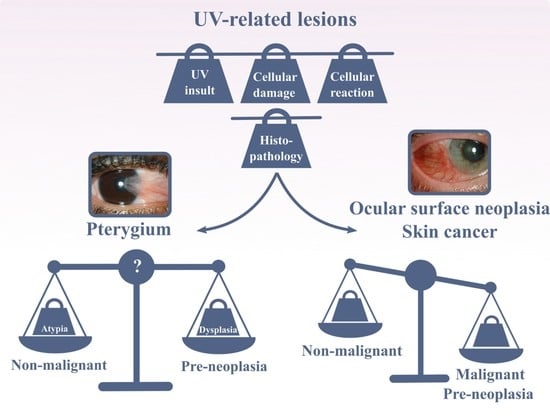
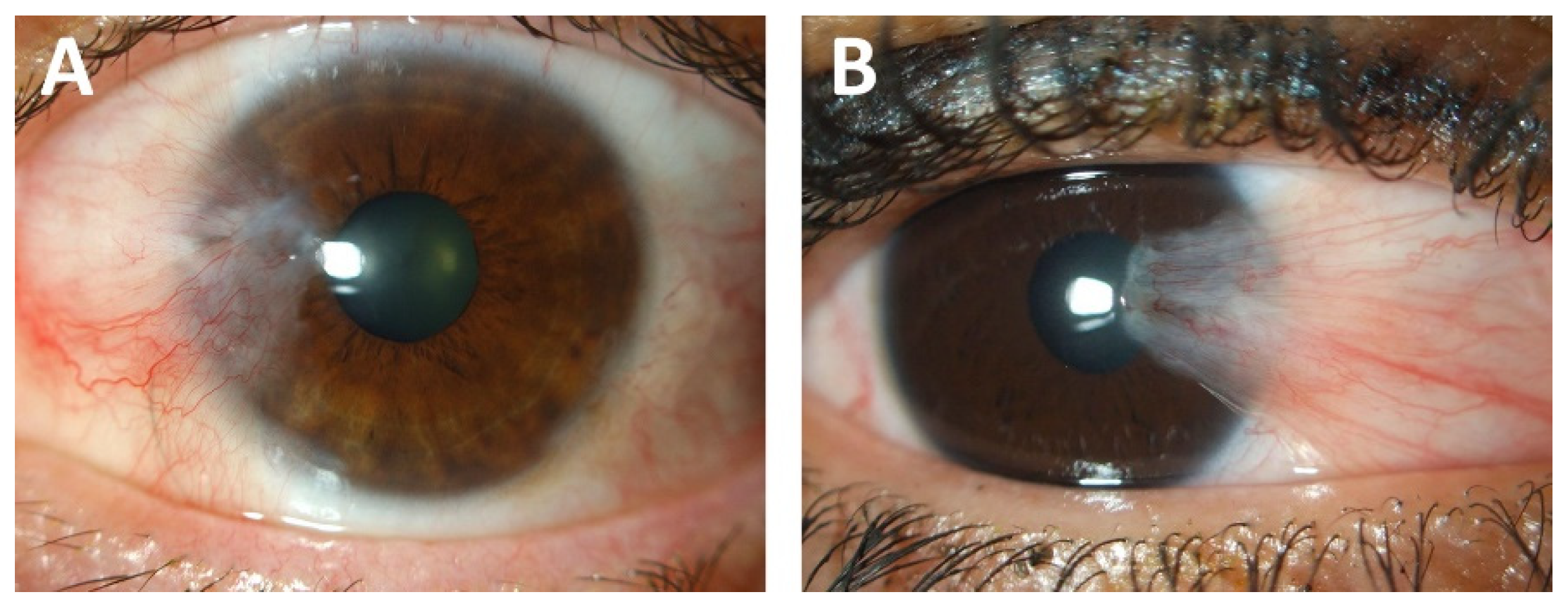
Pterygium—The Good, the Bad, and the Ugly
Abstract
1. Introduction
2. Risk Factors for Pterygium Development
3. UV-Induced Damage
3.1. Biochemical Pathway Disruption
3.2. DNA Damage
4. UV-Induced Cellular Reactions
4.1. Nuclear Repair Mechanisms
4.2. Autophagy
4.3. Apoptosis
4.4. Cellular Adaptations
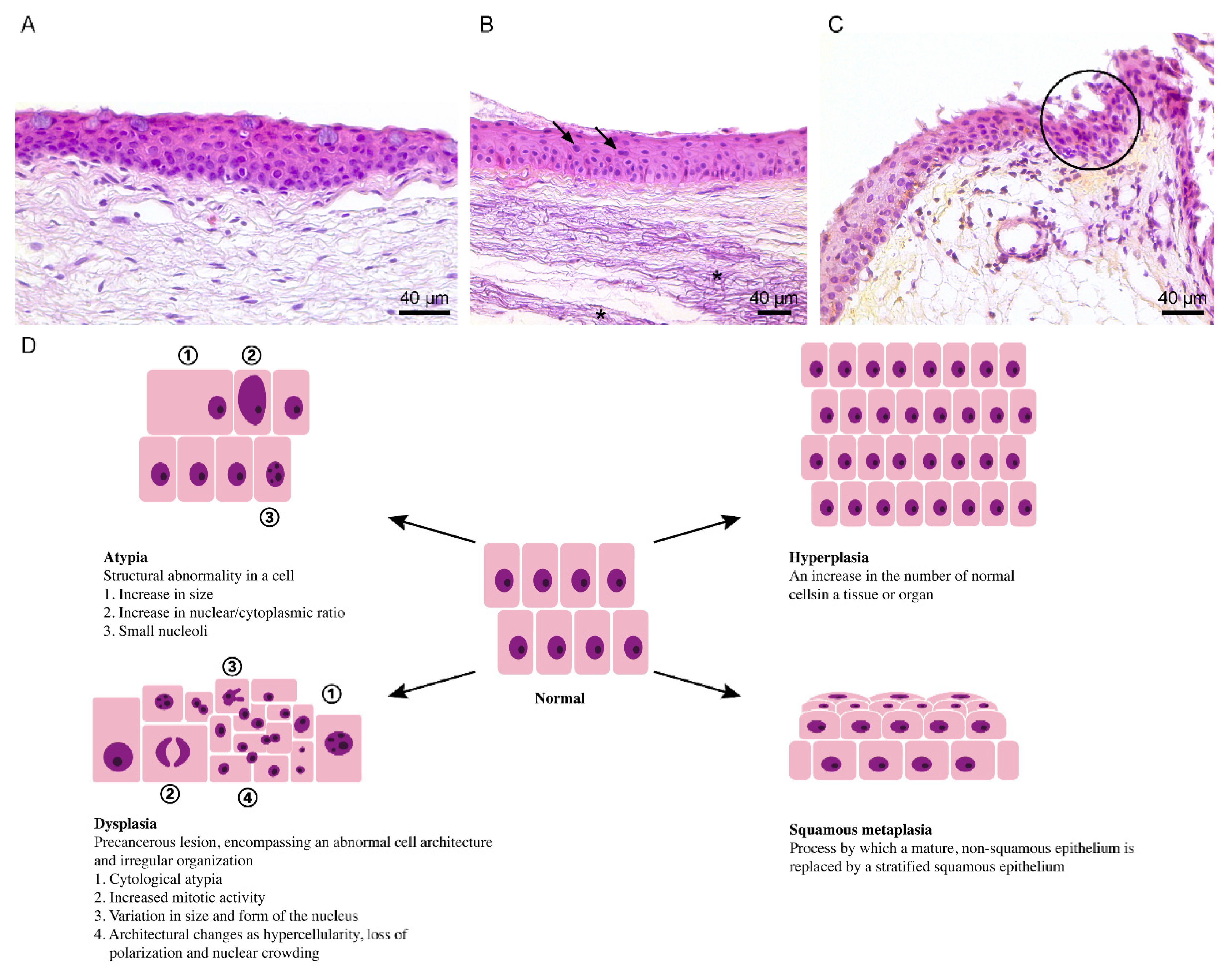
5. Dysplasia and Ocular Surface Squamous Neoplasia
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G. Smolin and Thoft’s The Cornea: Scientific Foundations and Clinical Practice, 4th ed.; Foster, C.S., Azar, D.T., Dohlman, C.H., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Cameron, M.E. Histology of pterygium: An electron microscopic study. Br. J. Ophthalmol. 1983, 67, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J.; Alvarado, J. Pterygium and pinguecula: Electron microscopic study. Arch. Ophthalmol. 1967, 78, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.; Coroneo, M.T.; Tat, L.T.; Crouch, R.; Wakefield, D.; Di Girolamo, N. Ophthalmic pterygium: A stem cell disorder with premalignant features. Am. J. Pathol. 2011, 178, 817–827. [Google Scholar] [CrossRef]
- Weinstein, O.; Rosenthal, G.; Zirkin, H.; Monos, T.; Lifshitz, T.; Argov, S. Overexpression of p53 tumor suppressor gene in pterygia. Eye 2002, 16, 619–621. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, J.C. Current approaches and future directions in the management of pterygium. Int. J. Ophthalmol. 2018, 11, 709–711. [Google Scholar] [CrossRef]
- Johnston, S.C.; Williams, P.B.; Sheppard, J.D.J. A Comprehensive System for Pterygium Classification. Investig. Ophthamol. Vis. Sci. 2004, 45, 2940. [Google Scholar]
- Miller, M.A.; Zachary, J.F. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. In Pathologic Basis of Veterinary Disease; Mosby: Maryland Heights, MO, USA, 2017; pp. 2–43.e19. [Google Scholar] [CrossRef]
- Zhou, W.P.; Zhu, Y.F.; Zhang, B.; Qiu, W.Y.; Yao, Y.F. The role of ultraviolet radiation in the pathogenesis of pterygia (Review). Mol. Med. Rep. 2016, 14, 3–15. [Google Scholar] [CrossRef]
- Cameron, M.E. Book Review. Clin. Exp. Optom. 1965, 48, 150. [Google Scholar] [CrossRef]
- Coroneo, M. Ultraviolet radiation and the anterior eye. Eye Contact Lens 2011, 37, 214–224. [Google Scholar] [CrossRef] [PubMed]
- King, T.C. Cell Injury, Cellular Responses to Injury, and Cell Death. In Elsevier’s Integrated Pathology; Mosby: Maryland Heights, MO, USA, 2007; pp. 1–20. [Google Scholar]
- Poljsak, B.; Milisav, I. Clinical implications of cellular stress responses. Bosn. J. Basic Med. Sci. 2012, 12, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Coroneo, M.; Wakefield, D. Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation. Am. J. Pathol. 2005, 167, 489–503. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Coroneo, M.T.; Wakefield, D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK-dependent pathway. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4705–4714. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Wakefield, D.; Coroneo, M.T. UVB-mediated induction of cytokines and growth factors in pterygium epithelial cells involves cell surface receptors and intracellular signaling. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2430–2437. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Sava, M.P.; Raica, M. DNA damage in human pterygium: One-shot multiple targets. Mol. Vis. 2013, 19, 348–356. [Google Scholar]
- Tsai, Y.Y.; Cheng, Y.W.; Lee, H.; Tsai, F.J.; Tseng, S.H.; Lin, C.L.; Chang, K.C. Oxidative DNA damage in pterygium. Mol. Vis. 2005, 11, 71–75. [Google Scholar]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Feehan, R.P.; Shantz, L.M. Molecular signaling cascades involved in nonmelanoma skin carcinogenesis. Biochem. J. 2016, 473, 2973–2994. [Google Scholar] [CrossRef] [PubMed]
- Cheepala, S.B.; Yin, W.; Syed, Z.; Gill, J.N.; McMillian, A.; Kleiner, H.E.; Lynch, M.; Loganantharaj, R.; Trutschl, M.; Cvek, U.; et al. Identification of the B-Raf/Mek/Erk MAP kinase pathway as a target for all-trans retinoic acid during skin cancer promotion. Mol. Cancer 2009, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Dushku, N.; John, M.K.; Schultz, G.S.; Reid, T.W. Pterygia pathogenesis: Corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch. Ophthalmol. 2001, 119, 695–706. [Google Scholar] [CrossRef]
- Van Acker, S.I.; Haagdorens, M.; Roelant, E.; Rozema, J.; Possemiers, T.; Van Gerwen, V.; Tassignon, M.J.; De Groot, V.; Ni Dhubhghaill, S.; Koppen, C.; et al. Pterygium Pathology: A Prospective Case-Control Study on Tear Film Cytokine Levels. Mediat. Inflamm. 2019, 2019, 9416262. [Google Scholar] [CrossRef] [PubMed]
- Abeyama, K.; Eng, W.; Jester, J.V.; Vink, A.A.; Edelbaum, D.; Cockerell, C.J.; Bergstresser, P.R.; Takashima, A. A role for NF-kappaB-dependent gene transactivation in sunburn. J. Clin. Investig. 2000, 105, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, J.K.; Joo, C.K. Translocation of nuclear factor-kappaB on corneal epithelial cells induced by ultraviolet B irradiation. Ophthalmic Res. 2005, 37, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-kappaB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.X.; Hawk, N.V.; Chen, W.; Coupar, J.; Lee, S.K.; Petersen, D.W.; Meltzer, P.S.; Montemarano, A.; Braun, M.; Chen, Z.; et al. Targeting Notch1 and IKKalpha Enhanced NF-kappaB Activation in CD133(+) Skin Cancer Stem Cells. Mol. Cancer Ther. 2018, 17, 2034–2048. [Google Scholar] [CrossRef]
- Liu, C.; Song, Y.; Wang, X.; Lai, Z.; Li, C.; Wan, P.; Xu, N.; Huang, D.; Liu, Y.; Wang, Z. The Key Role of VEGF in the Cross Talk between Pterygium and Dry Eye and Its Clinical Significance. Ophthalmic Res. 2020, 63, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Siak, J.J.; Ng, S.L.; Seet, L.F.; Beuerman, R.W.; Tong, L. The nuclear-factor kappaB pathway is activated in pterygium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 230–236. [Google Scholar] [CrossRef]
- Torres, J.; Enriquez-de-Salamanca, A.; Fernandez, I.; Rodriguez-Ares, M.T.; Quadrado, M.J.; Murta, J.; Benitez del Castillo, J.M.; Stern, M.E.; Calonge, M. Activation of MAPK signaling pathway and NF-kappaB activation in pterygium and ipsilateral pterygium-free conjunctival specimens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5842–5852. [Google Scholar] [CrossRef] [PubMed]
- Zaheryani, S.M.S.; Ebrahimi, M.E.; Kasaei, A.; Roointan, A.; Nejabat, M.; Dianatpour, M.; Meisam, M.; Talebnejad, M.R.; Naghibalhossaini, F. Expression of Inflammatory-Related NFkappaB Genes in Iranian Patients with Pterygium: A Case-Control Study. Int. J. Mol. Cell Med. 2018, 7, 169–175. [Google Scholar] [CrossRef]
- Nijsten, T.; Colpaert, C.G.; Vermeulen, P.B.; Harris, A.L.; Van Marck, E.; Lambert, J. Cyclooxygenase-2 expression and angiogenesis in squamous cell carcinoma of the skin and its precursors: A paired immunohistochemical study of 35 cases. Br. J. Dermatol. 2004, 151, 837–845. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Shimmura, S.; Kawakita, T.; Miyashita, H.; Ogawa, Y.; Yoshida, S.; Higa, K.; Okano, H.; Tsubota, K. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1511–1517. [Google Scholar] [CrossRef]
- Meshkani, S.E.; Kooshan, N.; Moghadam, A.B.; Falanji, F.; Adli, A.; Baghbani-Arani, F.; Arian, A.G.; Rad, A. Signaling roadmap to epithelial-mesenchymal transition in pterygium, TWIST1 centralized. J. Cell. Physiol. 2019, 234, 18146–18155. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Dong, S.; Qiao, C.; Yang, G.; Zhang, S.; Wang, C.; Xu, Y.; Zheng, F.; Yan, M. MiR-199a-3p/5p participated in TGF-beta and EGF induced EMT by targeting DUSP5/MAP3K11 in pterygium. J. Transl. Med. 2020, 18, 332. [Google Scholar] [CrossRef]
- Wu, C.W.; Peng, M.L.; Yeh, K.T.; Tsai, Y.Y.; Chiang, C.C.; Cheng, Y.W. Inactivation of p53 in pterygium influence miR-200a expression resulting in ZEB1/ZEB2 up-regulation and EMT processing. Exp. Eye Res. 2016, 146, 206–211. [Google Scholar] [CrossRef]
- Engelsvold, D.H.; Utheim, T.P.; Olstad, O.K.; Gonzalez, P.; Eidet, J.R.; Lyberg, T.; Troseid, A.M.; Dartt, D.A.; Raeder, S. miRNA and mRNA expression profiling identifies members of the miR-200 family as potential regulators of epithelial-mesenchymal transition in pterygium. Exp. Eye Res. 2013, 115, 189–198. [Google Scholar] [CrossRef][Green Version]
- Ling, S.; Liang, L.; Lin, H.; Li, W.; Xu, J. Increasing lymphatic microvessel density in primary pterygia. Arch. Ophthalmol. 2012, 130, 735–742. [Google Scholar] [CrossRef][Green Version]
- Martin-Lopez, J.; Perez-Rico, C.; Garcia-Honduvilla, N.; Bujan, J.; Pascual, G. Elevated blood/lymphatic vessel ratio in pterygium and its relationship with vascular endothelial growth factor (VEGF) distribution. Histol. Histopathol. 2019, 34, 917–929. [Google Scholar] [CrossRef]
- Zhao, F.; Cai, S.; Huang, Z.; Ding, P.; Du, C. Optical Coherence Tomography Angiography in Pinguecula and Pterygium. Cornea 2020, 39, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, M.; Lee, Y.; Choi, S.; Yang, J. Chondrocyte-derived extracellular matrix suppresses pathogenesis of human pterygium epithelial cells by blocking the NF-kappaB signaling pathways. Mol. Vis. 2016, 22, 1490–1502. [Google Scholar]
- Uthaithammarat, L.; Kasetsuwan, N.; Chongpison, Y.; Kasetsuwan, P.; Reinprayoon, U.; Nilyanimit, P.; Poovorawan, Y. Lack of HPV in pterygium with no evidence of autoinoculation and the role of cytokines in pterygium with dry eye. Sci. Rep. 2021, 11, 2842. [Google Scholar] [CrossRef]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef]
- Ateenyi-Agaba, C.; Dai, M.; Le Calvez, F.; Katongole-Mbidde, E.; Smet, A.; Tommasino, M.; Franceschi, S.; Hainaut, P.; Weiderpass, E. TP53 mutations in squamous-cell carcinomas of the conjunctiva: Evidence for UV-induced mutagenesis. Mutagenesis 2004, 19, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Betancourt, N.; Field, M.G.; Davila-Alquisiras, J.H.; Karp, C.L.; Hernandez-Zimbron, L.F.; Garcia-Vazquez, R.; Vazquez-Romo, K.A.; Wang, G.; Fromow-Guerra, J.; Hernandez-Quintela, E.; et al. Whole exome profiling and mutational analysis of Ocular Surface Squamous Neoplasia. Ocul. Surf. 2020, 18, 627–632. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Cheng, Y.W.; Lee, H.; Tsai, F.J.; Tseng, S.H.; Chang, K.C. P53 gene mutation spectrum and the relationship between gene mutation and protein levels in pterygium. Mol. Vis. 2005, 11, 50–55. [Google Scholar]
- Kamiya, H.; Iwai, S.; Kasai, H. The (6-4) photoproduct of thymine-thymine induces targeted substitution mutations in mammalian cells. Nucleic Acids Res. 1998, 26, 2611–2617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Douki, T.; von Koschembahr, A.; Cadet, J. Insight in DNA Repair of UV-induced Pyrimidine Dimers by Chromatographic Methods. Photochem. Photobiol. 2017, 93, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Balci, M.; Sahin, S.; Mutlu, F.M.; Yagci, R.; Karanci, P.; Yildiz, M. Investigation of oxidative stress in pterygium tissue. Mol. Vis. 2011, 17, 443–447. [Google Scholar]
- Mamalis, A.; Fiadorchanka, N.; Adams, L.; Serravallo, M.; Heilman, E.; Siegel, D.; Brody, N.; Jagdeo, J. An immunohistochemical panel to assess ultraviolet radiation-associated oxidative skin injury. J. Drugs Dermatol. 2014, 13, 574–578. [Google Scholar] [PubMed]
- Ibrahim, O.M.; Kojima, T.; Wakamatsu, T.H.; Dogru, M.; Matsumoto, Y.; Ogawa, Y.; Ogawa, J.; Negishi, K.; Shimazaki, J.; Sakamoto, Y.; et al. Corneal and retinal effects of ultraviolet-B exposure in a soft contact lens mouse model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2403–2413. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Wood, R.D. DNA repair in eukaryotes. Annu. Rev. Biochem. 1996, 65, 135–167. [Google Scholar] [CrossRef]
- Kemp, M.G.; Sancar, A. DNA excision repair: Where do all the dimers go? Cell Cycle 2012, 11, 2997–3002. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, J.E. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat. Rev. Cancer 2005, 5, 564–573. [Google Scholar] [CrossRef]
- Kraemer, K.H.; Lee, M.M.; Andrews, A.D.; Lambert, W.C. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch. Dermatol. 1994, 130, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Patton, L.L.; Valdez, I.H. Xeroderma pigmentosum: Review and report of a case. Oral Surg. Oral Med. Oral Pathol. 1991, 71, 297–300. [Google Scholar] [CrossRef]
- Goyal, J.L.; Rao, V.A.; Srinivasan, R.; Agrawal, K. Oculocutaneous manifestations in xeroderma pigmentosa. Br. J. Ophthalmol. 1994, 78, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.P.; Thompson, A.H.; Bishop, R.J.; Clayton, J.A.; Chan, C.C.; Tsilou, E.T.; Zein, W.M.; Tamura, D.; Khan, S.G.; Ueda, T.; et al. Ocular manifestations of xeroderma pigmentosum: Long-term follow-up highlights the role of DNA repair in protection from sun damage. Ophthalmology 2013, 120, 1324–1336. [Google Scholar] [CrossRef]
- Ramkumar, H.L.; Brooks, B.P.; Cao, X.; Tamura, D.; Digiovanna, J.J.; Kraemer, K.H.; Chan, C.C. Ophthalmic manifestations and histopathology of xeroderma pigmentosum: Two clinicopathological cases and a review of the literature. Surv. Ophthalmol. 2011, 56, 348–361. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Geng, J.; Yuan, Z.; Huang, D. Geographical prevalence and risk factors for pterygium: A systematic review and meta-analysis. BMJ Open 2013, 3, e003787. [Google Scholar] [CrossRef]
- Kao, A.A.; Galor, A.; Karp, C.L.; Abdelaziz, A.; Feuer, W.J.; Dubovy, S.R. Clinicopathologic correlation of ocular surface squamous neoplasms at Bascom Palmer Eye Institute: 2001 to 2010. Ophthalmology 2012, 119, 1773–1776. [Google Scholar] [CrossRef]
- Chiang, C.C.; Tsai, Y.Y.; Bau, D.T.; Cheng, Y.W.; Tseng, S.H.; Wang, R.F.; Tsai, F.J. Pterygium and genetic polymorphisms of the DNA repair enzymes XRCC1, XPA, and XPD. Mol. Vis. 2010, 16, 698–704. [Google Scholar]
- Kau, H.C.; Tsai, C.C.; Hsu, W.M.; Liu, J.H.; Wei, Y.H. Genetic polymorphism of hOGG1 and risk of pterygium in Chinese. Eye 2004, 18, 635–639. [Google Scholar] [CrossRef][Green Version]
- Wang, T.C.; Smith, K.C. Postreplication repair in ultraviolet-irradiated human fibroblasts: Formation and repair of DNA double-strand breaks. Carcinogenesis 1986, 7, 389–392. [Google Scholar] [CrossRef]
- Negritto, M.C. Repairing Double-Strand DNA Breaks. Nat. Educ. 2010, 3, 26. [Google Scholar]
- Tsai, Y.Y.; Bau, D.T.; Chiang, C.C.; Cheng, Y.W.; Tseng, S.H.; Tsai, F.J. Pterygium and genetic polymorphism of DNA double strand break repair gene Ku70. Mol. Vis. 2007, 13, 1436–1440. [Google Scholar]
- Lekawa-Ilczuk, A.; Antosz, H.; Rymgayllo-Jankowska, B.; Zarnowski, T. Expression of double strand DNA breaks repair genes in pterygium. Ophthalmic. Genet. 2011, 32, 39–47. [Google Scholar] [CrossRef]
- Han, J.; Colditz, G.A.; Samson, L.D.; Hunter, D.J. Polymorphisms in DNA double-strand break repair genes and skin cancer risk. Cancer Res. 2004, 64, 3009–3013. [Google Scholar] [CrossRef]
- Badadani, M. Autophagy Mechanism, Regulation, Functions, and Disorders. ISRN Cell Biol. 2012, 2012, 927064. [Google Scholar] [CrossRef]
- Sample, A.; He, Y.Y. Autophagy in UV Damage Response. Photochem. Photobiol. 2017, 93, 943–955. [Google Scholar] [CrossRef]
- Bustos, S.O.; Antunes, F.; Rangel, M.C.; Chammas, R. Emerging Autophagy Functions Shape the Tumor Microenvironment and Play a Role in Cancer Progression—Implications for Cancer Therapy. Front. Oncol. 2020, 10, 606436. [Google Scholar] [CrossRef]
- Zaarour, R.F.; Azakir, B.; Hajam, E.Y.; Nawafleh, H.; Zeinelabdin, N.A.; Engelsen, A.S.T.; Thiery, J.; Jamora, C.; Chouaib, S. Role of Hypoxia-Mediated Autophagy in Tumor Cell Death and Survival. Cancers 2021, 13, 533. [Google Scholar] [CrossRef]
- Luo, X.; Qiu, Y.; Dinesh, P.; Gong, W.; Jiang, L.; Feng, X.; Li, J.; Jiang, Y.; Lei, Y.L.; Chen, Q. The functions of autophagy at the tumour-immune interface. J. Cell. Mol. Med. 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; An, M. mTORC1 regulates apoptosis and cell proliferation in pterygium via targeting autophagy and FGFR3. Sci. Rep. 2017, 7, 7339. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.; Tang, W.Y.; Liu, Y.P.; Goh, H.S.; Smith, D.R. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br. J. Ophthalmol. 2000, 84, 212–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turan, M.; Turan, G. Bcl-2, p53, and Ki-67 expression in pterygium and normal conjunctiva and their relationship with pterygium recurrence. Eur. J. Ophthalmol. 2020, 1120672120945903. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ananthaswamy, H.N.; Muller, H.K.; Kripke, M.L. p53 protects against skin cancer induction by UV-B radiation. Oncogene 1999, 18, 4247–4253. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Rebel, H.; Mosnier, L.O.; Berg, R.J.; Westerman-de Vries, A.; van Steeg, H.; van Kranen, H.J.; de Gruijl, F.R. Early p53-positive foci as indicators of tumor risk in ultraviolet-exposed hairless mice: Kinetics of induction, effects of DNA repair deficiency, and p53 heterozygosity. Cancer Res. 2001, 61, 977–983. [Google Scholar]
- Hanel, W.; Moll, U.M. Links between mutant p53 and genomic instability. J. Cell Biochem. 2012, 113, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Sigal, A.; Rotter, V. Oncogenic mutations of the p53 tumor suppressor: The demons of the guardian of the genome. Cancer Res. 2000, 60, 6788–6793. [Google Scholar] [PubMed]
- Buschmann, T.; Minamoto, T.; Wagle, N.; Fuchs, S.Y.; Adler, V.; Mai, M.; Ronai, Z. Analysis of JNK, Mdm2 and p14(ARF) contribution to the regulation of mutant p53 stability. J. Mol. Biol. 2000, 295, 1009–1021. [Google Scholar] [CrossRef]
- Esrig, D.; Spruck, C.H., 3rd; Nichols, P.W.; Chaiwun, B.; Steven, K.; Groshen, S.; Chen, S.C.; Skinner, D.G.; Jones, P.A.; Cote, R.J. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am. J. Pathol. 1993, 143, 1389–1397. [Google Scholar] [PubMed]
- Kaye, P.V.; Ilyas, M.; Soomro, I.; Haider, S.A.; Atwal, G.; Menon, S.; Gill, S.; Richards, C.; Harrison, R.; West, K.; et al. Dysplasia in Barrett’s oesophagus: p53 immunostaining is more reproducible than haematoxylin and eosin diagnosis and improves overall reliability, while grading is poorly reproducible. Histopathology 2016, 69, 431–440. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Chang, K.C.; Lin, C.L.; Lee, H.; Tsai, F.J.; Cheng, Y.W.; Tseng, S.H. p53 Expression in pterygium by immunohistochemical analysis: A series report of 127 cases and review of the literature. Cornea 2005, 24, 583–586. [Google Scholar] [CrossRef]
- Schneider, B.G.; John-Aryankalayil, M.; Rowsey, J.J.; Dushku, N.; Reid, T.W. Accumulation of p53 protein in pterygia is not accompanied by TP53 gene mutation. Exp. Eye Res. 2006, 82, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Sasaki, M.; Maki, C.G. Regulation of p53 nuclear export through sequential changes in conformation and ubiquitination. J. Biol. Chem. 2007, 282, 14616–14625. [Google Scholar] [CrossRef]
- Cao, D.; Ng, T.K.; Yip, Y.W.Y.; Young, A.L.; Pang, C.P.; Chu, W.K.; Jhanji, V. p53 inhibition by MDM2 in human pterygium. Exp. Eye Res. 2018, 175, 142–147. [Google Scholar] [CrossRef]
- Cao, D.; Chu, W.K.; Ng, T.K.; Yip, Y.W.Y.; Young, A.L.; Pang, C.P.; Jhanji, V. Cellular Proliferation and Migration of Human Pterygium Cells: Mitomycin Versus Small-Molecule Inhibitors. Cornea 2018, 37, 760–766. [Google Scholar] [CrossRef]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar] [CrossRef]
- Choi, S.; Myers, J.N. Molecular pathogenesis of oral squamous cell carcinoma: Implications for therapy. J. Dent. Res. 2008, 87, 14–32. [Google Scholar] [CrossRef]
- Poh, C.F.; Ng, S.; Berean, K.W.; Williams, P.M.; Rosin, M.P.; Zhang, L. Biopsy and histopathologic diagnosis of oral premalignant and malignant lesions. J. Can. Dent. Assoc. 2008, 74, 283–288. [Google Scholar]
- Giroux, V.; Rustgi, A.K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat. Rev. Cancer 2017, 17, 594–604. [Google Scholar] [CrossRef]
- Scott, T.L.; Christian, P.A.; Kesler, M.V.; Donohue, K.M.; Shelton, B.; Wakamatsu, K.; Ito, S.; D’Orazio, J. Pigment-independent cAMP-mediated epidermal thickening protects against cutaneous UV injury by keratinocyte proliferation. Exp. Dermatol. 2012, 21, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, H.; Torronen, K.; Kumlin, T.; Rilla, K.; Tammi, M.I.; Tammi, R.H. Chronic UVR causes increased immunostaining of CD44 and accumulation of hyaluronan in mouse epidermis. J. Histochem. Cytochem. 2011, 59, 908–917. [Google Scholar] [CrossRef][Green Version]
- Turan, M.; Turan, G. Overexpression of fractalkine and its histopathological characteristics in primary pterygium. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.M.; Shaaban, Y.M.M.; Saad El-Din, S.A. Histopathological Parameters in Pterygia and Significant Clinical Correlations. J. Ophthalmic Vis. Res. 2018, 13, 110–118. [Google Scholar] [CrossRef]
- Chan, C.M.L.; Liu, Y.P.; Tan, D.T.H. Ocular surface changes in pterygium. Cornea 2002, 21, 38–42. [Google Scholar] [CrossRef]
- Tirado, A.G.; de los Bueis, A.B.; Jara, L.R. Ocular surface changes in recurrent pterygium cases post-operatively treated with 5-fluorouracil subconjunctival injections. Eur. J. Ophthalmol. 2019, 29, 9–14. [Google Scholar] [CrossRef]
- Soria, J.; Acera, A.; Duran, J.A.; Boto-de-los-Bueis, A.; Del-Hierro-Zarzuelo, A.; Gonzalez, N.; Reigada, R.; Suarez, T. The analysis of human conjunctival epithelium proteome in ocular surface diseases using impression cytology and 2D-DIGE. Exp. Eye Res. 2018, 167, 31–43. [Google Scholar] [CrossRef]
- Endo, H.; Kase, S.; Suzuki, Y.; Kase, M. Coincidence of Inflamed Conjunctival Carcinoma in situ and Primary Pterygium. Case Rep. Ophthalmol. 2016, 7, 208–212. [Google Scholar] [CrossRef]
- Telgote, V.; Karole, C.; Varma, P.; Meshram, P. Conjunctival Impression Cytology in Diagnosis of Dry Eye in Presence of Normal Tear Film Function. J. Evol. Med. Dent. Sci. 2016, 5, 4272–4276. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Wang, G.L.; Liu, Z.; Chen, P.; Yang, Q.C.; Dong, N.; Wu, H.P.; Liu, Z.G.; Li, W. APR-246/PRIMA-1(Met) Inhibits and Reverses Squamous Metaplasia in Human Conjunctival Epithelium. Investig. Ophth. Vis. Sci. 2016, 57, 444–452. [Google Scholar] [CrossRef]
- Han, S.B.; Yang, H.K.; Hyon, J.Y.; Wee, W.R. Conjunctival metaplasia after pterygium excision and limbal autograft. Optom. Vis. Sci. 2015, 92, 324–328. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Nag, D.; Mondal, S.K.; Gangopadhyay, S.; Bagchi, K.; Bhaduri, G. Ocular surface disorder in pterygium: Role of conjunctival impression cytology. Indian J. Pathol. Microbiol. 2010, 53, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Lai, W.T.; Liou, S.W.; Chiu, C.Z.; Hu, F.R.; Kao, W.W.Y.; Hung, P.T. Impression cytology of pterygium. J. Ocul. Pharmacol. Ther. 2000, 16, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P. Chapter 12—Female Genital Tract. In Histopathology of Preclinical Toxicity Studies, 4th ed.; Greaves, P., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 667–723. [Google Scholar]
- Messmer, E.M.; Mackert, M.J.; Zapp, D.M.; Kampik, A. In vivo confocal microscopy of normal conjunctiva and conjunctivitis. Cornea 2006, 25, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.B.; Kretz, M.; Ridky, T.W.; Kimmel, R.; Khavari, P.A. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006, 20, 3185–3197. [Google Scholar] [CrossRef]
- Yoon, S.; Leube, R.E. Keratin intermediate filaments: Intermediaries of epithelial cell migration. Essays Biochem. 2019, 63, 521–533. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Merjava, S.; Neuwirth, A.; Tanzerova, M.; Jirsova, K. The spectrum of cytokeratins expressed in the adult human cornea, limbus and perilimbal conjunctiva. Histol. Histopathol. 2011, 26, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Sha, X.Y.; Liu, Y.; Yang, R.M.; Wen, Y. Pterygium epithelium abnormal differentiation related to activation of extracellular signal-regulated kinase signaling pathway in vitro. Int. J. Ophthalmol. 2015, 8, 1118–1125. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Green, W.R.; Luckenbach, M.; Chan, C.C. Conjunctival lesions in adults. A clinical and histopathologic review. Cornea 1987, 6, 78–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Hirst, L.W. Ocular surface squamous neoplasia. Surv. Ophthalmol. 1995, 39, 429–450. [Google Scholar] [CrossRef]
- Colby, J.K.; Klein, R.D.; McArthur, M.J.; Conti, C.J.; Kiguchi, K.; Kawamoto, T.; Riggs, P.K.; Pavone, A.I.; Sawicki, J.; Fischer, S.M. Progressive metaplastic and dysplastic changes in mouse pancreas induced by cyclooxygenase-2 overexpression. Neoplasia 2008, 10, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R. 6—Malignant neoplasms of the larynx, hypopharynx, and trachea. In Head and Neck Pathology, 2nd ed.; Thompson, L.D.R., Goldblum, J.R., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 144–179. [Google Scholar]
- Oellers, P.; Karp, C.L.; Sheth, A.; Kao, A.A.; Abdelaziz, A.; Matthews, J.L.; Dubovy, S.R.; Galor, A. Prevalence, treatment, and outcomes of coexistent ocular surface squamous neoplasia and pterygium. Ophthalmology 2013, 120, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Karp, C.L.; Oellers, P.; Kao, A.A.; Abdelaziz, A.; Feuer, W.; Dubovy, S.R. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology 2012, 119, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Artornsombudh, P.; Sanpavat, A.; Tinnungwattana, U.; Tongkhomsai, V.; Sansopha, L.; Tulvatana, W. Prevalence and clinicopathologic findings of conjunctival epithelial neoplasia in pterygia. Ophthalmology 2013, 120, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Hirst, L.W.; Axelsen, R.A.; Schwab, I. Pterygium and associated ocular surface squamous neoplasia. Arch. Ophthalmol. 2009, 127, 31–32. [Google Scholar] [CrossRef]
- Lomeli-Linares, D.; Garcia-Salgado, L.; Riancho-Sanchez, G.; Lopez-Star, E.; Lansingh, V.C.; Corredor-Casas, S. Frequency of conjunctival epithelial dysplasia in patients with pterygium. Arq. Bras. Oftalmol. 2020, 83, 323–328. [Google Scholar] [CrossRef]
- Singh, R.; Joseph, A.; Umapathy, T.; Tint, N.L.; Dua, H.S. Impression cytology of the ocular surface. Br. J. Ophthalmol. 2005, 89, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.C.; Lin, C.L.; Chen, Z.T.; Hu, F.R.; Sung, F.C.; Wang, I.J. Risk of skin cancer in patients with pterygium: A nationwide population-based cohort study in Taiwan. Ocul. Surf. 2014, 12, 69–76. [Google Scholar] [CrossRef]
- Ayanniyi, A.A.; Badmos, K.B.; Olatunji, F.O.; Owoeye, J.; Sanni, T.O. Blindness Caused by Pterygium—A Case Report. Sierra Leone J. Biomed. Res. 2011, 3, 60–62. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach. In The Development and Causes of Cancer; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9963/ (accessed on 1 June 2021).
- Heinze, K.; Pham, C.; Lin, A.; Setabutr, P. Malignant Conversion of Eyelid Capillary Hemangioma to Cutaneous Angiosarcoma. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e120–e122. [Google Scholar] [CrossRef] [PubMed]
- Nathenson, M.J.; Molavi, D.; Aboulafia, A. Angiosarcoma arising in a patient with a 10-year-old hemangioma. Case Rep. Oncol. Med. 2014, 2014, 185323. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Esposito, G.; Pilozzi, E.; Purchiaroni, F.; Corleto, V.D.; Di Giulio, E.; Annibale, B. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand. J. Gastroenterol. 2015, 50, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Liang, P.S.; Bang, S.J.; Hwang, J.H. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest. Endosc. 2016, 84, 18–28. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Acker, S.I.; Van den Bogerd, B.; Haagdorens, M.; Siozopoulou, V.; Ní Dhubhghaill, S.; Pintelon, I.; Koppen, C. Pterygium—The Good, the Bad, and the Ugly. Cells 2021, 10, 1567. https://doi.org/10.3390/cells10071567
Van Acker SI, Van den Bogerd B, Haagdorens M, Siozopoulou V, Ní Dhubhghaill S, Pintelon I, Koppen C. Pterygium—The Good, the Bad, and the Ugly. Cells. 2021; 10(7):1567. https://doi.org/10.3390/cells10071567
Chicago/Turabian StyleVan Acker, Sara I., Bert Van den Bogerd, Michel Haagdorens, Vasiliki Siozopoulou, Sorcha Ní Dhubhghaill, Isabel Pintelon, and Carina Koppen. 2021. "Pterygium—The Good, the Bad, and the Ugly" Cells 10, no. 7: 1567. https://doi.org/10.3390/cells10071567
APA StyleVan Acker, S. I., Van den Bogerd, B., Haagdorens, M., Siozopoulou, V., Ní Dhubhghaill, S., Pintelon, I., & Koppen, C. (2021). Pterygium—The Good, the Bad, and the Ugly. Cells, 10(7), 1567. https://doi.org/10.3390/cells10071567