Emerging Role and Clinicopathological Significance of AEG-1 in Different Cancer Types: A Concise Review
Abstract
1. Introduction
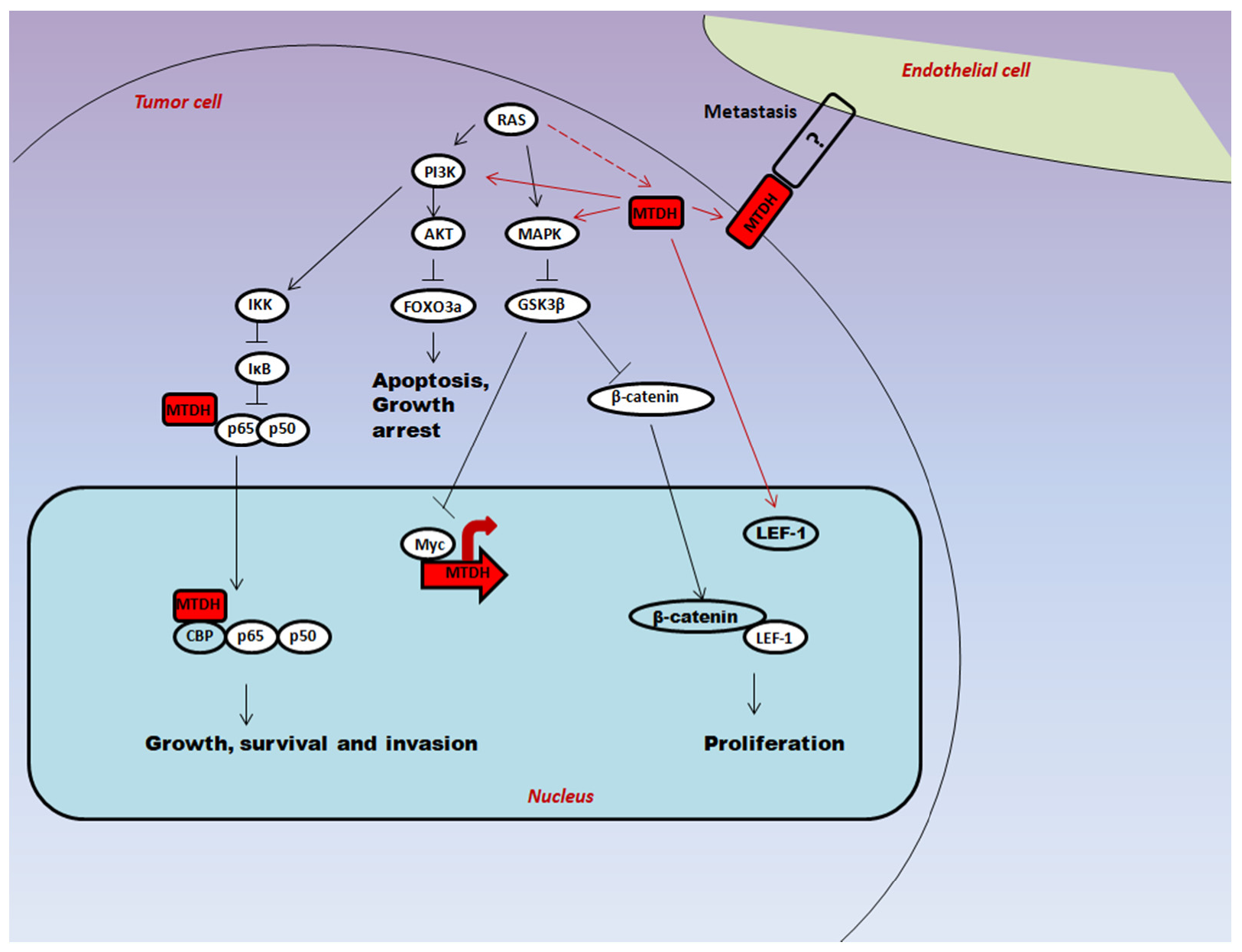
2. Molecular Mechanism of Multifunctional Protein AEG-1 in Carcinogenesis
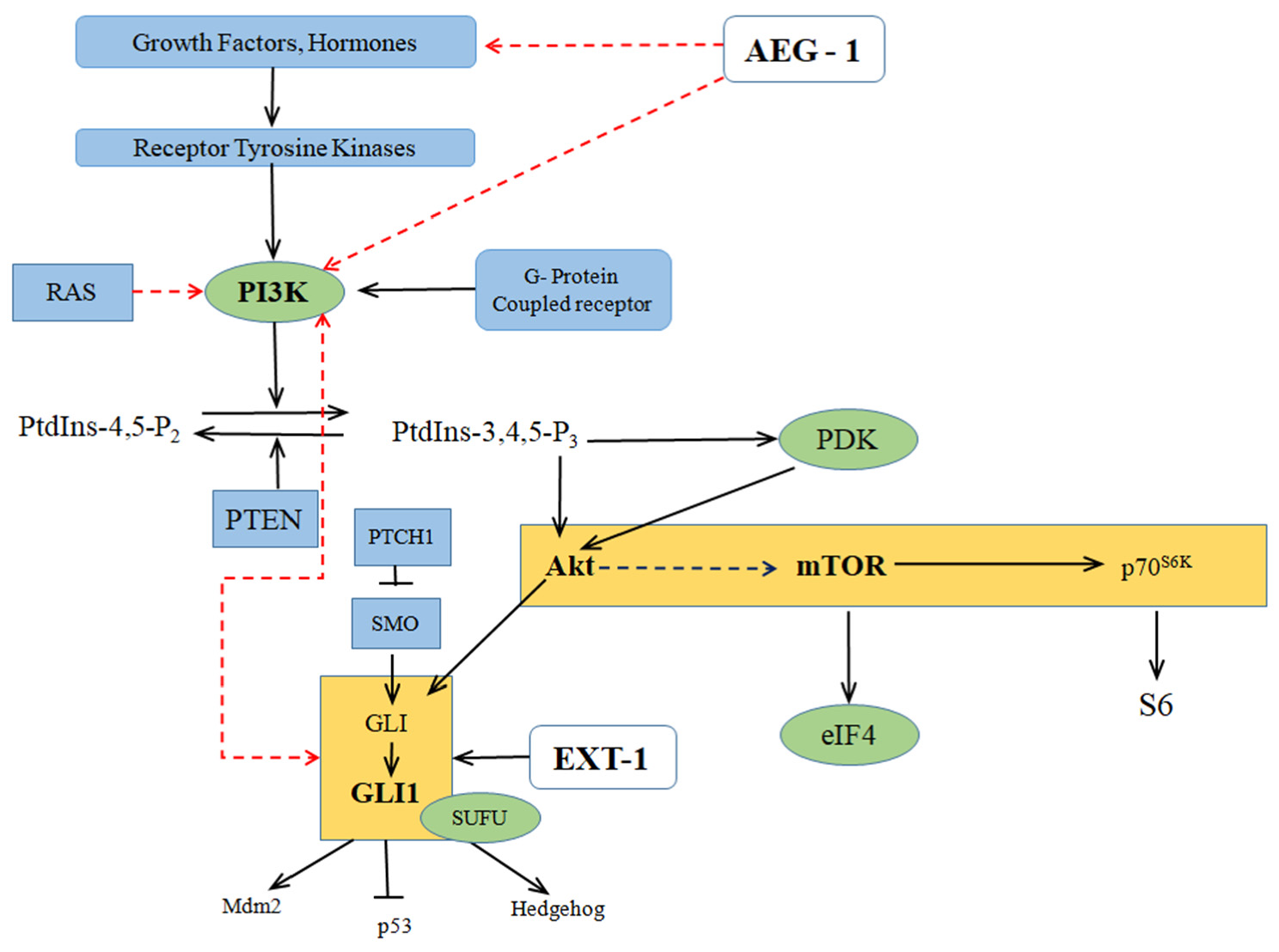
2.1. Oncogenic Ha-Ras Induces AEG-1 via PI3K/AKT Signaling
2.2. Regulation of Downstream Target Proteins of PI3K/AKT Signaling by AEG-1
2.3. Crosstalk between the PI3K/AKT/mTOR and Hedgehog Signaling
3. Multifaceted Functions of AEG-1 in Cancer Progression
3.1. Proliferation
3.2. Angiogenesis
3.3. Metastasis
4. Expression of AEG-1 in Different Cancer Types
4.1. Esophageal Carcinoma
4.2. Gastric Cancer
4.3. Colorectal Cancer
4.4. Hepatocellular Carcinoma
4.5. Gall Bladder Carcinoma
4.6. Breast Cancer
4.7. Prostate Cancer
4.8. Non-Small Cell Lung Cancer
4.9. Glioma Cancer
4.10. Osteosarcoma
5. Overview on Clinicopathological Significance of AEG-1 in Different Cancer Types
6. MicroRNAs and Silencing RNA as Effective AEG-1 Inhibitors
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britt, D.E.; Yang, D.-F.; Flanagan, D.; Callanan, H.; Lim, Y.-P.; Lin, S.-H.; Hixson, D.C. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp. Cell Res. 2004, 300, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Gnosa, S.; Shen, Y.-M.; Wang, C.-J.; Zhang, H.; Stratmann, J.; Arbman, G.; Sun, X.-F. Expression of AEG-1 mRNA and protein in colorectal cancer patients and colon cancer cell lines. J. Transl. Med. 2012, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, K.; Zheng, H.; Guo, X.; Jia, W.; Li, M.; Zeng, M.; Li, J.; Song, L. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis 2009, 30, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Jian-Bo, X.; Hui, W.; Yu-Long, H.; Chang-Hua, Z.; Long-Juan, Z.; Shi-Rong, C.; Wen-Hua, Z. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Med. Oncol. 2010, 28, 455–462. [Google Scholar] [CrossRef]
- Gnosa, S.; Zhang, H.; Brodin, V.P.; Carstensen, J.; Adell, G.; Sun, X.-F. AEG-1 expression is an independent prognostic factor in rectal cancer patients with preoperative radiotherapy: A study in a Swedish clinical trial. Br. J. Cancer 2014, 111, 166–173. [Google Scholar] [CrossRef]
- Yoo, B.K.; Emdad, L.; Su, Z.-Z.; Villanueva, A.; Chiang, D.Y.; Mukhopadhyay, N.D.; Mills, A.S.; Waxman, S.; Fisher, R.A.; Llovet, J.M.; et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J. Clin. Investig. 2009, 119, 465–477. [Google Scholar] [CrossRef]
- Srivastava, J.; Siddiq, A.; Emdad, L.; Santhekadur, P.K.; Chen, D.; Gredler, R.; Shen, X.N.; Robertson, C.L.; Dumur, C.I.; Hylemon, P.B.; et al. Astrocyte elevated gene-1 (AEG-1) promotes hepatocarcinogenesis: Novel insights from a mouse model. Hepatology 2012, 56, 1782–1791. [Google Scholar] [CrossRef]
- Li, M.; Dai, Y.; Wang, L.; Li, L. Astrocyte elevated gene-1 promotes the proliferation and invasion of breast cancer cells by activating the Wnt/β-catenin signaling pathway. Oncol. Lett. 2017, 13, 2385–2390. [Google Scholar] [CrossRef][Green Version]
- Lee, H.J.; Jung, D.B.; Sohn, E.J.; Kim, H.H.; Park, M.N.; Lew, J.H.; Lee, S.G.; Kim, B.L.; Kim, S.H. Inhibition of Hypoxia Inducible Factor Alpha and Astrocyte Elevated Gene-1 Mediates Cryptotanshinone Exerted Antitumor Activity in Hypoxic PC-3 Cells. Evid. Based Complement. Alt. Med. 2012, 13, 390957. [Google Scholar] [CrossRef]
- Milhem, M.M.; Knutson, T.; Yang, S.; Zhu, D.; Wang, X.; Leslie, K.K.; Meng, X. Correlation of MTDH/AEG-1 and HOTAIR Expression with Metastasis and Response to Treatment in Sarcoma Patients. J. Cancer Sci. Ther. 2012, 1. [Google Scholar] [CrossRef]
- Lee, S.-G.; Su, Z.-Z.; Emdad, L.; Sarkar, D.; Fisher, P.B. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc. Natl. Acad. Sci. USA 2006, 103, 17390–17395. [Google Scholar] [CrossRef]
- Sarkar, D.; Park, E.S.; Emdad, L.; Lee, S.G.; Su, Z.Z.; Fisher, P.B. Molecular basis of nuclear factor-kappa-B activation by astrocyte elevated gene-1. Cancer Res. 2008, 68, 1478–1484. [Google Scholar] [CrossRef]
- Emdad, L.; Lee, S.-G.; Su, Z.Z.; Jeon, H.Y.; Boukerche, H.; Sarkar, D.; Fisher, P.B. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21300–21305. [Google Scholar] [CrossRef]
- Yoo, B.K.; Emdad, L.; Lee, S.-G.; Su, Z.-Z.; Santhekadur, P.; Chen, D.; Gredler, R.; Fisher, P.B.; Sarkar, D. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol. Ther. 2011, 130, 1–8. [Google Scholar] [CrossRef]
- Kong, B.; Zhao, Y.; Moran, M.S.; Yang, Q.; Liu, Q.; Yuan, C.; Hong, S. Metadherin regulates radioresistance in cervical cancer cells. Oncol. Rep. 2012, 27, 1520–1526. [Google Scholar] [CrossRef]
- Brown, D.M.; Ruoslahti, E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 2004, 5, 365–374. [Google Scholar] [CrossRef]
- Ostad, S.N.; Parsa, M. Breast Cancer from Molecular Point of View: Pathogenesis and Biomarkers, Breast Cancer—Focusing Tumor Microenvironment, Stem cells and Metastasis; Gunduz, M., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-766-6. [Google Scholar] [CrossRef]
- Kauffmann-Zeh, A.; Rodriquez-Viciana, P.; Elrich, E.; Gilbert, C.; Coffer, P.; Downward, J.; Evan, G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI (3) K and PKB. Nature 1997, 385, 544–548. [Google Scholar] [CrossRef]
- Ying, Z.; Li, J.; Li, M. Astrocyte elevated gene 1: Biological functions and molecular mechanism in cancer and beyond. Cell Biosci. 2011, 1, 36. [Google Scholar] [CrossRef]
- Lee, S.-G.; Su, Z.-Z.; Emdad, L.; Sarkar, D.; Franke, T.F.; Fisher, P.B. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 2007, 27, 1114–1121. [Google Scholar] [CrossRef]
- Noch, E.; Bookland, M.; Khalili, K. Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose deprivation in glioblastoma. Cancer Biol. Ther. 2011, 11, 32–39. [Google Scholar] [CrossRef]
- Li, J.; Yang, L.; Song, L.; Xiong, H.; Wang, L.; Yan, X.; Yuan, J.; Wu, J.; Li, M. Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene 2009, 28, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ren, M.; Jiang, H.; Cui, S.; Wang, S.; Jiang, H.; Qi, Y.; Wang, J.; Wang, X.; Dong, G.; et al. Downregulated AEG-1 together with inhibited PI3K/Akt pathway is associated with reduced viability of motor neurons in an ALS model. Mol. Cell. Neurosci. 2015, 68, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Kikuno, N.; Shiina, H.; Urakami, S.; Kawamoto, K.; Hirata, H.; Tanaka, Y.; Place, R.F.; Pookot, D.; Majid, S.; Igawa, M.; et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene 2007, 26, 7647–7655. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Emdad, L.; Bacolod, M.D.; Kegelman, T.P.; Shen, X.-N.; Alzubi, M.A.; Das, S.K.; Sarkar, D.; Fisher, P.B. Astrocyte elevated gene-1 interacts with akt isoform 2 to control glioma growth, survival, and pathogenesis. Cancer Res. 2014, 74, 7321–7332. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Wei, L.; Xu, Z. Cross-signaling among phosphinositide-3 kinase, mitogen-activated protein kinase and sonic hedgehog pathways exists in esophageal cancer. Int. J. Cancer 2010, 129, 275–284. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Q.; Yen, C.-J.; Xia, W.; Izzo, J.G.; Lang, J.-Y.; Li, C.-W.; Hsu, J.L.; Miller, S.A.; Wang, X.; et al. The crosstalk of mTOR/S6K1 and hedgehog pathways. Cancer Cell 2012, 21, 374–387. [Google Scholar] [CrossRef]
- Das, S.; Samant, R.S.; Shevde, L.A. Nonclassical activation of hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to smoothened-targeting hedgehog inhibition. J. Biol. Chem. 2013, 288, 11824–11833. [Google Scholar] [CrossRef]
- Yoo, Y.A.; Kang, M.H.; Lee, H.J.; Kim, B.-H.; Park, J.K.; Kim, H.K.; Kim, J.S.; Oh, S.C. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011, 71, 7061–7070. [Google Scholar] [CrossRef]
- Kaylani, S.Z.; Xu, J.; Srivastava, R.K.; Kopelovich, L.; Pressey, J.G.; Athar, M. Rapamycin targeting mTOR and hedgehog signaling pathways blocks human rhabdomyosarcoma growth in xenograft murine model. Biochem. Biophys. Res. Commun. 2013, 435, 557–561. [Google Scholar] [CrossRef]
- Ng, J.M.Y.; Curran, T. The Hedgehog’s tale: Developing strategies for targeting cancer. Nat. Rev. Cancer 2011, 11, 493–501. [Google Scholar] [CrossRef]
- Justilien, V.; Fields, A.P. Molecular pathways: Novel approaches for improved therapeutic targeting of hedgehog signaling in cancer stem cells. Clin. Cancer Res. 2015, 21, 505–513. [Google Scholar] [CrossRef]
- Di Magno, L.; Coni, S.; Di Marcotullio, L.; Canettieri, G. Digging a hole under Hedgehog: Downstream inhibition as an emerging anticancer strategy. Biochim. Biophys. Acta. 2015, 1856, 62–72. [Google Scholar] [CrossRef]
- D’Amico, D.; Canettieri, G. Translating hedgehog in cancer: Controlling protein synthesis. Trends Mol. Med. 2016, 22, 851–862. [Google Scholar] [CrossRef]
- Dimitrova, V.; Arcaro, A. Targeting the PI3K/AKT/mTOR signaling pathway in medulloblastoma. Curr. Mol. Med. 2015, 15, 82–93. [Google Scholar] [CrossRef]
- Brechbiel, J.; Miller-Moslin, K.; Adjei, A.A. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat. Rev. 2014, 40, 750–759. [Google Scholar] [CrossRef]
- Larsen, L.J.; Møller, L.B. Crosstalk of Hedgehog and mTORC1 Pathways. Cells 2020, 9, 2316. [Google Scholar] [CrossRef]
- Sriramulu, S.; Malayaperumal, S.; Nandy, S.K.; Banerjee, A.; Essa, M.M.; Chidambaram, S.; Qoronfleh, M.W.; Pathak, S. Silencing of Astrocyte Elevated Gene-1 (AEG-1) inhibits the proliferative and invasive potential through interaction with Exostosin-1 (EXT-1) in primary and metastatic colon cancer cells. BioCell 2021, 45, 563–576. [Google Scholar] [CrossRef]
- Sriramulu, S.; Nandy, S.K.; Ganesan, H.; Banerjee, A.; Pathak, S. In silico analysis and prediction of transcription factors of the proteins interacting with astrocyte elevated gene-1. Comput. Biol. Chem. 2021, 92, 107478. [Google Scholar] [CrossRef]
- Agarwal, N.K.; Qu, C.; Kunkulla, K.; Liu, Y.; Vega, F. Transcriptional regulation of serine/threonine protein kinase (AKT) genes by glioma-associated oncogene homolog 1. J. Biol. Chem. 2013, 288, 15390–15401. [Google Scholar] [CrossRef]
- Pawłowski, K.M.; Majewska, A.; Szyszko, K.; Dolka, I.; Motyl, T.; Król, M. Gene expression pattern in canine mammary osteosarcoma. Pol. J. Vet. Sci. 2011, 14, 11–20. [Google Scholar] [CrossRef]
- Eckerdt, F.D.; Goldman, S.; Platanias, L.C. New insights into malignant cell survival mechanisms in medulloblastoma. Cancer Cell Microenviron. 2015, 1, 374. [Google Scholar] [CrossRef]
- Chaturvedi, N.K.; Kling, M.J.; Coulter, D.W.; McGuire, T.R.; Ray, S.; Kesherwani, V.; Joshi, S.S.; Sharp, J.G. Improved therapy for medulloblastoma: Targeting hedgehog and PI3K-mTOR signaling pathways in combination with chemotherapy. Oncotarget 2018, 9, 16619–16633. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X. The role of MTDH/AEG-1 in the progression of cancer. Int. J. Clin. Exp. Med. 2015, 8, 4795–4807. [Google Scholar]
- Ke, Z.-F.; Mao, X.; Zeng, C.; He, S.; Li, S.; Wang, L.-T. AEG-1 expression characteristics in human non-small cell lung cancer and its relationship with apoptosis. Med. Oncol. 2013, 30, 1–9. [Google Scholar] [CrossRef]
- Kang, D.-C.; Su, Z.-Z.; Sarkar, D.; Emdad, L.; Volsky, D.J.; Fisher, P.B. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene 2005, 353, 8–15. [Google Scholar] [CrossRef]
- Nikpour, M.; Emadi-Baygi, M.; Fischer, U.; Niegisch, G.; Schulz, W.A.; Nikpour, P. MTDH/AEG-1 contributes to central features of the neoplastic phenotype in bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 670–677. [Google Scholar] [CrossRef]
- Emdad, L.; Sarkar, D.; Lee, S.-G.; Su, Z.Z.; Yoo, B.K.; Dash, R.; Yacoub, A.; Fuller, C.E.; Shah, K.; Dent, P.; et al. Astrocyte elevated gene-1: A novel target for human glioma therapy. Mol. Cancer Ther. 2010, 9, 79–88. [Google Scholar] [CrossRef]
- Liu, H.; Song, X.; Liu, C.; Xie, L.; Wei, L.; Sun, R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. J. Exp. Clin. Cancer Res. 2009, 28, 19. [Google Scholar] [CrossRef]
- Su, P.; Zhang, Q.; Yang, Q. Immunohistochemical analysis of Metadherin in proliferative and cancerous breast tissue. Diagn. Pathol. 2010, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, X.; Huo, Q.; Sun, M.; Cai, C.; Liu, Z.; Hu, G.; Yang, Q. MicroRNA-30a suppresses breast tumor growth and metastasis by targeting metadherin. Oncogene 2013, 33, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, R.; Song, H.; Wang, D.; Feng, T.; Yu, X.; Zhao, Y.; Liu, J.; Yu, X.; Wang, Y.; et al. Significance of aeg-1 expression in correlation with vegf, microvessel density and clinicopathological characteristics in triple-negative breast cancer. J. Surg. Oncol. 2011, 103, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Jeon, H.-Y.; Su, Z.-Z.; Richards, J.E.; Vozhilla, N.; Sarkar, D.; Van Maerken, T.; Fisher, P.B. Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene 2009, 28, 2476–2484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, S.; Ke, Z.; Wang, F.; Li, S.; Chen, W.; Han, A.; Wang, Z.; Shi, H.; Wang, L.-T.; Chen, X. Overexpression of astrocyte-elevated gene-1 is closely correlated with poor prognosis in human non–small cell lung cancer and mediates its metastasis through up-regulation of matrix metalloproteinase-9 expression. Hum. Pathol. 2012, 43, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ke, Z.-F.; Sun, S.-J.; Chen, W.-F.; Yang, S.-C.; Li, S.-H.; Mao, X.-P.; Wang, L.-T. Oncogenic roles of astrocyte elevated gene-1 (AEG-1) in osteosarcoma progression and prognosis. Cancer Biol. Ther. 2011, 12, 539–548. [Google Scholar] [CrossRef]
- Hu, G.; Chong, R.A.; Yang, Q.; Wei, Y.; Blanco, M.A.; Li, F.; Reiss, M.; Au, J.L.-S.; Haffty, B.G.; Kang, Y. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell 2009, 15, 9–20. [Google Scholar] [CrossRef]
- Zhou, Z.; Deng, H.; Yan, W.; Huang, H.; Deng, Y.; Li, Y.; Tian, D. Expression of metadherin/AEG-1 gene is positively related to orientation chemotaxis and adhesion of human hepatocellular carcinoma cell lines of different metastatic potentials. Acta Acad. Med. Wuhan 2012, 32, 353–357. [Google Scholar] [CrossRef]
- Jiang, T.; Zhu, A.; Zhu, Y.; Piao, D. Clinical implications of AEG-1 in liver metastasis of colorectal cancer. Med. Oncol. 2012, 29, 2858–2863. [Google Scholar] [CrossRef]
- Liu, D.C.; Yang, Z.L. MTDH and EphA7 are markers for metastasis and poor prognosis of gallbladder adenocarcinoma. Diagn Cytopathol. 2013, 41, 199–205. [Google Scholar] [CrossRef]
- Chen, W.; Ke, Z.; Shi, H.; Yang, S.; Wang, L. Overexpression of AEG-1 in renal cell carcinoma and its correlation with tumor nuclear grade and progression. Neoplasma 2010, 57, 522–529. [Google Scholar] [CrossRef][Green Version]
- Wan, L.; Hu, G.; Wei, Y.; Yuan, M.; Bronson, R.T.; Yang, Q.; Siddiqui, J.; Pienta, K.J.; Kang, Y. Genetic ablation of metadherin inhibits autochthonous prostate cancer progression and metastasis. Cancer Res. 2014, 74, 5336–5347. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Lu, R.; Yu, G.; Wang, X.; Zhao, Y.; Song, H.; Lin, P.; Sun, X.; Yu, X.; et al. AEG -1 overexpression: A novel indicator for peritoneal dissemination and lymph node metastasis in epithelial ovarian cancers. Int. J. Gynecol. Cancer 2011, 21, 602–608. [Google Scholar] [CrossRef]
- Turley, E.A.; Veiseh, M.; Radisky, D.C.; Bissell, M.J. Mechanisms of Disease: Epithelial–mesenchymal transition—does cellular plasticity fuel neoplastic progression? Nat. Clin. Pract. Oncol. 2008, 5, 280–290. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Huo, Q.; Guo, H.; Yan, S.; Yuan, C.; Moran, M.S.; Shao, C.; Yang, Q. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci. 2011, 102, 1151–1157. [Google Scholar] [CrossRef]
- Ward, A.; Balwierz, A.; Zhang, J.D.; Küblbeck, M.; Pawitan, Y.; Hielscher, T.; Wiemann, S. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene 2012, 32, 1173–1182. [Google Scholar] [CrossRef]
- Liu, K.; Guo, L.; Miao, L.; Bao, W.; Yang, J.; Li, X.; Xi, T.; Zhao, W. Ursolic acid inhibits epithelial mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human non small cell lung cancer A549 cells. Anticancer Drugs 2013, 24, 494–503. [Google Scholar] [CrossRef]
- Zhu, K.; Dai, Z.; Pan, Q.; Wang, Z.; Yang, G.-H.; Yu, L.; Ding, Z.-B.; Shi, G.-M.; Ke, A.-W.; Yang, X.-R.; et al. Metadherin Promotes Hepatocellular Carcinoma Metastasis through Induction of Epithelial–Mesenchymal Transition. Clin. Cancer Res. 2011, 17, 7294–7302. [Google Scholar] [CrossRef]
- Tang, J.; Shen, L.; Yang, Q.; Zhang, C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014, 47, 427–434. [Google Scholar] [CrossRef]
- Yu, C.; Liu, Y.; Tan, H.; Li, G.; Su, Z.; Ren, S.; Zhu, G.; Tian, Y.; Qiu, Y.; Zhang, X. Metadherin regulates metastasis of squamous cell carcinoma of the head and neck via AKT signalling pathway-mediated epithelial–mesenchymal transition. Cancer Lett. 2014, 343, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Brachova, P.; Yang, S.; Xiong, Z.; Zhang, Y.; Thiel, K.W.; Leslie, K.K. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS ONE 2011, 6, e20920. [Google Scholar] [CrossRef] [PubMed]
- Nohata, N.; Hanazawa, T.; Kikkawa, N.; Mutallip, M.; Sakurai, D.; Fujimura, L.; Kawakami, K.; Chiyomaru, T.; Yoshino, H.; Enokida, H.; et al. Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC). J. Hum. Genet. 2011, 56, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.I.; Devesa, S.S. Cancer burden in the year 2000. The global picture. Eur. J. Cancer 2001, 37, S4–S66. [Google Scholar] [CrossRef]
- Sarkar, D.; Emdad, L.; Lee, S.-G.; Yoo, B.K.; Su, Z.-Z.; Fisher, P.B. Astrocyte elevated gene-1: Far more than just a gene regulated in astrocytes. Cancer Res. 2009, 69, 8529–8535. [Google Scholar] [CrossRef]
- Hu, G.; Wei, Y.; Kang, Y. The multifaceted role of MTDH/AEG-1 in cancer progression: Figure 1. Clin. Cancer Res. 2009, 15, 5615–5620. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, G.; Kang, Y. Metadherin as a link between metastasis and chemoresistance. Cell Cycle 2009, 8, 2131–2137. [Google Scholar] [CrossRef]
- Yoo, B.K.; Gredler, R.; Vozhilla, N.; Su, Z.-Z.; Chen, D.; Forcier, T.; Shah, K.; Saxena, U.; Hansen, U.; Fisher, P.B.; et al. Identification of genes conferring resistance to 5-fluorouracil. Proc. Natl. Acad. Sci. USA 2009, 106, 12938–12943. [Google Scholar] [CrossRef]
- Sutherland, H.G.; Lam, Y.W.; Briers, S.; Lamond, A.; A Bickmore, W. 3D3/lyric: A novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Exp. Cell Res. 2004, 294, 94–105. [Google Scholar] [CrossRef]
- Emdad, L.; Sarkar, D.; Su, Z.Z.; Randolph, A.; Boukerche, H.; Valerie, K.; Fisher, P.B. Activation of nuclear factor kappa B pathway by astrocyte elevated gene-1: Implications for tumor progression and metastasis. Cancer Res. 2006, 66, 1509–1516. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kosuge, T.; Shimada, K.; Sano, T.; Hibi, T.; Yamamoto, J.; Takayama, T.; Makuuchi, M. Clinical significance of extra hepatic bile duct resection for advanced gallbladder cancer. J. Surg. Oncol. 2006, 94, 298–306. [Google Scholar] [CrossRef]
- Dixon, E.; Vollmer, C.M., Jr.; Sahajpal, A.; Cattral, M.; Grant, D.; Doig, C.; Hemming, A.; Taylor, B.; Langer, B.; Greig, P.; et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: A 12-year study at a North American Center. Ann. Surg. 2005, 241, 385–394. [Google Scholar] [CrossRef]
- Lai, E.C.H.; Lau, W.Y. Aggressive Surgical Resection for Carcinoma of Gallbladder. ANZ J. Surg. 2005, 75, 441–444. [Google Scholar] [CrossRef]
- Amaro, J.; Severo, M.; Vilela, S.; Fonseca, S.; Fontes, F.; La Vecchia, C.; Lunet, N. Patterns of breast cancer mortality trends in Europe. Breast 2013, 22, 244–253. [Google Scholar] [CrossRef]
- Justo, N.; Wilking, N.; Jönsson, B.; Luciani, S.; Cazap, E. A Review of Breast Cancer Care and Outcomes in Latin America. Oncologist 2013, 18, 248–256. [Google Scholar] [CrossRef]
- Li, N.; Zheng, R.-S.; Zhang, S.-W.; Zou, X.-N.; Zeng, H.-M.; Dai, Z.; Chen, W.-Q. Analysis and prediction of breast cancer incidence trend in China. Zhonghua Yu Fang Yi Xue Za Zhi Chin. J. Prev. Med. 2012, 46, 703–707. [Google Scholar]
- Dodiyi Manuel, A.; Wakama, I.E. Predispositions of carcinoma of the breast: A review. Niger J. Med. 2014, 23, 7–12. [Google Scholar]
- Nasr, Z.; Robert, F.; Porco, J.A.; Muller, W.J.; Pelletier, J. eIF4F suppression in breast cancer affects maintenance and progression. Oncogene 2012, 32, 861–871. [Google Scholar] [CrossRef]
- Xu, Z.-D.; Li, Q.-Q.; Cheng, Y.-Y.; Xu, J.-W.; Tao, L.-L.; Yu, J.; Chen, Q.; Liu, X.-P. Involvement of EGFR in the promotion of malignant properties in multidrug resistant breast cancer cells. Int. J. Oncol. 2011, 39, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Huang, O.; Zhang, W.; Zhi, Q.; Xue, X.; Liu, H.; Shen, D.; Geng, M.; Xie, Z.; Jiang, M. Featured Article: Teriflunomide, an immunomodulatory drug, exerts anticancer activity in triple negative breast cancer cells. Exp. Biol. Med. 2015, 240, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Shargh, S.A.; Sakizli, M.; Khalaj, V.; Movafagh, A.; Yazdi, H.; Hagigatjou, E.; Sayad, A.; Mansouri, N.; Mortazavi-Tabatabaei, S.A.; Khorshid, H.R.K. Downregulation of E-cadherin expression in breast cancer by promoter hypermethylation and its relation with progression and prognosis of tumor. Med. Oncol. 2014, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Kang, Y. Pleiotropic roles of AEG 1/MTDH/LYRIC in breast cancer. Adv. Cancer Res. 2013, 120, 113–134. [Google Scholar]
- Emdad, L.; Das, S.K.; Dasgupta, S.; Hu, B.; Sarkar, D.; Fisher, P.B. AEG 1/MTDH/LYRIC: Signaling pathways, downstream genes, interacting proteins, and regulation of tumor angiogenesis. Adv. Cancer Res. 2013, 120, 75–111. [Google Scholar]
- Xu, C.; Kong, X.; Wang, H.; Zhang, N.; Kong, X.; Ding, X.; Li, X.; Yang, Q. MTDH mediates estrogen-independent growth and tamoxifen resistance by down-regulating PTEN in MCF-7 breast cancer Cells. Cell. Physiol. Biochem. 2014, 33, 1557–1567. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Q. Astrocyte elevated gene-1 and breast cancer (Review). Oncol. Lett. 2011, 2, 399–405. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.Z.; Kim, S.H.; Park, W.H. Arsenic trioxide inhibits the growth of Calu-6 cells via inducing a G2 arrest of the cell cycle and apoptosis accompanied with the depletion of GSH. Cancer Lett. 2008, 18, 40–55. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef]
- Lan, L.; Luo, Y.; Cui, D.; Shi, B.-Y.; Deng, W.; Huo, L.-L.; Chen, H.-L.; Zhang, G.-Y.; Deng, L.-L. Epithelial-mesenchymal transition triggers cancer stem cell generation in human thyroid cancer cells. Int. J. Oncol. 2013, 43, 113–120. [Google Scholar] [CrossRef]
- Gao, C.; Cao, W.; Bao, L.; Zuo, W.; Xie, G.; Cai, T.; Fu, W.; Zhang, J.; Wu, W.; Zhang, X.; et al. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 2010, 12, 781–790. [Google Scholar] [CrossRef]
- Nelson, J.; Bagnato, A.; Battistini, B.; Nisen, P. The endothelin axis: Emerging role in cancer. Nat. Rev. Cancer 2003, 3, 110–116. [Google Scholar] [CrossRef]
- Felx, M.; Guyot, M.C.; Isler, M.; Turcotte, R.E.; Doyon, J.; Khatib, A.M.; Leclerc, S.; Moreau, A.; Moldovan, F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-κB in human osteosarcoma. Clin. Sci. Lond. 2006, 110, 645–654. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, Q.; Zhu, Y.; Long, H. Endothelin-1 Promotes osteosarcoma cell invasion and survival against cisplatin-induced apoptosis. Clin. Orthop. Relat. Res. 2011, 469, 3190–3199. [Google Scholar] [CrossRef]
- Dong, L.; Qin, S.; Li, Y.; Zhao, L.; Dong, S.; Wang, Y.; Zhang, C.; Han, S. High expression of astrocyte elevated gene-1 is associated with clinical staging, metastasis, and unfavorable prognosis in gastric carcinoma. Tumor Biol. 2014, 36, 2169–2178. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Khan, M.; Sarkar, D. The Scope of Astrocyte Elevated Gene-1/Metadherin (AEG-1/MTDH) in Cancer Clinicopathology: A Review. Genes Basel. 2021, 12, 308. [Google Scholar] [CrossRef]
- Jung, H.I.; Ahn, T.; Bae, S.H.; Chung, J.C.; Kim, H.; Chin, S.; Jeong, D.; Cho, H.D.; Lee, M.S.; Kim, H.C.; et al. Astrocyte elevated gene-1 overexpression in hepatocellular carcinoma: An independent prognostic factor. Ann. Surg. Treat. Res. 2015, 88, 77–85. [Google Scholar] [CrossRef]
- Xu, S.-T.; Ma, Y.-C.; Wang, C.-H.; Xu, Y.; Gu, G.-J. Prognostic and clinicopathologic significance of AEG-1/MTDH and E-cadherin expression in human gallbladder carcinoma. Int. J. Clin. Exp. Pathol 2018, 11, 6025–6031. [Google Scholar]
- van ’t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Song, L.-B.; Liao, W.-T.; Jiang, L.-L.; Gong, L.-Y.; Wu, J.; Yuan, J.; Zhang, H.-Z.; Zeng, M.-S.; et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin. Cancer Res. 2008, 14, 3319–3326. [Google Scholar] [CrossRef] [PubMed]
- Thirkettle, H.J.; Girling, J.; Warren, A.Y.; Mills, I.G.; Sahadevan, K.; Leung, H.; Hamdy, F.; Whitaker, H.C.; Neal, D. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin. Cancer Res. 2009, 15, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, W.; Zhang, H.; Liao, W.; Dai, T.; Yu, C.; Ding, X.; Zhang, L.; Li, J. Over-expression of AEG-1 significantly associates with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. J. Pathol. 2009, 219, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, J.; Ying, Z.; Chen, B.; Han, A.; Liang, Y.; Song, L.; Yuan, J.; Li, J.; Li, M. Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9 and induces human glioma invasion. Cancer Res. 2010, 70, 3750–3759. [Google Scholar] [CrossRef]
- He, Z.; He, M.; Wang, C.; Xu, B.; Tong, L.; He, J.; Sun, B.; Wei, L.; Chu, M. Prognostic significance of astrocyte elevated gene-1 in human astrocytomas. Int. J. Clin. Exp. Pathol. 2014, 7, 5038–5044. [Google Scholar] [PubMed]
- Ding, Z.; Zhang, Z.; Jin, X.; Chen, P.; Lv, F.; Liu, D.; Shen, Y.; Li, Y.; Gu, X. Interaction with AEG-1 and MDM2 is associated with glioma development and progression and correlates with poor prognosis. Cell Cycle 2019, 18, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.-F.; He, S.; Li, S.; Luo, D.; Feng, C.; Zhou, W. Expression characteristics of astrocyte elevated gene-1 (AEG-1) in tongue carcinoma and its correlation with poor prognosis. Cancer Epidemiol. 2013, 37, 179–185. [Google Scholar] [CrossRef]
- Fan, Y.-Z.; Sun, W.; Xi, H.; Lu, X.-S.; Ye, C.; Zhang, J.-T. Astrocyte elevated gene-1 overexpression in human primary gallbladder carcinomas: An unfavorable and independent prognostic factor. Oncol. Rep. 2011, 26, 1133–1142. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Wang, Z.; Yin, C.; Zhang, W. Metadherin is a novel prognostic marker for bladder cancer progression and overall patient survival. Asia-Pac. J. Clin. Oncol. 2012, 8, e42–e48. [Google Scholar] [CrossRef]
- Tokunaga, E.; Nakashima, Y.; Yamashita, N.; Hisamatsu, Y.; Okada, S.; Akiyoshi, S.; Aishima, S.; Kitao, H.; Morita, M.; Maehara, Y. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer 2014, 21, 341–349. [Google Scholar] [CrossRef]
- Song, H.; Li, C.; Lu, R.; Zhang, Y.; Geng, J. Expression of astrocyte elevated gene-1: A novel marker of the pathogenesis, progression, and poor prognosis for endometrial cancer. Int. J. Gynecol. Cancer. 2010, 20, 1188–1196. [Google Scholar] [CrossRef]
- Li, C.; Chen, K.; Cai, J.; Shi, Q.-T.; Li, Y.; Li, L.; Song, H.; Qiu, H.; Qin, Y.; Geng, J.-S. Astrocyte elevated gene-1: A novel independent prognostic biomarker for metastatic ovarian tumors. Tumor Biol. 2013, 35, 3079–3085. [Google Scholar] [CrossRef]
- Agendia. Available online: www.agendia.com/healthcare-professionals/breast-cancer/mammaprint (accessed on 5 April 2021).
- Yan, J.J.; Chang, Y.; Zhang, Y.N.; Lin, J.S.; He, X.X.; Huang, H.J. miR-195 inhibits cell proliferation via targeting AEG-1 in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 3118–3126. [Google Scholar] [CrossRef]
- Hu, C.; Cui, S.; Zheng, J.; Yin, T.; Lv, J.; Long, J.; Zhang, W.; Wang, X.; Sheng, S.; Zhang, H.; et al. MiR-875-5p inhibits hepatocellular carcinoma cell proliferation and migration by repressing astrocyte elevated gene-1 (AEG-1) expression. Transl. Cancer Res. 2018, 7, 158–169. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Sun, F.-F.; Feng, J.-Q.; Peng, J.-J.; Li, H.; Wang, C.; Wang, D.; Liu, Y.; Bai, Y.-D.; et al. MicroRNA-375 represses tumor angiogenesis and reverses resistance to sorafenib in hepatocarcinoma. Cancer Gene Ther. 2021, 28, 126–140. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Lv, Z.; Li, Q.; Gong, W.; Wu, H. MicroRNA-342-3p Inhibits the Proliferation, Migration, and Invasion of Osteosarcoma Cells by Targeting Astrocyte-Elevated Gene-1 (AEG-1). Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 1505–1515. [Google Scholar] [CrossRef]
- Yan, J.-J.; Zhang, Y.-N.; Liao, J.-Z.; Ke, K.-P.; Chang, Y.; Li, P.-Y.; Wang, M.; Lin, J.-S.; He, X.-X. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget 2015, 6, 29527–29542. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Huang, Y.; Wu, J.; Chen, M.; Cui, P.; Zhang, W.; Zhang, Y. HBx Elevates Oncoprotein AEG-1 Expression to Promote Cell Migration by Downregulating miR-375 and miR-136 in Malignant Hepatocytes. DNA Cell Biol. 2014, 33, 715–722. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, L.; Shao, Y.; Ma, Q.; Lv, H. microRNA-377 inhibits non-small-cell lung cancer through targeting AEG-1. Int. J. Clin. Exp. Pathol. 2015, 8, 13853–13863. [Google Scholar]
- Yang, Y.; Wu, J.; Guan, H.; Cai, J.; Fang, L.; Li, J.; Li, M. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Lett. 2012, 586, 3608–3612. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, D.; Meng, L.; Wang, B. MicroRNA-124 inhibits proliferation, invasion, migration and epithelial-mesenchymal transition of cervical carcinoma cells by targeting astrocyte-elevated gene-1. Oncol. Rep. 2016, 36, 2321–2328. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Z.-L.; Jiang, K.-W.; Zhao, G.; Wang, C.-Y.; Yan, Y.-C.; Yang, Y.; Zhang, J.-Z.; Shen, C.; Gao, Z.-D.; et al. MicroRNA-217 functions as a prognosis predictor and inhibits colorectal cancer cell proliferation and invasion via an AEG-1 dependent mechanism. BMC Cancer 2015, 15, 437. [Google Scholar] [CrossRef]
- Wang, L.; Lyu, X.; Ma, Y.; Wu, F.; Wang, L. MicroRNA-504 targets AEG-1 and inhibits cell proliferation and invasion in retinoblastoma. Mol. Med. Rep. 2019, 19, 2935–2942. [Google Scholar] [CrossRef]
- Miao, S.; Mao, X.; Zhao, S.; Song, K.; Xianguang, Y.; Lv, Y.; Jiang, H.; Wang, L.; Li, B.; Yang, X.; et al. miR-217 inhibits laryngeal cancer metastasis by repressing AEG-1 and PD-L1 expression. Oncotarget 2017, 8, 62143–62153. [Google Scholar] [CrossRef]
- Zou, M.; Duan, Y.; Wang, P.; Gao, R.; Chen, X.; Ou, Y.; Zhu, H. DYT-40, a novel synthetic 2-styryl-5-nitroimidazole derivative, blocks malignant glioblastoma growth and invasion by inhibiting AEG-1 and NF-κB signaling pathways. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Jimeno, J.M.; Acosta, G.; Molina, M.; Molina-Vila, M.Á.; Karachaliou, N.; Teixidó, C.; Obiol, C.; Villacañas, O.; Bertran, J.; Rouco, M.S.; et al. Abstract 4601: Astrocytic elevated gene 1 (AEG1) a target for pharmacological anticancer intervention. Cancer Res. 2014, 74, 4601. [Google Scholar] [CrossRef]
- Yang, X.; Song, S. Silencing of Astrocyte elevated gene-1 (AEG-1) inhibits proliferation, migration, and invasiveness, and promotes apoptosis in pancreatic cancer cells. Biochem. Cell Biol. 2019, 97, 165–175. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Liu, H.; Feng, Y.; Zhu, T.; Zhang, L.; Zhu, B.; Zhang, Y. Knockdown of astrocyte elevated gene-1 (AEG-1) in cervical cancer cells decreases their invasiveness, epithelial to mesenchymal transition, and chemoresistance. Cell Cycle 2014, 13, 1702–1707. [Google Scholar] [CrossRef]
- Salman, M.M.; Marsh, G.; Kusters, I.; Delincé, M.; Di Caprio, G.; Upadhyayula, S.; De Nola, G.; Hunt, R.; Ohashi, K.G.; Gray, T.; et al. Design and Validation of a Human Brain Endothelial Microvessel-on-a-Chip Open Microfluidic Model Enabling Advanced Optical Imaging. Front. Bioeng. Biotechnol. 2020, 8, 573775. [Google Scholar] [CrossRef]
- Jackson, E.L.; Lu, H. Three-dimensional models for studying development and disease: Moving on from organisms to organs-on-a-chip and organoids. Integr. Biol. 2016, 8, 672–683. [Google Scholar] [CrossRef]
- Moffitt, J.R.; Hao, J.; Wang, G.; Chen, K.H.; Babcock, H.P.; Zhuang, X. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl. Acad. Sci. USA 2016, 113, 11046–11051. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, J.R.; Bambah-Mukku, D.; Eichhorn, S.W.; Vaughn, E.; Shekhar, K.; Perez, J.D.; Rubinstein, N.D.; Hao, J.; Regev, A.; Dulac, C.; et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018, 362, eaau5324. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Clarke, P.; Raynaud, F.; Van Montfort, R.L. Drugging the PI3 Kinome: From Chemical Tools to Drugs in the Clinic. Cancer Res. 2010, 70, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Navon, A.; Ciechanover, A. The 26 S proteasome: From basic mechanisms to drug targeting. J. Biol. Chem. 2009, 284, 33713–33718. [Google Scholar] [CrossRef]
- Aldewachi, H.; Al-Zidan, R.; Conner, M.; Salman, M. High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering 2021, 8, 30. [Google Scholar] [CrossRef]
- Salman, M.; Al-Obaidi, Z.; Kitchen, P.; Loreto, A.; Bill, R.; Wade-Martins, R. Advances in Applying Computer-Aided Drug Design for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4688. [Google Scholar] [CrossRef]
| AEG-1 Inhibitors | Type of Molecule | Associated Conditions | Reference |
|---|---|---|---|
| miR-195 | miRNA | Hepatocellular carcinoma | [125] |
| miR-875-5p | miRNA | Hepatocellular carcinoma | [126] |
| miR-375 | miRNA | Hepatocellular carcinoma | [127,129] |
| miR-136 | miRNA | Hepatocellular carcinoma, Glioma | [130,132] |
| miR-874 | miRNA | Retinoblastoma | [131] |
| miR-124 | miRNA | Cervical cancer | [133] |
| miR-217 | miRNA | Colorectal cancer | [134] |
| miR-30 | miRNA | Breast cancer | [52] |
| miR-504 | miRNA | Retinoblastoma | [135] |
| miR-448 | miRNA | Laryngeal cancer | [136] |
| DYT-40 | Novel synthetic compound | Malignant glioblastoma | [137] |
| PB0412-3 (PB3) | small molecule polyheterocyclic compound | Brain tumor | [138] |
| Cryptotanshinone | Natural compound (abietane-diterpene derivative) | Prostate cancer- Hypoxia | [9] |
| shRNA | shRNA | Pancreatic cancer | [139] |
| RNAi | siRNA | Cervical cancer | [140] |
| miR-377 | miRNA | Non-small cell lung cancer | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriramulu, S.; Sun, X.-F.; Malayaperumal, S.; Ganesan, H.; Zhang, H.; Ramachandran, M.; Banerjee, A.; Pathak, S. Emerging Role and Clinicopathological Significance of AEG-1 in Different Cancer Types: A Concise Review. Cells 2021, 10, 1497. https://doi.org/10.3390/cells10061497
Sriramulu S, Sun X-F, Malayaperumal S, Ganesan H, Zhang H, Ramachandran M, Banerjee A, Pathak S. Emerging Role and Clinicopathological Significance of AEG-1 in Different Cancer Types: A Concise Review. Cells. 2021; 10(6):1497. https://doi.org/10.3390/cells10061497
Chicago/Turabian StyleSriramulu, Sushmitha, Xiao-Feng Sun, Sarubala Malayaperumal, Harsha Ganesan, Hong Zhang, Murugesan Ramachandran, Antara Banerjee, and Surajit Pathak. 2021. "Emerging Role and Clinicopathological Significance of AEG-1 in Different Cancer Types: A Concise Review" Cells 10, no. 6: 1497. https://doi.org/10.3390/cells10061497
APA StyleSriramulu, S., Sun, X.-F., Malayaperumal, S., Ganesan, H., Zhang, H., Ramachandran, M., Banerjee, A., & Pathak, S. (2021). Emerging Role and Clinicopathological Significance of AEG-1 in Different Cancer Types: A Concise Review. Cells, 10(6), 1497. https://doi.org/10.3390/cells10061497







