesicular, Bi-Directional Trafficking of Lipids
4.2. The Cycle of PI4P at the ER-Golgi Interface
4.3. Sources of DAG at the TGN for PKD Recruitment and Activation
4.4. SM Metabolism and Cab45-Mediated Secretory Cargo Sorting at the TGN
5. Signaling at the Golgi Membranes for the Regulation of Protein Secretion
5.1. GPCR-Mediated Signaling
5.2. Sac1-Mediated Signaling
5.3. Mechanical Signals
6. Summary and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spoelstra, W.K.; Deshpande, S.; Dekker, C. Tailoring the appearance: What will synthetic cells look like? Curr. Opin. Biotechnol. 2018, 51, 47–56. [Google Scholar] [CrossRef]
- Spang, A. The Road not Taken: Less Traveled Roads from the TGN to the Plasma Membrane. Membranes 2015, 5, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, R.; Sticco, L.; Luini, A. Regulation of cargo export and sorting at the trans-Golgi network. FEBS Lett. 2019, 593, 2306–2318. [Google Scholar] [CrossRef]
- Luini, A.; Parashuraman, S. Signaling at the Golgi: Sensing and controlling the membrane fluxes. Curr. Opin. Cell Biol. 2016, 39, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cancino, J.; Jung, J.E.; Luini, A. Regulation of Golgi signaling and trafficking by the KDEL receptor. Histochem. Cell Biol. 2013, 140, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The Mechanisms of Vesicle Budding and Fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Matlin, K.S. Spatial expression of the genome: The signal hypothesis at forty. Nat. Rev. Mol. Cell Biol. 2011, 12, 333–340. [Google Scholar] [CrossRef]
- Barlowe, C.; Orci, L.; Yeung, T.; Hosobuchi, M.; Hamamoto, S.; Salama, N.; Rexach, M.F.; Ravazzola, M.; Amherdt, M.; Schekman, R. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 1994, 77, 895–907. [Google Scholar] [CrossRef]
- Emr, S.; Glick, B.S.; Linstedt, A.D.; Lippincott-Schwartz, J.; Luini, A.; Malhotra, V.; Marsh, B.J.; Nakano, A.; Pfeffer, S.R.; Rabouille, C.; et al. Journeys through the Golgi—Taking stock in a new era. J. Cell Biol. 2009, 449–453. [Google Scholar] [CrossRef]
- Klumperman, J. Architecture of the mammalian Golgi. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef]
- Lorente-Rodríguez, A.; Barlowe, C. Entry and exit mechanisms at the cis-face of the Golgi complex. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lujan, P.; Campelo, F. Should I stay or should I go? Golgi membrane spatial organization for protein sorting and retention. Arch. Biochem. Biophys. 2021, 707, 108921. [Google Scholar] [CrossRef]
- Welch, L.G.; Munro, S. A tale of short tails, through thick and thin: Investigating the sorting mechanisms of Golgi enzymes. FEBS Lett. 2019, 593, 2452–2465. [Google Scholar] [CrossRef]
- Banfield, D.K. Mechanisms of protein retention in the Golgi. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stalder, D.; Gershlick, D.C. Direct trafficking pathways from the Golgi apparatus to the plasma membrane. Semin. Cell Dev. Biol. 2020, 107, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sirkis, D.W.; Schekman, R. Protein sorting at the trans-Golgi network. Annu. Rev. Cell Dev. Biol. 2014, 30, 169–206. [Google Scholar] [CrossRef] [PubMed]
- von Blume, J.; Hausser, A. Lipid-dependent coupling of secretory cargo sorting and trafficking at the trans-Golgi network. FEBS Lett. 2019, 593, 2412–2427. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, C.; von Blume, J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014, 24, 584–593. [Google Scholar] [CrossRef]
- Ramazanov, B.R.; Tran, M.L.; von Blume, J. Sending out molecules from the TGN. Curr. Opin. Cell Biol. 2021, 71, 55–62. [Google Scholar] [CrossRef]
- Boncompain, G.; Weigel, A.V. Transport and sorting in the Golgi complex: Multiple mechanisms sort diverse cargo. Curr. Opin. Cell Biol. 2018, 50, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, P.A.; Yucel, J.K.; Veit, B.; Faulkner, D.J.; Deerinck, T.; Soto, G.; Ellisman, M.; Malhotra, V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 1993, 73, 1079–1090. [Google Scholar] [CrossRef]
- Jamora, C.; Takizawa, P.A.; Zaarour, R.F.; Denesvre, C.; Faulkner, D.J.; Malhotra, V. Regulation of Golgi structure through heterotrimeric G proteins. Cell 1997, 91, 617–626. [Google Scholar] [CrossRef]
- Jamora, C.; Yamanouye, N.; Van Lint, J.; Laudenslager, J.; Vandenheede, J.R.; Faulkner, D.J.; Malhotra, V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 1999, 98, 59–68. [Google Scholar] [CrossRef]
- Prestle, J.; Pfizenmaier, K.; Brenner, J.; Johannes, F.J. Protein kinase C μ is located at the Golgi compartment. J. Cell Biol. 1996, 134, 1401–1410. [Google Scholar] [CrossRef]
- Liljedahl, M.; Maeda, Y.; Colanzi, A.; Ayala, I.; Van Lint, J.; Malhotra, V. Protein Kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 2001, 104, 409–420. [Google Scholar] [CrossRef]
- Yeaman, C.; Ayala, M.I.; Wright, J.R.; Bard, F.; Bossard, C.; Ang, A.; Maeda, Y.; Seufferlein, T.; Mellman, I.; Nelson, W.J.; et al. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 2004, 6, 106–112. [Google Scholar] [CrossRef]
- Bossard, C.; Bresson, D.; Polishchuk, R.S.; Malhotra, V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J. Cell Biol. 2007, 179, 1123–1131. [Google Scholar] [CrossRef]
- Eiseler, T.; Döppler, H.; Yan, I.K.; Goodison, S.; Storz, P. Protein kinase D1 regulates matrix metalloproteinase expression and inhibits breast cancer cell invasion. Breast Cancer Res. 2009, 11, R13. [Google Scholar] [CrossRef] [PubMed]
- Eiseler, T.; Wille, C.; Koehler, C.; Illing, A.; Seufferlein, T. Protein kinase D2 assembles a multiprotein complex at the trans-Golgi network to regulate matrix metalloproteinase secretion. J. Biol. Chem. 2016, 291, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Bard, F.; Malhotra, V. The formation of TGN-to-plasma-membrane transport carriers. Annu. Rev. Cell Dev. Biol. 2006, 22, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, V.; Campelo, F. PKD regulates membrane fission to generate TGN to cell surface transport carriers. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Beznoussenko, G.V.; Van Lint, J.; Mironov, A.A.; Malhotra, V. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J. 2001, 20, 5982–5990. [Google Scholar] [CrossRef]
- Sumara, G.; Formentini, I.; Collins, S.; Sumara, I.; Windak, R.; Bodenmiller, B.; Ramracheya, R.; Caille, D.; Jiang, H.; Platt, K.A.; et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell 2009, 136, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Gehart, H.; Goginashvili, A.; Beck, R.; Morvan, J.; Erbs, E.; Formentini, I.; De Matteis, M.A.; Schwab, Y.; Wieland, F.T.; Ricci, R. The BAR Domain Protein Arfaptin-1 Controls Secretory Granule Biogenesis at the trans-Golgi Network. Dev. Cell 2012, 23, 756–768. [Google Scholar] [CrossRef]
- von Wichert, G.; Edenfeld, T.; von Blume, J.; Krisp, H.; Krndija, D.; Schmid, H.; Oswald, F.; Lother, U.; Walther, P.; Adler, G.; et al. Protein kinase D2 regulates chromogranin A secretion in human BON neuroendocrine tumour cells. Cell. Signal. 2008, 20, 925–934. [Google Scholar] [CrossRef]
- Cruz-Garcia, D.; Ortega-Bellido, M.; Scarpa, M.; Villeneuve, J.; Jovic, M.; Porzner, M.; Balla, T.; Seufferlein, T.; Malhotra, V. Recruitment of arfaptins to the trans-Golgi network by PI(4)P and their involvement in cargo export. EMBO J. 2013, 32, 1717–1729. [Google Scholar] [CrossRef]
- Bonazzi, M.; Spanò, S.; Turacchio, G.; Cericola, C.; Valente, C.; Colanzi, A.; Kweon, H.S.; Hsu, V.W.; Polishchuck, E.V.; Polishchuck, R.S.; et al. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 2005, 7, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Pagliuso, A.; Valente, C.; Giordano, L.L.; Filograna, A.; Li, G.; Circolo, D.; Turacchio, G.; Marzullo, V.M.; Mandrich, L.; Zhukovsky, M.A.; et al. Golgi membrane fission requires the CtBP1-S/BARS-induced activation of lysophosphatidic acid acyltransferase δ. Nat. Commun. 2016, 7, 12148. [Google Scholar] [CrossRef]
- Wakana, Y.; van Galen, J.; Meissner, F.; Scarpa, M.; Polishchuk, R.S.; Mann, M.; Malhotra, V. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. EMBO J. 2012, 31, 3976–3990. [Google Scholar] [CrossRef]
- Wakana, Y.; Villeneuve, J.; van Galen, J.; Cruz-Garcia, D.; Tagaya, M.; Malhotra, V. Kinesin-5/Eg5 is important for transport of CARTS from the trans-Golgi network to the cell surface. J. Cell Biol. 2013, 202, 241–250. [Google Scholar] [CrossRef]
- Wakana, Y.; Hayashi, K.; Nemoto, T.; Watanabe, C.; Taoka, M.; Angulo-Capel, J.; Garcia-Parajo, M.F.; Kumata, H.; Umemura, T.; Inoue, H.; et al. The ER cholesterol sensor SCAP promotes CARTS biogenesis at ER-Golgi membrane contact sites. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Rivera-Molina, F.E.; Toomre, D.K.; Burd, C.G. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc. Natl. Acad. Sci. USA 2016, 113, 6677–6682. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Pakdel, M.; Blank, B.; Sundberg, E.L.; Burd, C.G.; von Blume, J. Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network. Dev. Cell 2018, 47, 464–478.e8. [Google Scholar] [CrossRef] [PubMed]
- von Blume, J.; Alleaume, A.M.; Cantero-Recasens, G.; Curwin, A.; Carreras-Sureda, A.; Zimmermann, T.; van Galen, J.; Wakana, Y.; Valverde, M.A.; Malhotra, V. ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell 2011, 20, 652–662. [Google Scholar] [CrossRef] [PubMed]
- von Blume, J.; Alleaume, A.M.; Kienzle, C.; Carreras-Sureda, A.; Valverde, M.; Malhotra, V. Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol. 2012, 199, 1057–1066. [Google Scholar] [CrossRef]
- von Blume, J.; Duran, J.M.; Forlanelli, E.; Alleaume, A.M.; Egorov, M.; Polishchuk, R.; Molina, H.; Malhotra, V. Actin remodeling by ADF/cofilin is required for cargo sorting at the trans-Golgi network. J. Cell Biol. 2009, 187, 1055–1069. [Google Scholar] [CrossRef]
- Crevenna, A.H.; Blank, B.; Maiser, A.; Emin, D.; Prescher, J.; Beck, G.; Kienzle, C.; Bartnik, K.; Habermann, B.; Pakde, M.; et al. Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J. Cell Biol. 2016, 213, 305–314. [Google Scholar] [CrossRef]
- Sundberg, E.L.; Deng, Y.; Burd, C.G. Syndecan-1 Mediates Sorting of Soluble Lipoprotein Lipase with Sphingomyelin-Rich Membrane in the Golgi Apparatus. Dev. Cell 2019, 51, 387–398.e4. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, I.; Splinter, D.; Keijzer, N.; Wulf, P.S.; Demmers, J.; Ohtsuka, T.; Modesti, M.; Maly, I.V.; Grosveld, F.; Hoogenraad, C.C.; et al. Rab6 Regulates Transport and Targeting of Exocytotic Carriers. Dev. Cell 2007, 13, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Miserey-Lenkei, S.; Chalancon, G.; Bardin, S.; Formstecher, E.; Goud, B.; Echard, A. Rab and actomyosin-dependent fission of transport vesicles at the golgi complex. Nat. Cell Biol. 2010, 12, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, I.; Yu, K.L.; Martinez-Sanchez, E.; Serra-Marques, A.; Smal, I.; Meijering, E.; Demmers, J.; Peränen, J.; Pasterkamp, R.J.; van Der Sluijs, P.; et al. Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr. Biol. 2011, 21, 967–974. [Google Scholar] [CrossRef]
- Eisler, S.A.; Curado, F.; Link, G.; Schulz, S.; Noack, M.; Steinke, M.; Olayioye, M.A.; Hausser, A. A Rho signaling network links microtubules to PKD controlled carrier transport to focal adhesions. Elife 2018, 7, e35907. [Google Scholar] [CrossRef]
- Fourriere, L.; Kasri, A.; Gareil, N.; Bardin, S.; Bousquet, H.; Pereira, D.; Perez, F.; Goud, B.; Boncompain, G.; Miserey-Lenkei, S. RAB6 and microtubules restrict protein secretion to focal adhesions. J. Cell Biol. 2019, 218, 2215–2231. [Google Scholar] [CrossRef]
- Micaroni, M.; Stanley, A.C.; Khromykh, T.; Venturato, J.; Wong, C.X.F.; Lim, J.P.; Marsh, B.J.; Storrie, B.; Gleeson, P.A.; Stow, J.L. Rab6a/a’ Are Important Golgi Regulators of Pro-Inflammatory TNF Secretion in Macrophages. PLoS ONE 2013, 8, e57034. [Google Scholar] [CrossRef]
- Johns, H.L.; Gonzalez-Lopez, C.; Sayers, C.L.; Hollinshead, M.; Elliott, G. Rab6 Dependent Post-Golgi Trafficking of HSV1 Envelope Proteins to Sites of Virus Envelopment. Traffic 2014, 15, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Huet-Calderwood, C.; Rivera-Molina, F.; Iwamoto, D.V.; Kromann, E.B.; Toomre, D.; Calderwood, D.A. Novel ecto-tagged integrins reveal their trafficking in live cells. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stehbens, S.J.; Paszek, M.; Pemble, H.; Ettinger, A.; Gierke, S.; Wittmann, T. CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat. Cell Biol. 2014, 16, 558–570. [Google Scholar] [CrossRef]
- Fu, Y.; Rubin, C.S. Protein kinase D: Coupling extracellular stimuli to the regulation of cell physiology. EMBO Rep. 2011, 12, 785–796. [Google Scholar] [CrossRef]
- Reinhardt, R.; Truebestein, L.; Schmidt, H.A.; Leonard, T.A. It Takes Two to Tango: Activation of Protein Kinase D by Dimerization. BioEssays 2020, 42, 1900222. [Google Scholar] [CrossRef] [PubMed]
- Döppler, H.; Storz, P. A novel tyrosine phosphorylation site in protein kinase D contributes to oxidative stress-mediated activation. J. Biol. Chem. 2007, 282, 31873–31881. [Google Scholar] [CrossRef]
- Zhang, Z.; Meszaros, G.; He, W.T.; Xu, Y.; de Fatima Magliarelli, H.; Mailly, L.; Mihlan, M.; Liu, Y.; Gámez, M.P.; Goginashvili, A.; et al. Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J. Exp. Med. 2017, 214, 2671–2693. [Google Scholar] [CrossRef] [PubMed]
- Baron, C.L.; Malhotra, V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 2002, 295, 325–328. [Google Scholar] [CrossRef]
- Griner, E.M.; Kazanietz, M.G. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 2007, 7, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Fielitz, J.; Kim, M.S.; Shelton, J.M.; Qi, X.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc. Natl. Acad. Sci. USA 2008, 105, 3059–3063. [Google Scholar] [CrossRef]
- Matthews, S.A.; Navarro, M.N.; Sinclair, L.V.; Emslie, E.; Feijoo-Carnero, C.; Cantrell, D.A. Unique functions for protein kinase D1 and protein kinase D2 in mammalian cells. Biochem. J. 2010, 432, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Atik, N.; Kunii, M.; Avriyanti, E.; Furumoto, N.; Inami, K.; Yoshimura, S.I.; Harada, R.; Harada, A. The role of PKD in cell polarity, biosynthetic pathways, and organelle/f-actin distribution. Cell Struct. Funct. 2014, 39, 61–77. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yin, M.; Pei, X.; Hausser, A.; Ishikawa, E.; Yamasaki, S.; Jin, Z.G. Deletion of Protein Kinase D3 Promotes Liver Fibrosis in Mice. Hepatology 2020, 72, 1717–1734. [Google Scholar] [CrossRef]
- Rozengurt, E. Protein kinase D signaling: Multiple biological functions in health and disease. Physiology 2011, 26, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, J.; Rykx, A.; Maeda, Y.; Vantus, T.; Sturany, S.; Malhotra, V.; Vandenheede, J.R.; Seufferlein, T. Protein kinase D: An intracellular traffic regulator on the move. Trends Cell Biol. 2002, 12, 193–200. [Google Scholar] [CrossRef]
- Elsner, D.J.; Siess, K.M.; Gossenreiter, T.; Hartl, M.; Leonard, T.A. A ubiquitin-like domain controls protein kinase D dimerization and activation by trans-autophosphorylation. J. Biol. Chem. 2019, 294, 14422–14441. [Google Scholar] [CrossRef]
- Aicart-Ramos, C.; He, S.D.Q.; Land, M.; Rubin, C.S. A novel conserved domain mediates dimerization of protein kinase D (PKD) isoforms: Dimerization is essential for PKD-dependent regulation of secretion and innate immunity. J. Biol. Chem. 2016, 291, 23516–23531. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, F.; Li, J.; Wang, Q.J. Selective binding of phorbol esters and diacylglycerol by individual C1 domains of the PKD family. Biochem. J. 2008, 411, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Pusapati, G.V.; Krndija, D.; Armacki, M.; von Wichert, G.; von Blume, J.; Malhotra, V.; Adler, G.; Seufferlein, T. Role of the second cysteine-rich domain and Pro275 in protein kinase D2 interaction with ADP-ribosylation factor 1, trans-Golgi network recruitment, and protein transport. Mol. Biol. Cell 2010, 21, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, T.; Rozengurt, E. Protein kinase D activation by mutations within its pleckstrin homology domain. J. Biol. Chem. 1998, 273, 410–416. [Google Scholar] [CrossRef]
- Sánchez-Ruiloba, L.; Cabrera-Poch, N.; Rodríguez-Martínez, M.; López-Menéndez, C.; Jean-Mairet, R.M.; Higuero, A.M.; Iglesias, T. Protein kinase D intracellular localization and activity control kinase D-interacting substrate of 220-kDa traffic through a postsynaptic density-95/discs large/zonula occludens-1-binding motif. J. Biol. Chem. 2006, 281, 18888–18900. [Google Scholar] [CrossRef]
- Wang, Q.J. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 2006, 27, 317–323. [Google Scholar] [CrossRef]
- Zhang, X.; Connelly, J.; Chao, Y.; Wang, Q.J. Multifaceted functions of protein kinase d in pathological processes and human diseases. Biomolecules 2021, 11, 483. [Google Scholar] [CrossRef]
- Pasquier, A.; Vivot, K.; Erbs, E.; Spiegelhalter, C.; Zhang, Z.; Aubert, V.; Liu, Z.; Senkara, M.; Maillard, E.; Pinget, M.; et al. Lysosomal degradation of newly formed insulin granules contributes to β cell failure in diabetes. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Weigert, R.; Silletta, M.G.; Spano, S.; Turacchio, G.; Cericola, C.; Colanzi, A.; Senatore, S.; Mancini, R.; Polishchuk, E.V.; Salmona, M.; et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 1999, 402, 429–433. [Google Scholar] [CrossRef]
- Spanfò, S.; Silletta, M.G.; Colanzi, A.; Alberti, S.; Fiucci, G.; Valente, C.; Fusella, A.; Salmona, M.; Mironov, A.; Luini, A.; et al. Molecular cloning and functional characterization of brefeldin A-ADP-ribosylated substrate: A novel protein involved in the maintenance of the golgi structure. J. Biol. Chem. 1999, 274, 17705–17710. [Google Scholar] [CrossRef]
- Valente, C.; Turacchio, G.; Mariggió, S.; Pagliuso, A.; Gaibisso, R.; Di Tullio, G.; Santoro, M.; Formiggini, F.; Spanó, S.; Piccini, D.; et al. A 14-3-3γ 3 dimer-based scaffold bridges CtBP1-S/BARS to PI(4)KIIIβ 2 to regulate post-Golgi carrier formation. Nat. Cell Biol. 2012, 14, 343–354. [Google Scholar] [CrossRef]
- Miserey-Lenkei, S.; Bousquet, H.; Pylypenko, O.; Bardin, S.; Dimitrov, A.; Bressanelli, G.; Bonifay, R.; Fraisier, V.; Guillou, C.; Bougeret, C.; et al. Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Hausser, A.; Storz, P.; Martens, S.; Link, G.; Toker, A.; Pfizenmaier, K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat. Cell Biol. 2005, 7, 880–886. [Google Scholar] [CrossRef]
- Hausser, A.; Link, G.; Hoene, M.; Russo, C.; Selchow, O.; Pfizenmaier, K. Phospho-specific binding of 14-3-3 proteins to phosphatidylinositol 4-kinase III β protects from dephosphorylation and stabilizes lipid kinase activity. J. Cell Sci. 2006, 119, 3613–3621. [Google Scholar] [CrossRef]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. The structure and function of acylglycerophosphate acyltransferase 4/lysophosphatidic acid acyltransferase delta (AGPAT4/LPAATδ). Front. Cell Dev. Biol. 2019, 7, 147. [Google Scholar] [CrossRef]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Phosphatidic acid in membrane rearrangements. FEBS Lett. 2019, 593, 2428–2451. [Google Scholar] [CrossRef] [PubMed]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Protein Amphipathic Helix Insertion: A Mechanism to Induce Membrane Fission. Front. Cell Dev. Biol. 2019, 7, 291. [Google Scholar] [CrossRef]
- Valente, C.; Luini, A.; Corda, D. Components of the CtBP1/BARS-dependent fission machinery. Histochem. Cell Biol. 2013, 140, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Bottanelli, F.; Kilian, N.; Ernst, A.M.; Rivera-Molina, F.; Schroeder, L.K.; Kromann, E.B.; Lessard, M.D.; Erdmann, R.S.; Schepartz, A.; Baddeley, D.; et al. A novel physiological role for ARF1 in the formation of bidirectional tubules from the Golgi. Mol. Biol. Cell 2017, 28, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Campelo, F.; Malhotra, V. Membrane fission: The biogenesis of transport carriers. Annu. Rev. Biochem. 2012, 81, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Jacamo, R.; Sinnett-Smith, J.; Rey, O.; Waldron, R.T.; Rozengurt, E. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: Differential regulation of activation loop Ser744 and Ser748 phosphorylation. J. Biol. Chem. 2008, 283, 12877–12887. [Google Scholar] [CrossRef] [PubMed]
- Diaz Anel, A.M.; Malhotra, V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 2005, 169, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, M.T.; Newton, A.C. Calcium transduces plasma membrane receptor signals to produce diacylglycerol at Golgi membranes. J. Biol. Chem. 2010, 285, 22748–22752. [Google Scholar] [CrossRef]
- Fugmann, T.; Hausser, A.; Schöffler, P.; Schmid, S.; Pfizenmaier, K.; Olayioye, M.A. Regulation of secretory transport by protein kinase D-mediated phosphorylation of the ceramide transfer protein. J. Cell Biol. 2007, 178, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Kumagai, K.; Yasuda, S.; Miura, Y.; Kawano, M.; Fukasawa, M.; Nishijima, M. Molecular machinery for non-vesicular trafficking of ceramide. Nature 2003, 426, 803–809. [Google Scholar] [CrossRef]
- Nhek, S.; Ngo, M.; Yang, X.; Ng, M.M.; Field, S.J.; Asara, J.M.; Ridgway, N.D.; Toker, A. Regulation of oxysterol-binding protein Golgi localization through protein kinase D-mediated phosphorylation. Mol. Biol. Cell 2010, 21, 2327–2337. [Google Scholar] [CrossRef]
- Mesmin, B.; Bigay, J.; Moser von Filseck, J.; Lacas-Gervais, S.; Drin, G.; Antonny, B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi Tether OSBP. Cell 2013, 155, 830. [Google Scholar] [CrossRef]
- Antonny, B.; Bigay, J.; Mesmin, B. The Oxysterol-Binding Protein Cycle: Burning off PI(4)P to Transport Cholesterol. Annu. Rev. Biochem. 2018, 87, 809–837. [Google Scholar] [CrossRef]
- Sharp, D.J.; McDonald, K.L.; Brown, H.M.; Matthies, H.J.; Walczak, C.; Vale, R.D.; Mitchison, T.J.; Scholey, J.M. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J. Cell Biol. 1999, 144, 125–138. [Google Scholar] [CrossRef]
- Kapoor, T.M.; Mitchison, T.J. Eg5 is static in bipolar spindles relative to tubulin: Evidence for a static spindle matrix. J. Cell Biol. 2001, 154, 1125–1133. [Google Scholar] [CrossRef]
- Kwok, B.H.; Yang, J.G.; Kapoor, T.M. The rate of bipolar spindle assembly depends on the microtubule-gliding velocity of the mitotic kinesin Eg5. Curr. Biol. 2004, 14, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Perlman, Z.E.; Burbank, K.S.; Groen, A.C.; Mitchison, T.J. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J. Cell Biol. 2004, 167, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Kapitein, L.C.; Peterman, E.J.G.; Kwok, B.H.; Kim, J.H.; Kapoor, T.M.; Schmidt, C.F. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 2005, 435, 114–118. [Google Scholar] [CrossRef]
- Brust-Mascher, I.; Sommi, P.; Cheerambathur, D.K.; Scholey, J.M. Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis. Mol. Biol. Cell 2009, 20, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hu, R.; Brissova, M.; Stein, R.W.; Powers, A.C.; Gu, G.; Kaverina, I. Microtubules Negatively Regulate Insulin Secretion in Pancreatic β Cells. Dev. Cell 2015, 34, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. Cargo carriers from the Golgi to the cell surface. EMBO J. 2012, 31, 3954–3955. [Google Scholar] [CrossRef]
- Pakdel, M.; von Blume, J. Exploring new routes for secretory protein export from the trans-Golgi network. Mol. Biol. Cell 2018, 29, 235–240. [Google Scholar] [CrossRef]
- Wakana, Y.; Kotake, R.; Oyama, N.; Murate, M.; Kobayashi, T.; Arasaki, K.; Inoue, H.; Tagaya, M. CARTS biogenesis requires VAP–lipid transfer protein complexes functioning at the endoplasmic reticulum–Golgi interface. Mol. Biol. Cell 2015, 26, 4686–4699. [Google Scholar] [CrossRef]
- Duran, J.M.; Campelo, F.; van Galen, J.; Sachsenheimer, T.; Sot, J.; Egorov, M.V.; Rentero, C.; Enrich, C.; Polishchuk, R.S.; Goñi, F.M.; et al. Sphingomyelin organization is required for vesicle biogenesis at the Golgi complex. EMBO J. 2012, 31, 4535–4546. [Google Scholar] [CrossRef]
- van Galen, J.; Campelo, F.; Martínez-Alonso, E.; Scarpa, M.; Martínez-Menárguez, J.A.; Malhotra, V. Sphingomyelin homeostasis is required to form functional enzymatic domains at the trans-Golgi network. J. Cell Biol. 2014, 206, 609–618. [Google Scholar] [CrossRef]
- Keller, P.; Simons, K. Cholesterol Is Required for Surface Transport of Influenza Virus Hemagglutinin. J. Cell Biol. 1998, 140, 1357–1367. [Google Scholar] [CrossRef]
- Klemm, R.W.; Ejsing, C.S.; Surma, M.A.; Kaiser, H.J.; Gerl, M.J.; Sampaio, J.L.; De Robillard, Q.; Ferguson, C.; Proszynski, T.J.; Shevchenko, A.; et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J. Cell Biol. 2009, 185, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Liu, P.; Lagerholm, B.C. The Lateral Organization and Mobility of Plasma Membrane Components. Cell 2019, 177, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Campelo, F.; van Galen, J.; Turacchio, G.; Parashuraman, S.; Kozlov, M.M.; García-Parajo, M.F.; Malhotra, V. Sphingomyelin metabolism controls the shape and function of the Golgi cisternae. Elife 2017, 6. [Google Scholar] [CrossRef]
- Capasso, S.; Sticco, L.; Rizzo, R.; Pirozzi, M.; Russo, D.; Dathan, N.A.; Campelo, F.; Galen, J.; Hölttä-Vuori, M.; Turacchio, G.; et al. Sphingolipid metabolic flow controls phosphoinositide turnover at the trans-Golgi network. EMBO J. 2017, 36, 1736–1754. [Google Scholar] [CrossRef]
- Peretti, D.; Dahan, N.; Shimoni, E.; Hirschberg, K.; Lev, S. Coordinated lipid transfer between the endoplasmic reticulum and the golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol. Biol. Cell 2008, 19, 3871–3884. [Google Scholar] [CrossRef]
- Lujan, P.; Angulo-Capel, J.; Chabanon, M.; Campelo, F. Interorganelle communication and membrane shaping in the early secretory pathway. Curr. Opin. Cell Biol. 2021, 71, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Kovacs, D.; D’Angelo, G. Lipid exchange and signaling at ER–Golgi contact sites. Curr. Opin. Cell Biol. 2019, 57, 8–15. [Google Scholar] [CrossRef]
- Venditti, R.; Masone, M.C.; De Matteis, M.A. ER-Golgi membrane contact sites. Biochem. Soc. Trans. 2020, 48, 187–197. [Google Scholar] [CrossRef]
- Phillips, M.J.; Voeltz, G.K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 2016, 17, 69–82. [Google Scholar] [CrossRef]
- Litvak, V.; Dahan, N.; Ramachandran, S.; Sabanay, H.; Lev, S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol. 2005, 7, 225–234. [Google Scholar] [CrossRef]
- Lin, X.; Mattjus, P.; Pike, H.M.; Windebank, A.J.; Brown, R.E. Cloning and expression of glycolipid transfer protein from bovine and porcine brain. J. Biol. Chem. 2000, 275, 5104–5110. [Google Scholar] [CrossRef]
- Mikitova, V.; Levine, T.P. Analysis of the Key Elements of FFAT-Like Motifs Identifies New Proteins That Potentially Bind VAP on the ER, Including Two AKAPs and FAPP2. PLoS ONE 2012, 7, e30455. [Google Scholar] [CrossRef]
- D’Angelo, G.; Polishchuk, E.; Di Tullio, G.; Santoro, M.; Di Campli, A.; Godi, A.; West, G.; Bielawski, J.; Chuang, C.C.; van der Spoel, A.C.; et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 2007, 449, 62–67. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Uemura, T.; Chuang, C.C.; Polishchuk, E.; Santoro, M.; Ohvo-Rekilä, H.; Sato, T.; Di Tullio, G.; Varriale, A.; D’Auria, S.; et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature 2013, 501, 116–120. [Google Scholar] [CrossRef]
- Venditti, R.; Masone, M.C.; Rega, L.R.; Di Tullio, G.; Santoro, M.; Polishchuk, E.; Serrano, I.C.; Olkkonen, V.M.; Harada, A.; Medina, D.L.; et al. The activity of Sac1 across ER–TGN contact sites requires the four-phosphate-adaptor-protein-1. J. Cell Biol. 2019, 218, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Beznoussenko, G.V.; Parashuraman, S.; Rizzo, R.; Polishchuk, R.; Martella, O.; Di Giandomenico, D.; Fusella, A.; Spaar, A.; Sallese, M.; Capestrano, M.G.; et al. Transport of soluble proteins through the Golgi occurs by diffusion via continuities across cisternae. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.M.; Syed, S.A.; Zaki, O.; Bottanelli, F.; Zheng, H.; Hacke, M.; Xi, Z.; Rivera-Molina, F.; Graham, M.; Rebane, A.A.; et al. S-Palmitoylation Sorts Membrane Cargo for Anterograde Transport in the Golgi. Dev. Cell 2018, 47, 479–493.e7. [Google Scholar] [CrossRef]
- Reinisch, K.M.; Prinz, W.A. Mechanisms of nonvesicular lipid transport. J. Cell Biol. 2021, 220. [Google Scholar] [CrossRef]
- Balla, A.; Balla, T. Phosphatidylinositol 4-kinases: Old enzymes with emerging functions. Trends Cell Biol. 2006, 16, 351–361. [Google Scholar] [CrossRef]
- Boura, E.; Nencka, R. Phosphatidylinositol 4-kinases: Function, structure, and inhibition. Exp. Cell Res. 2015, 337, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Weixel, K.M.; Blumental-Perry, A.; Watkins, S.C.; Aridor, M.; Weisz, O.A. Distinct golgi populations of phosphatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases. J. Biol. Chem. 2005, 280, 10501–10508. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Meyers, R.; Cantley, L.C. Subcellular locations of phosphatidylinositol 4-kinase isoforms. J. Biol. Chem. 1997, 272, 13236–13241. [Google Scholar] [CrossRef]
- Carrasco, S.; Merida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef]
- Díaz Añel, A.M. Phospholipase C β3 is a key component in the Gβγ/PKCη/ PKD-mediated regulation of trans-Golgi network to plasma membrane transport. Biochem. J. 2007, 406, 157–165. [Google Scholar] [CrossRef]
- Sicart, A.; Katan, M.; Egea, G.; Sarri, E. PLCγ1 Participates in Protein Transport and Diacylglycerol Production Triggered by cargo Arrival at the Golgi. Traffic 2015, 16, 250–266. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Zimmerberg, J.; Kozlov, M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006, 7, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.A.; Gutowski, S.; Moomaw, C.R.; Slaughter, C.; Sternweis, P.C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 1993, 75, 1137–1144. [Google Scholar] [CrossRef]
- Riebeling, C.; Morris, A.J.; Shields, D. Phospholipase D in the Golgi apparatus. Biochim. Biophys. Acta 2009, 1791, 876–880. [Google Scholar] [CrossRef]
- Sonoda, H.; Okada, T.; Jahangeer, S.; Nakamura, S.I. Requirement of phospholipase D for ilimaquinone-induced Golgi membrane fragmentation. J. Biol. Chem. 2007, 282, 34085–34092. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Roth, M.G.; Ktistakis, N.T. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr. Biol. 1997, 7, 301–307. [Google Scholar] [CrossRef]
- Hecht, T.K.H.; Blank, B.; Steger, M.; Lopez, V.; Beck, G.; Ramazanov, B.; Mann, M.; Tagliabracci, V.; von Blume, J. Fam20C regulates protein secretion by Cab45 phosphorylation. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Eiseler, T.; Döppler, H.; Yan, I.K.; Kitatani, K.; Mizuno, K.; Storz, P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat. Cell Biol. 2009, 11, 545–556. [Google Scholar] [CrossRef]
- Curwin, A.J.; von Blume, J.; Malhotra, V. Cofilin-mediated sorting and export of specific cargo from the Golgi apparatus in yeast. Mol. Biol. Cell 2012, 23, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Apodaca, G.; Brown, W.J. Membrane traffic research: Challenges for the next decade. Front. Cell Dev. Biol. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Galli, T. Reciprocal link between cell biomechanics and exocytosis. Traffic 2018, 19, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Irannejad, R.; Wedegaertner, P.B. Regulation of constitutive cargo transport from the trans-golgi network to plasma membrane by golgi-localized G protein bg subunits. J. Biol. Chem. 2010. [Google Scholar] [CrossRef]
- Solis, G.P.; Bilousov, O.; Koval, A.; Lüchtenborg, A.M.; Lin, C.; Katanaev, V.L. Golgi-Resident Gαo Promotes Protrusive Membrane Dynamics. Cell 2017, 170, 939–955.e24. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Karunarathne, W.K.; Angaswamy, N.; Saini, D.; Cho, J.H.; Kalyanaraman, V.; Gautam, N. Regulation of Golgi structure and secretion by receptor-induced G protein betagamma complex translocation. Proc. Natl. Acad. Sci. USA 2010, 107, 11417–11422. [Google Scholar] [CrossRef] [PubMed]
- Capitani, M.; Sallese, M. The KDEL receptor: New functions for an old protein. FEBS Lett. 2009, 583, 3863–3871. [Google Scholar] [CrossRef]
- Giannotta, M.; Ruggiero, C.; Grossi, M.; Cancino, J.; Capitani, M.; Pulvirenti, T.; Consoli, G.M.L.; Geraci, C.; Fanelli, F.; Luini, A.; et al. The KDEL receptor couples to Gα q/11 to activate Src kinases and regulate transport through the Golgi. EMBO J. 2012, 31, 2869–2881. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, T.; Giannotta, M.; Capestrano, M.; Capitani, M.; Pisanu, A.; Polishchuk, R.S.; Pietro, E.S.; Beznoussenko, G.V.; Mironov, A.A.; Turacchio, G.; et al. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat. Cell Biol. 2008, 10, 912–922. [Google Scholar] [CrossRef]
- Weller, S.G.; Capitani, M.; Cao, H.; Micaroni, M.; Luini, A.; Sallese, M.; McNiven, M.A. Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2. Proc. Natl. Acad. Sci. USA 2010, 107, 5863–5868. [Google Scholar] [CrossRef]
- Cancino, J.; Capalbo, A.; DiCampli, A.; Giannotta, M.; Rizzo, R.; Jung, J.E.; DiMartino, R.; Persico, M.; Heinklein, P.; Sallese, M.; et al. Control systems of membrane transport at the interface between the endoplasmic reticulum and the Golgi. Dev. Cell 2014, 30, 280–294. [Google Scholar] [CrossRef]
- Di Martino, R.; Capalbo, A.; Sticco, L.; Varavallo, A.; Kunnathully, V.; De Luca, V.; Iyengar, N.R.; Monte, M.L.; Henklein, P.; Cancino, J.; et al. Autoregulatory circuit regulating basolateral cargo export from the TGN: Role of the orphan receptor GPRC5A in PKD signaling and cell polarity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Subramanian, A.; Capalbo, A.; Iyengar, N.R.; Rizzo, R.; di Campli, A.; Di Martino, R.; Lo Monte, M.; Beccari, A.R.; Yerudkar, A.; del Vecchio, C.; et al. Auto-regulation of Secretory Flux by Sensing and Responding to the Folded Cargo Protein Load in the Endoplasmic Reticulum. Cell 2019, 176, 1461–1476.e23. [Google Scholar] [CrossRef] [PubMed]
- Blagoveshchenskaya, A.; Fei, Y.C.; Rohde, H.M.; Glover, G.; Knödler, A.; Nicolson, T.; Boehmelt, G.; Mayinger, P. Integration of Golgi trafficking and growth factor signaling by the lipid phosphatase SAC1. J. Cell Biol. 2008, 180, 803–812. [Google Scholar] [CrossRef]
- Casas, M.; Fadó, R.; Domínguez, J.L.; Roig, A.; Kaku, M.; Chohnan, S.; Solé, M.; Unzeta, M.; Miñano-Molina, A.J.; Rodríguez-Álvarez, J.; et al. Sensing of nutrients by CPT1C controls SAC1 activity to regulate AMPA receptor trafficking. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Venditti, R.; Rega, L.R.; Masone, M.C.; Santoro, M.; Polishchuk, E.; Sarnataro, D.; Paladino, S.; D’Auria, S.; Varriale, A.; Olkkonen, V.M.; et al. Molecular determinants of ER–Golgi contacts identified through a new FRET–FLIM system. J. Cell Biol. 2019, 218. [Google Scholar] [CrossRef]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef] [PubMed]
- Romani, P.; Brian, I.; Santinon, G.; Pocaterra, A.; Audano, M.; Pedretti, S.; Mathieu, S.; Forcato, M.; Bicciato, S.; Manneville, J.B.; et al. Extracellular matrix mechanical cues regulate lipid metabolism through Lipin-1 and SREBP. Nat. Cell Biol. 2019, 21, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.N.; Ponik, S.M.; Garcia-Mendoza, M.G.; Pehlke, C.A.; Inman, D.R.; Eliceiri, K.W.; Keely, P.J. Microtubules regulate GEF-H1 in response to extracellular matrix stiffness. Mol. Biol. Cell 2012, 23, 2583–2592. [Google Scholar] [CrossRef]
- Meiri, D.; Marshall, C.B.; Mokady, D.; LaRose, J.; Mullin, M.; Gingras, A.C.; Ikura, M.; Rottapel, R. Mechanistic insight into GPCR-mediated activation of the microtubule-associated RhoA exchange factor GEF-H1. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Lopes-da-Silva, M.; McCormack, J.J.; Burden, J.J.; Harrison-Lavoie, K.J.; Ferraro, F.; Cutler, D.F. A GBF1-Dependent Mechanism for Environmentally Responsive Regulation of ER-Golgi Transport. Dev. Cell 2019, 49, 786–801.e6. [Google Scholar] [CrossRef]
| Transport Carriers | Protein Name | Topology | Secretion Type | References |
|---|---|---|---|---|
| PKD-dependent carriers (General) | CD4 | TM | Constitutive | [25] |
| TGN46 | TM | Constitutive | [25] | |
| Furin | TM | Constitutive | [32] | |
| VSV-G (Basolateral PM) | TM | Constitutive | [25,26] | |
| ss-HRP | Soluble | Constitutive | [27] | |
| Insulin | Soluble | Regulated | [33,34] | |
| Chromogranin A | Soluble | Regulated | [35,36] | |
| ß1-integrin (Basolateral PM) | TM | Constitutive | [26] | |
| E-cadherin (Basolateral PM) | TM | Constitutive | [26] | |
| CtBP1/BARS-dependent carriers | VSV-G | TM | Constitutive | [37] |
| Low-density lipoprotein (LDL) receptor | TM | Constitutive | [38] | |
| Human growth hormone (hGH) | Soluble | Constitutive | [38] | |
| CARTS | TGN46 | TM | Constitutive | [39] |
| ss-HRP | Soluble | Constitutive | [39,40] | |
| PAUF | Soluble | Constitutive | [39] | |
| Lysozyme C | Soluble | Constitutive | [39] | |
| Synaptotagmin II | TM | Constitutive | [39] | |
| FM4-GPI (CD58) | GPI-AP | Constitutive | [41] | |
| EQ-SM (SM reporter protein) | MA | Constitutive | [41] | |
| SM-rich transport carriers | EQ-SM (SM reporter protein) | MA | Constitutive | [42] |
| FM4-GPI (CD58) | GPI-AP | Constitutive | [42] | |
| Cab45 | Soluble | Constitutive | [43,44,45] | |
| ss-HRP | Soluble | Constitutive | [44,46] | |
| Lysozyme C | Soluble | Constitutive | [45,47] | |
| Cartilage oligomeric matrix protein (COMP) | Soluble | Constitutive | [45,47] | |
| Lipoprotein lipase | MA | Constitutive | [48] | |
| Syndecan-1 | TM | Constitutive | [48] | |
| Rab6-positive carriers (General) | VSV-G | TM | Constitutive | [49,50] |
| Neuropeptide Y (NPY) | Soluble | Constitutive | [49,51] | |
| TNFα | Soluble | Constitutive | [52,53,54] | |
| Herpes Simplex virus 1 (HSV1) glycoproteins gD/gE | TM | Constitutive | [55] | |
| Collagen type X | Soluble | Constitutive | [53] | |
| CD59 | GPI-AP | Constitutive | [53] | |
| Placenta alkaline phosphatase (PLAP) | GPI-AP | Constitutive | [53] | |
| ß1-integrin | TM | Constitutive | [56] | |
| Membrane type 1-matrix metalloproteinase (MT1-MMP) | TM | Constitutive | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakana, Y.; Campelo, F. The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers. Cells 2021, 10, 1618. https://doi.org/10.3390/cells10071618
Wakana Y, Campelo F. The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers. Cells. 2021; 10(7):1618. https://doi.org/10.3390/cells10071618
Chicago/Turabian StyleWakana, Yuichi, and Felix Campelo. 2021. "The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers" Cells 10, no. 7: 1618. https://doi.org/10.3390/cells10071618
APA StyleWakana, Y., & Campelo, F. (2021). The PKD-Dependent Biogenesis of TGN-to-Plasma Membrane Transport Carriers. Cells, 10(7), 1618. https://doi.org/10.3390/cells10071618






