UNC-45A Is Highly Expressed in the Proliferative Cells of the Mouse Genital Tract and in the Microtubule-Rich Areas of the Mouse Nervous System
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation of Mouse CNS and Upper Genital Tract
2.2. Antibodies
2.3. Immunohistochemical Staining of Mouse Upper Genital Tract, Brain, and Spinal Cord
2.4. Bright-Field Imaging
2.5. Immunofluorescence (IF) Microscopy
2.6. Quantification of UNC-45A Expression Levels via Immunofluorescence and via Immunohistochemistry
3. Results
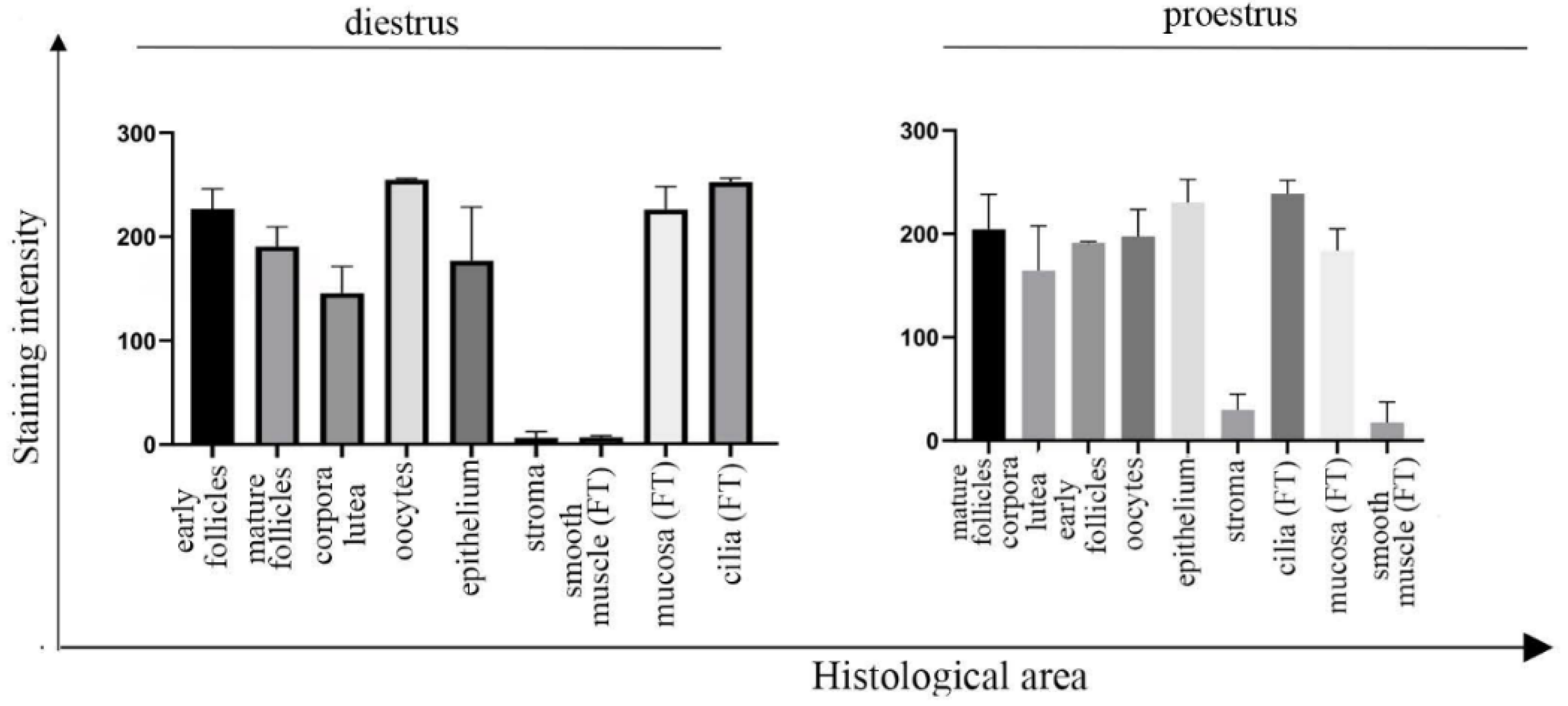
3.1. UNC-45A Is Expressed in the Mouse Upper Genital Tract with a Stronger Expression in Proliferating Cells and Cilia
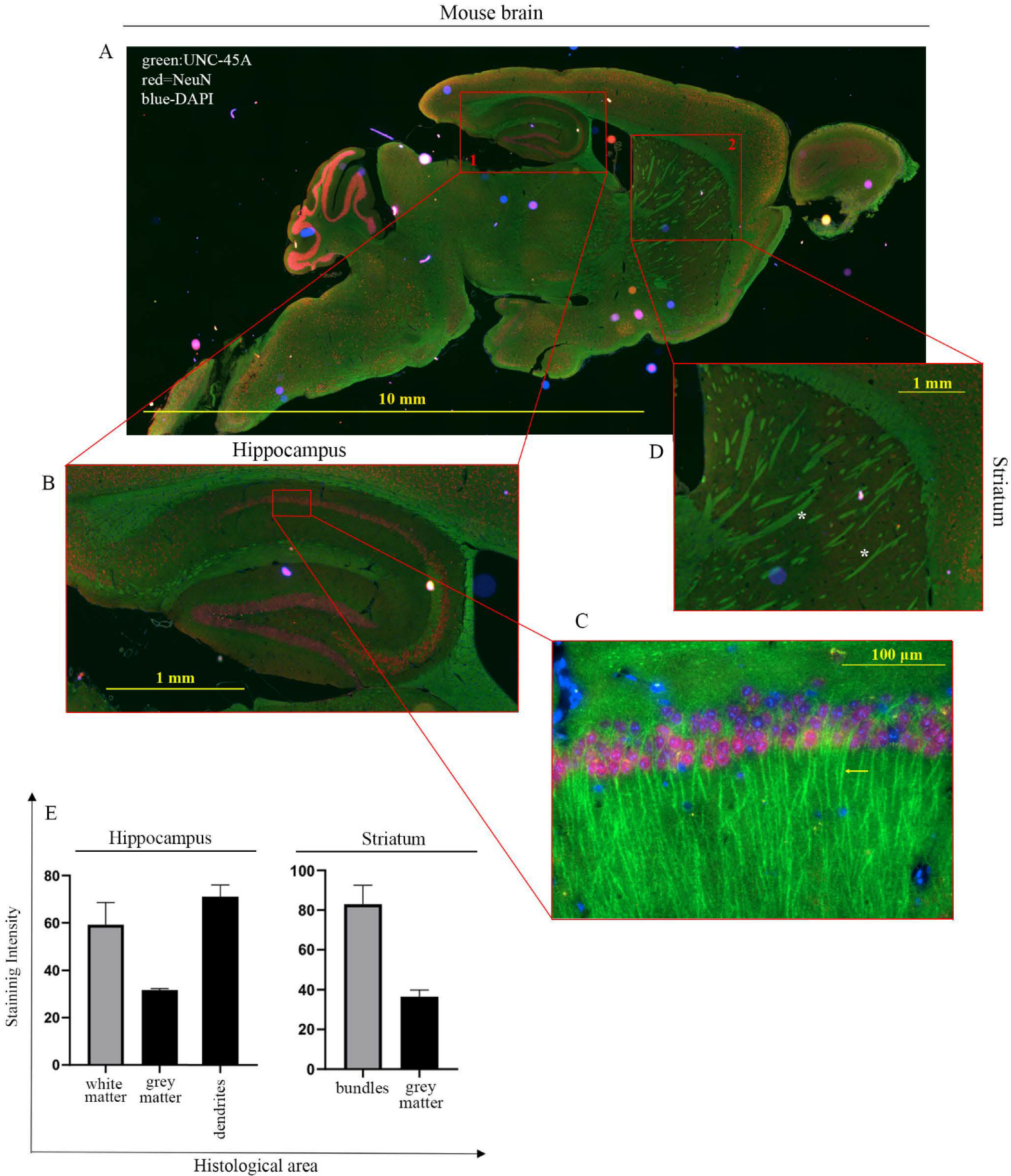
3.2. UNC-45A Is Expressed in the Microtubule-Rich Regions of the Mouse Central Nervous System (CNS) and Mouse Nerve Roots
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barral, J.M.; Hutagalung, A.H.; Brinker, A.; Hartl, F.U.; Epstein, H.F. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 2002, 295, 669–671. [Google Scholar] [CrossRef]
- Kachur, T.; Ao, W.; Berger, J.; Pilgrim, D. Maternal UNC-45 is involved in cytokinesis and colocalizes with non-muscle myosin in the early Caenorhabditis elegans embryo. J. Cell Sci. 2004, 117, 5313–5321. [Google Scholar] [CrossRef]
- Shi, H.; Blobel, G. UNC-45/CRO1/She4p (UCS) protein forms elongated dimer and joins two myosin heads near their actin binding region. Proc. Natl. Acad. Sci. USA 2010, 107, 21382–21387. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Fenix, A.; Kotila, T.M.; Balistreri, G.; Paavolainen, L.; Varjosalo, M.; Burnette, D.T.; Lappalainen, P. UNC-45a promotes myosin folding and stress fiber assembly. J. Cell Biol. 2017, 216, 4053–4072. [Google Scholar] [CrossRef] [PubMed]
- Habicht, J.; Mooneyham, A.; Shetty, M.; Zhang, X.; Shridhar, V.; Winterhoff, B.; Zhang, Y.; Cepela, J.; Starr, T.; Lou, E.; et al. UNC-45A is preferentially expressed in epithelial cells and binds to and co-localizes with interphase MTs. Cancer Biol. Ther. 2019, 20, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Mooneyham, A.; Iizuka, Y.; Yang, Q.; Coombes, C.; McClellan, M.; Shridhar, V.; Emmings, E.; Shetty, M.; Chen, L.; Ai, T.; et al. UNC-45A is a novel microtubule-associated protein and regulator of paclitaxel sensitivity in ovarian cancer cells. Mol. Cancer Res. 2019, 17, 370–383. [Google Scholar] [CrossRef]
- Habicht, J.; Mooneyham, A.; Hoshino, A.; Shetty, M.; Zhang, X.; Emmings, E.; Yang, Q.; Coombes, C.; Gardner, M.K.; Bazzaro, M. UNC-45A breaks mt lattice independent of its effect on non-muscle myosin II. J. Cell Sci. 2021. [Google Scholar] [CrossRef]
- Sarwar, M.; Sykes, P.H.; Chitcholtan, K.; Evans, J.J. Deciphering biophysical modulation in ovarian cancer cells. Cell Biophys. 2021, 79, 375–386. [Google Scholar] [CrossRef]
- McGrail, D.J.; Kieu, Q.M.N.; Dawson, M.R. Metastatic ovarian cancer cell malignancy is increased on soft matrices through a mechanosensitive Rho/ROCK pathway. J. Cell Sci. 2014, 127, 2621–2626. [Google Scholar] [CrossRef]
- Jeong, K.J.; Park, S.Y.; Cho, K.H.; Sohn, J.S.; Lee, J.; Kim, Y.K.; Kang, J.; Park, C.G.; Han, J.W.; Lee, H.Y. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene 2012, 31, 4279–4289. [Google Scholar] [CrossRef]
- Ohta, T.; Takahashi, T.; Shibuya, T.; Amita, M.; Henmi, N.; Takahashi, K.; Kurachi, H. Inhibition of the Rho/ROCK pathway enhances the efficacy of cisplatin through the blockage of hypoxia-inducible factor-1α in human ovarian cancer cells. Cancer Biol. Ther. 2012, 13, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Guérit, S.; Macrez, R.; Gosselet, F.; Sevin, E.; Lebas, H.; Maubert, E.; De Vries, H.E.; Bardou, I.; Vivien, D.; et al. Nonionotropic Action of Endothelial NMDA Receptors on Blood–Brain Barrier Permeability via Rho/ROCK-Mediated Phosphorylation of Myosin. J. Neurosci. 2020, 40, 1778–1787. [Google Scholar] [CrossRef]
- Stankiewicz, T.R.; Pena, C.; Bouchard, R.J.; Linseman, D.A. Dysregulation of Rac or Rho elicits death of motor neurons and activation of these GTPases is altered in the G93A mutant hSOD1 mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2020, 136, 104743. [Google Scholar] [CrossRef]
- Denton, K.R.; Lei, L.; Grenier, J.; Rodionov, V.; Blackstone, C.; Li, X.-J. Loss of spastin function results in disease-specific axonal defects in human pluripotent stem cell-based models of hereditary spastic paraplegia. Stem Cells 2014, 32, 414–423. [Google Scholar] [CrossRef]
- Tao, J.; Feng, C.; Rolls, M.M. The microtubule severing protein fidgetin acts after dendrite injury to promote degeneration. J. Cell Sci. 2016, 129, 3274–3281. [Google Scholar] [CrossRef] [PubMed]
- Fassier, C.; Fréal, A.; Gasmi, L.; Delphin, C.; Martin, D.T.; De Gois, S.; Tambalo, M.; Bosc, C.; Mailly, P.; Revenu, C.; et al. Motor axon navigation relies on Fidgetin-like 1–driven microtubule plus end dynamics. J. Cell Biol. 2018, 217, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Yu, W.; McNally, F.J.; Baas, P.W. An essential role for katanin in severing microtubules in the neuron. J. Cell Biol. 1999, 145, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Karabay, A.; Yu, W.; Solowska, J.M.; Baird, D.H.; Baas, P.W. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J. Neurosci. 2004, 24, 5778–5788. [Google Scholar] [CrossRef]
- Schiewek, J.; Schumacher, U.; Lange, T.; Joosse, S.A.; Wikman, H.; Pantel, K.; Mikhaylova, M.; Kneussel, M.; Linder, S.; Schmalfeldt, B.; et al. Clinical relevance of cytoskeleton associated proteins for ovarian cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2195–2205. [Google Scholar] [CrossRef]
- Price, D.K.; Ball, J.R.; Bahrani-Mostafavi, Z.; Vachris, J.C.; Kaufman, J.; Naumann, R.W.; Higgins, R.V.; Hall, J.B. The phosphoprotein Op18/stathmin is differentially expressed in ovarian cancer. Cancer Investig. 2000, 18, 722–730. [Google Scholar] [CrossRef]
- Su, D.; Smith, S.; Preti, M.; Schwartz, P.; Rutherford, T.J.; Menato, G.; Danese, S.; Ma, S.; Yu, H.; Katsaros, D. Stathmin and tubulin expression and survival of ovarian cancer patients receiving platinum treatment with and without paclitaxel. Cancer 2009, 115, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Lombino, F.L.; Muhia, M.; Lopez-Rojas, J.; Brill, M.S.; Thies, E.; Ruschkies, L.; Lutz, D.; Richter, M.; Hausrat, T.J.; Lopes, A.; et al. The microtubule severing protein katanin regulates proliferation of neuronal progenitors in embryonic and adult neurogenesis. Sci. Rep. 2019, 9, 15940. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, R.; Welsh, M.J.; Day, B.W. Altered levels and regulation of stathmin in paclitaxel-resistant ovarian cancer cells. Oncogene 2003, 22, 8924–8930. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, W.; Chen, D.; Fan, Z.; Epstein, H.F. Differential turnover of myosin chaperone UNC-45a isoforms increases in metastatic human breast cancer. J. Mol. Biol. 2011, 412, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Bazzaro, M.; Santillan, A.; Lin, Z.; Tang, T.; Lee, M.K.; Bristow, R.E.; Shih, I.-M.; Roden, R.B. Myosin II co-chaperone general cell UNC-45 overexpression is associated with ovarian cancer, rapid proliferation, and motility. Am. J. Pathol. 2007, 171, 1640–1649. [Google Scholar] [CrossRef]
- Eisa, N.H.; Jilani, Y.; Kainth, K.; Redd, P.; Lu, S.; Bougrine, O.; Sater, H.A.; Patwardhan, C.A.; Shull, A.; Shi, H.; et al. The co-chaperone UNC45A is essential for the expression of mitotic kinase NEK7 and tumorigenesis. J. Biol. Chem. 2019, 294, 5246–5260. [Google Scholar] [CrossRef]
- Jilani, Y.; Lu, S.; Lei, H.; Karnitz, L.M.; Chadli, A. UNC45A localizes to centrosomes and regulates cancer cell proliferation through ChK1 activation. Cancer Lett. 2015, 357, 114–120. [Google Scholar] [CrossRef]
- Iizuka, Y.; Cichocki, F.; Sieben, A.J.; Sforza, F.; Karim, R.; Coughlin, K.; Vogel, R.; Gavioli, R.; McCullar, V.; Lenvik, T.; et al. UNC-45A Is a nonmuscle myosin IIA chaperone required for NK cell cytotoxicity via control of lytic granule secretion. J. Immunol. 2015, 195, 4760–4770. [Google Scholar] [CrossRef]
- Iizuka, Y.; Mooneyham, A.; Sieben, A.; Chen, K.; Maile, M.; Hellweg, R.; Schütz, F.; Teckle, K.; Starr, T.; Thayanithy, V.; et al. UNC-45A is required for neurite extension via controlling NMII activation. Mol. Biol. Cell 2017, 28, 1337–1346. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pr. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Westwood, F.R. The female rat reproductive cycle: A practical histological guide to staging. Toxicol. Pathol. 2008, 36, 375–384. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: An integrated protocol. Bio-protocol 2019, 9, e3465. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.F. The cell biological basis of ciliary disease. J. Cell Biol. 2008, 180, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Ueno, H.; Omori, T.; Kikuchi, K. Cilia and centrosomes: Ultrastructural and mechanical perspectives. Semin. Cell Dev. Biol. 2021, 110, 61–69. [Google Scholar] [CrossRef]
- Kapitein, L.C.; Hoogenraad, C.C. Building the neuronal microtubule cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2004, 6, 204. [Google Scholar] [CrossRef]
- Gusel’nikova, V.V.; Korzhevskiy, D.E. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Naturae. 2015, 7, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, F.; Jin, Z.; Ying, X.; Liu, J. Microtubule-severing protein Katanin p60 ATPase-containing subunit A-like 1 is involved in pole-based spindle organization during mouse oocyte meiosis. Mol. Med. Rep. 2019, 20, 3573–3582. [Google Scholar] [CrossRef]
- Yang, H.-Y.; McNally, K.; McNally, F.J. MEI-1/katanin is required for translocation of the meiosis I spindle to the oocyte cortex in C. elegans. Dev. Biol. 2003, 260, 245–259. [Google Scholar] [CrossRef]
- McNally, K.; Audhya, A.; Oegema, K.; McNally, F.J. Katanin controls mitotic and meiotic spindle length. J. Cell Biol. 2006, 175, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Garzon, S.; Laganà, A.S.; Casarin, J.; Raffaelli, R.; Cromi, A.; Franchi, M.; Barra, F.; Alkatout, I.; Ferrero, S.; Ghezzi, F. Secondary and tertiary ovarian cancer recurrence: What is the best management? Gland. Surg. 2020, 9, 1118–1129. [Google Scholar] [CrossRef]
- Lagana, A.S.; Colonese, F.; Colonese, E.; Sofo, V.; Salmeri, F.M.; Granese, R.; Chiofalo, B.; Ciancimino, L.; Triolo, O. Cytogenetic analysis of epithelial ovarian cancer’s stem cells: An overview on new diagnostic and therapeutic perspectives. Eur. J. Gynaecol. Oncol. 2015, 36, 495–505. [Google Scholar]
- Price, M.G.; Landsverk, M.L.; Barral, J.M.; Epstein, H.F. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci. 2002, 115, 4013–4023. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Lam, A.J.; Tan, T.; Han, J.; Nowakowski, D.W.; Vershinin, M.; Simó, S.; Ori-McKenney, K.M.; McKenney, R.J. Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 2019, 21, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dong, S.; Gu, F.; Hu, Y.; Zhao, Z. Advances in the pathogenesis of Alzheimer’s Disease: Focusing on tau-mediated neurodegeneration. Transl. Neurodegener. 2012, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.W.; Rao, A.; Matamoros, A.J.; Leo, L. Stability properties of neuronal microtubules. Cytoskelet. 2016, 73, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.; Ratnakaran, N.; Koushika, S.P. Neurodegeneration and microtubule dynamics: Death by a thousand cuts. Front. Cell. Neurosci. 2015, 9, 343. [Google Scholar] [CrossRef]
- Hellal, F.; Hurtado, A.; Ruschel, J.; Flynn, K.C.; Laskowski, C.J.; Umlauf, M.; Kapitein, L.C.; Strikis, D.; Lemmon, V.; Bixby, J.; et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 2011, 331, 928–931. [Google Scholar] [CrossRef]
- Jean, D.C.; Baas, P.W. It cuts two ways: Microtubule loss during Alzheimer disease. EMBO J. 2013, 32, 2900–2902. [Google Scholar] [CrossRef]
- Ruschel, J.; Hellal, F.; Flynn, K.C.; Dupraz, S.; Elliott, D.A.; Tedeschi, A.; Bates, M.; Sliwinski, C.; Brook, G.; Dobrindt, K.; et al. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 2015, 348, 347–352. [Google Scholar] [CrossRef]
- Mimori, K.; Sadanaga, N.; Yoshikawa, Y.; Ishikawa, K.; Hashimoto, M.; Tanaka, F.; Sasaki, A.T.; Inoue, H.; Sugimachi, K.; Mori, M. Reduced tau expression in gastric cancer can identify candidates for successful Paclitaxel treatment. Br. J. Cancer 2006, 94, 1894–1897. [Google Scholar] [CrossRef]
- Plun-Favreau, H.; Lewis, P.A.; Hardy, J.; Martins, L.M.; Wood, N.W. Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea. PLoS Genet. 2010, 6, e1001257. [Google Scholar] [CrossRef]
- Owinsky, E.R.K.R.; Onehower, R.O.C.D. Paclitaxel (Taxol). N. Engl. J. Med. 1995, 332, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Duggal, P.; Mehan, S. Neuroprotective approach of anti-cancer microtubule stabilizers against tauopathy associated dementia: Current status of clinical and preclinical findings. J. Alzheimer’s Dis. Rep. 2019, 3, 179–218. [Google Scholar] [CrossRef]
- Esteve, C.; Francescatto, L.; Tan, P.L.; Bourchany, A.; De Leusse, C.; Marinier, E.; Blanchard, A.; Bourgeois, P.; Brochier-Armanet, C.; Bruel, A.-L.; et al. Loss-of-function mutations in UNC45A cause a syndrome associating cholestasis, diarrhea, impaired hearing, and bone fragility. Am. J. Hum. Genet. 2018, 102, 364–374. [Google Scholar] [CrossRef]
- Lee, L.; Ostrowski, L.E. Motile cilia genetics and cell biology: Big results from little mice. Cell. Mol. Life Sci. 2021, 78, 769–797. [Google Scholar] [CrossRef]
- Suciu, S.K.; Caspary, T. Cilia, neural development and disease. Semin. Cell Dev. Biol. 2021, 110, 34–42. [Google Scholar] [CrossRef]
- Higgins, M.; Obaidi, I.; McMorrow, T. Primary cilia and their role in cancer (Review). Oncol. Lett. 2019, 17, 3041–3047. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente, V.; Hoshino, A.; Meints, J.; Shetty, M.; Starr, T.; Lee, M.; Bazzaro, M. UNC-45A Is Highly Expressed in the Proliferative Cells of the Mouse Genital Tract and in the Microtubule-Rich Areas of the Mouse Nervous System. Cells 2021, 10, 1604. https://doi.org/10.3390/cells10071604
Clemente V, Hoshino A, Meints J, Shetty M, Starr T, Lee M, Bazzaro M. UNC-45A Is Highly Expressed in the Proliferative Cells of the Mouse Genital Tract and in the Microtubule-Rich Areas of the Mouse Nervous System. Cells. 2021; 10(7):1604. https://doi.org/10.3390/cells10071604
Chicago/Turabian StyleClemente, Valentino, Asumi Hoshino, Joyce Meints, Mihir Shetty, Tim Starr, Michael Lee, and Martina Bazzaro. 2021. "UNC-45A Is Highly Expressed in the Proliferative Cells of the Mouse Genital Tract and in the Microtubule-Rich Areas of the Mouse Nervous System" Cells 10, no. 7: 1604. https://doi.org/10.3390/cells10071604
APA StyleClemente, V., Hoshino, A., Meints, J., Shetty, M., Starr, T., Lee, M., & Bazzaro, M. (2021). UNC-45A Is Highly Expressed in the Proliferative Cells of the Mouse Genital Tract and in the Microtubule-Rich Areas of the Mouse Nervous System. Cells, 10(7), 1604. https://doi.org/10.3390/cells10071604







