Colesevelam Reduces Ethanol-Induced Liver Steatosis in Humanized Gnotobiotic Mice
Abstract
1. Introduction
2. Materials and Methods
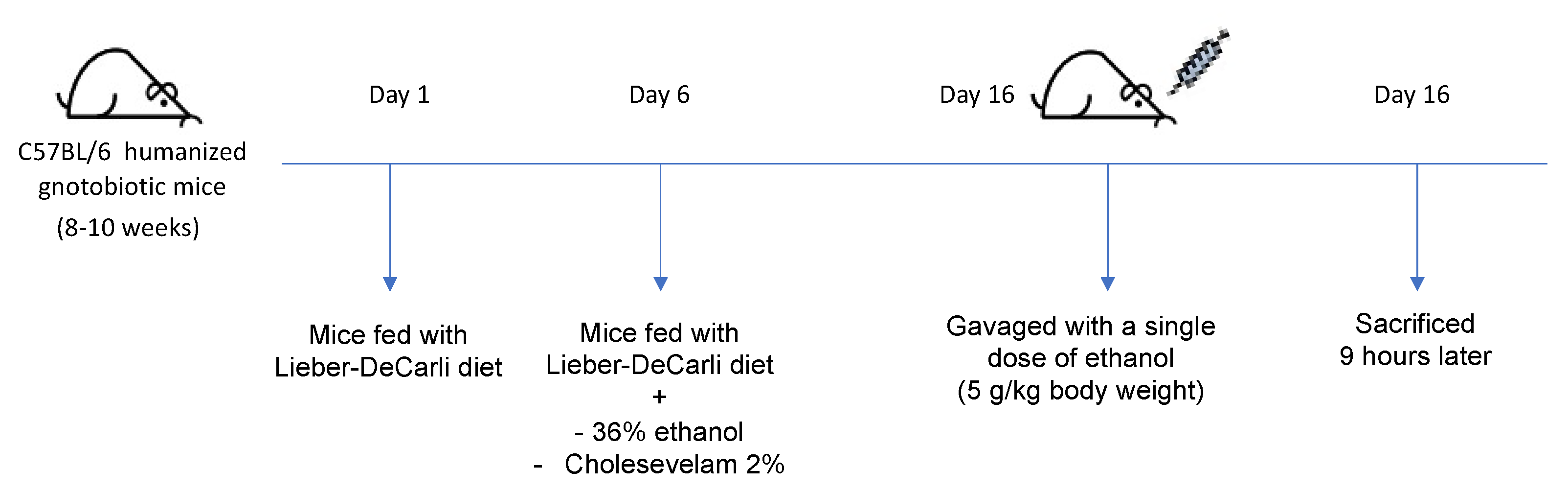
2.1. Mice
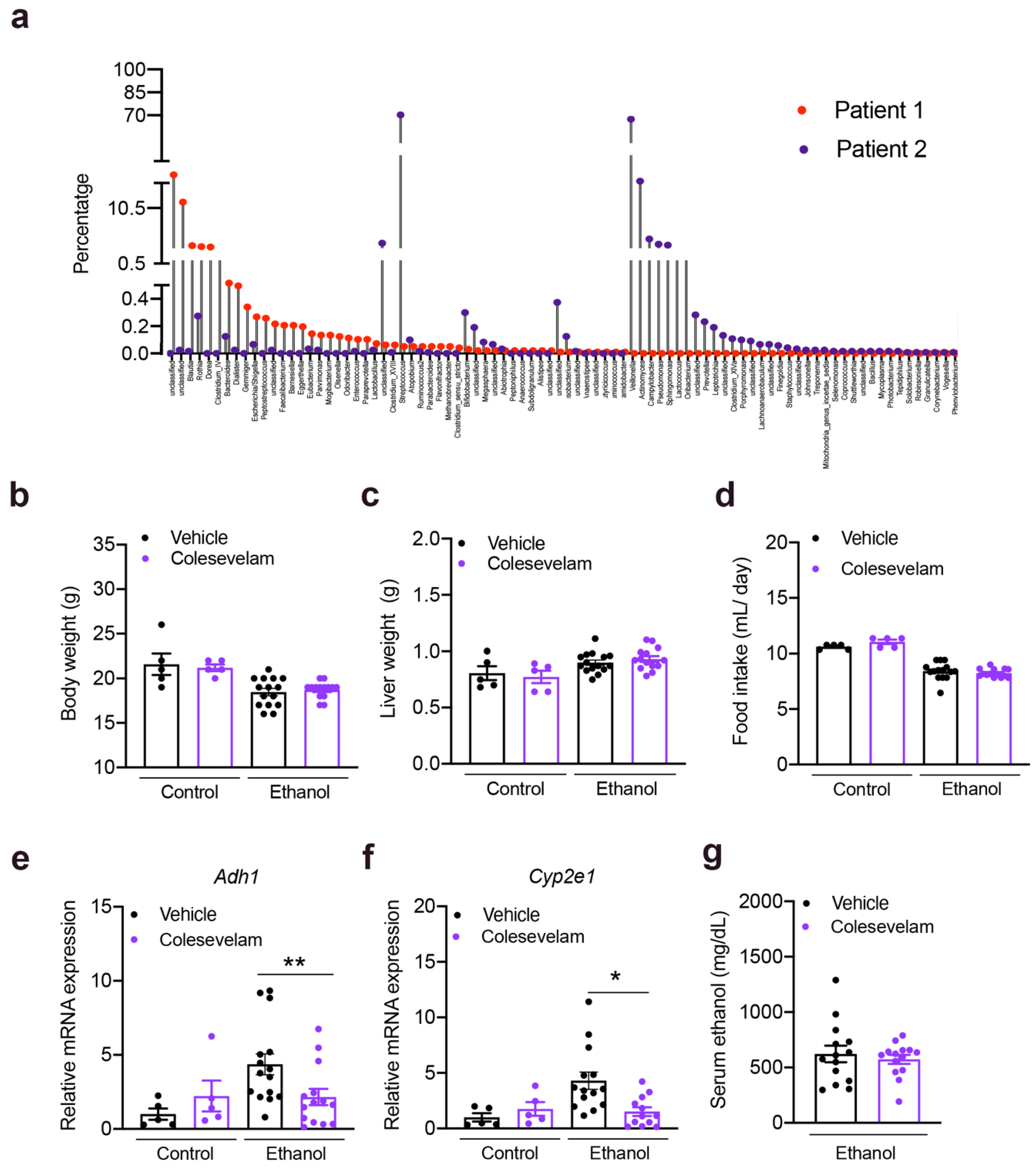
2.2. 16S rRNA Sequencing
2.3. Real-Time Quantitative PCR
2.4. Staining Procedures
2.5. Biochemical Analysis
2.6. Immunoblot Analyses
2.7. Statistical Analysis
3. Results
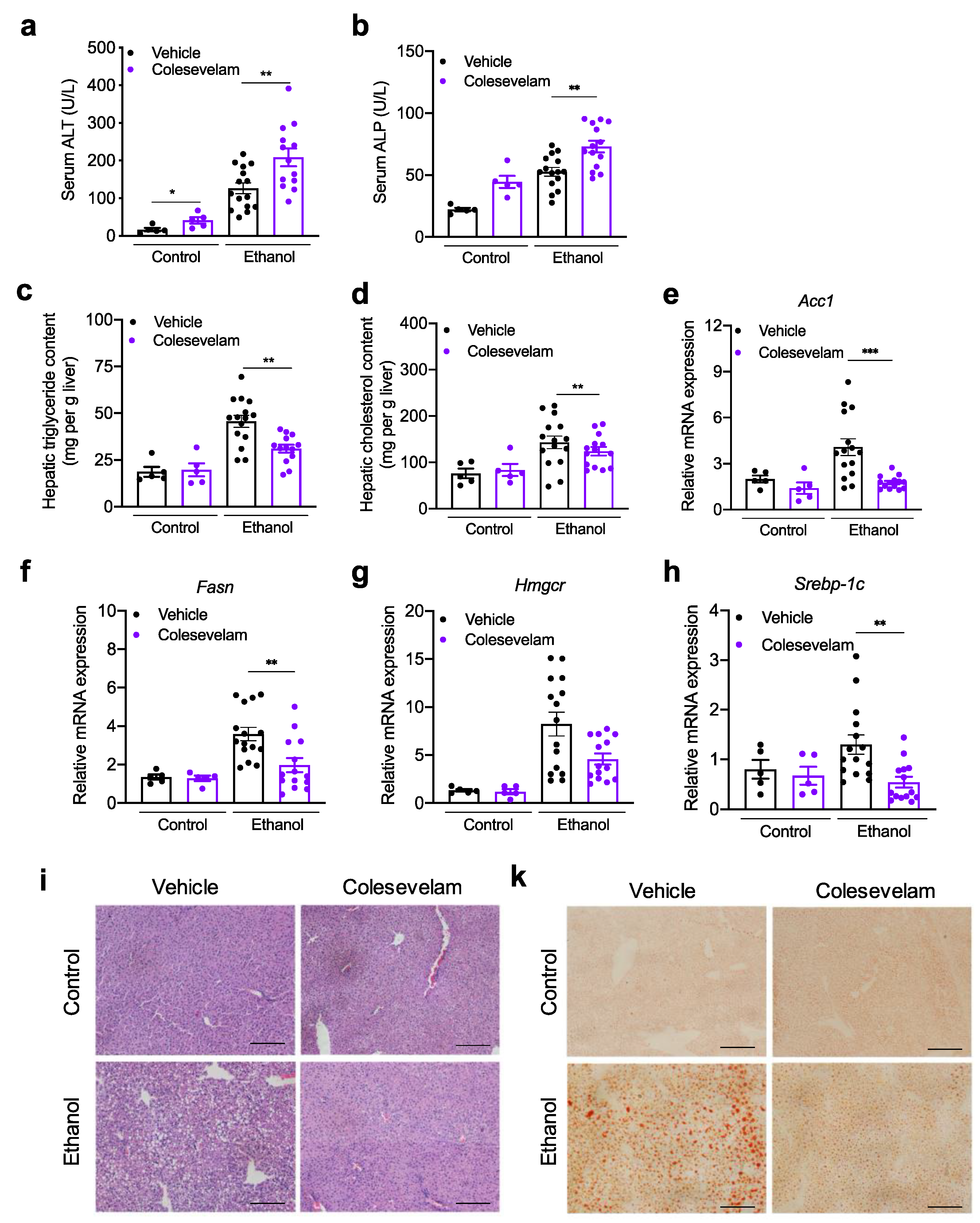
3.1. Colesevelam Reduces Ethanol-Induced Liver Steatosis in Gnotobiotic Mice
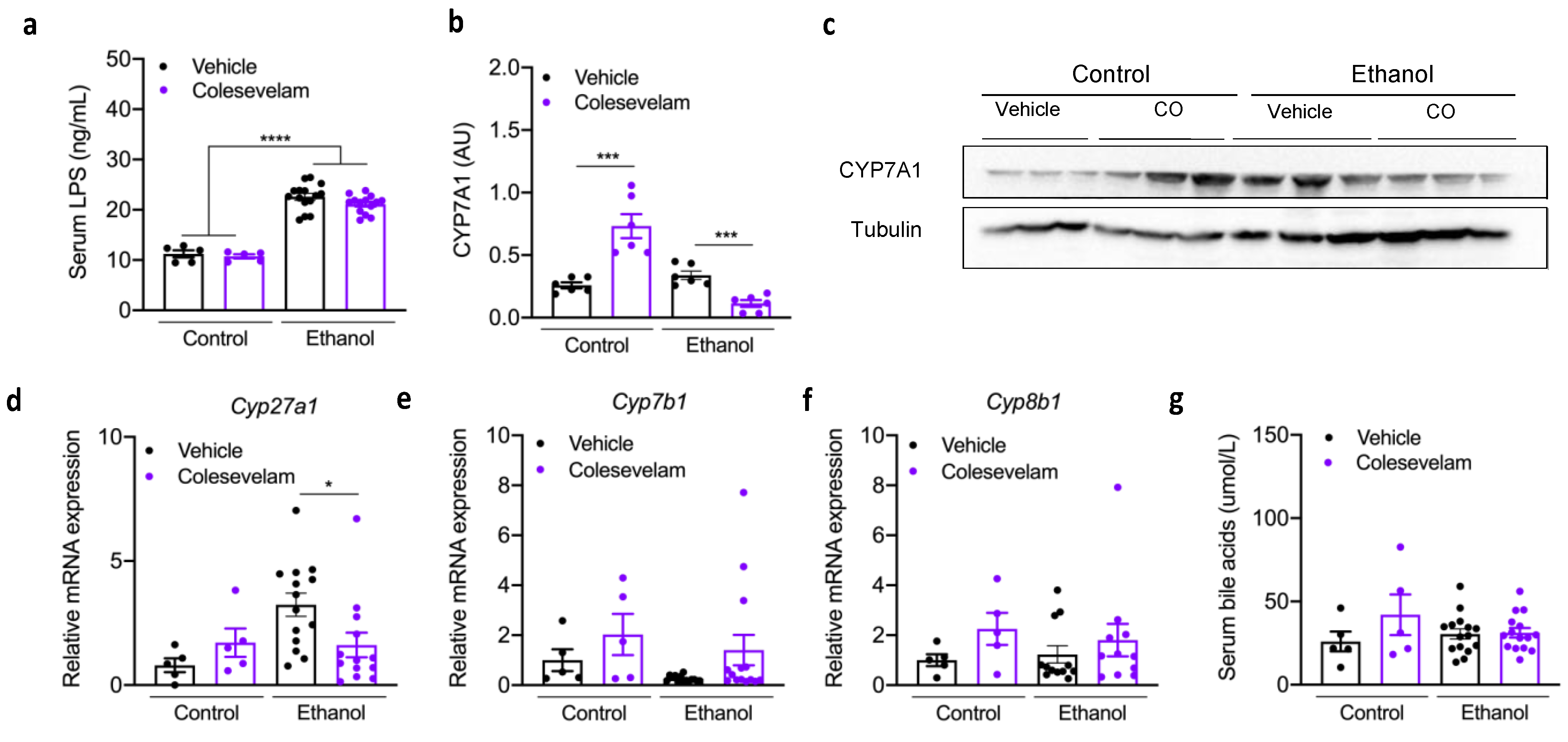
3.2. Effect of Colesevelam Treatment on Hepatic Inflammation
3.3. Colesevelam Treatment and Bile Acids
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masarone, M.; Rosato, V.; Dallio, M.; Abenavoli, L.; Federico, A.; Loguercio, C.; Persico, M. Epidemiology and Natural History of Alcoholic Liver Disease. Rev. Recent Clin. Trials 2016, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol. Res. 2017, 38, 147–161. [Google Scholar] [PubMed]
- Singal, A.K.; Kamath, P.S.; Gores, G.J.; Shah, V.H. Alcoholic Hepatitis: Current Challenges and Future Directions. Clin. Gastroenterol. Hepatol. 2014, 12, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Crabb, D.W.; Bataller, R.; Chalasani, N.P.; Kamath, P.S.; Lucey, M.; Mathurin, P.; McClain, C.; McCullough, A.; Mitchell, M.C.; Morgan, T.R.; et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients with Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016, 150, 785–790. [Google Scholar] [CrossRef]
- Louvet, A.; Thursz, M.R.; Kim, D.J.; Labreuche, J.; Atkinson, S.R.; Sidhu, S.S.; O’Grady, J.G.; Akriviadis, E.; Sinakos, E.; Carithers, R.L.; et al. Corticosteroids Reduce Risk of Death within 28 Days for Patients with Severe Alcoholic Hepatitis, Compared with Pentoxifylline or Placebo—A Meta-analysis of Individual Data From Controlled Trials. Gastroenterology 2018, 155, 458–468.e8. [Google Scholar] [CrossRef]
- Singh, S.; Murad, M.H.; Chandar, A.K.; Bongiorno, C.M.; Singal, A.K.; Atkinson, S.; Thursz, M.R.; Loomba, R.; Shah, V.H. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology 2015, 149, 958–970.e12. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, J.; López-Pelayo, H.; Michelena, J.; Jones, P.D.; Ortega, L.; Ginès, P.; Caballería, J.; Gual, A.; Bataller, R.; Lligoña, A. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology 2017, 66, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Labreuche, J.; Artru, F.; Bouthors, A.; Rolland, B.; Saffers, P.; Lollivier, J.; Lemaître, E.; Dharancy, S.; Lassailly, G.; et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology 2017, 66, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Chen, P.-H.; Haugen, C.; Hernaez, R.; Gurakar, A.; Philosophe, B.; Dagher, N.; Moore, S.A.; Li, Z.; Cameron, A.M. Three-year Results of a Pilot Program in Early Liver Transplantation for Severe Alcoholic Hepatitis. Ann. Surg. 2017, 265, 20–29. [Google Scholar] [CrossRef]
- Lee, B.P.; Mehta, N.; Platt, L.; Gurakar, A.; Rice, J.P.; Lucey, M.R.; Im, G.; Therapondos, G.; Han, H.; Victor, D.W.; et al. Outcomes of Early Liver Transplantation for Patients with Severe Alcoholic Hepatitis. Gastroenterology 2018, 155, 422–430.e1. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Chacko, K.R.; Reinus, J. Spectrum of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Brandl, K.; Hartmann, P.; Jih, L.J.; Pizzo, D.P.; Argemi, J.; Ventura-Cots, M.; Coulter, S.; Liddle, C.; Ling, L.; Rossi, S.J.; et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J. Hepatol. 2018, 69, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Hochrath, K.; Horvath, A.; Chen, P.; Seebauer, C.T.; Llorente, C.; Wang, L.; Alnouti, Y.; Fouts, D.E.; Stärkel, P.; et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2017, 67, 2150–2166. [Google Scholar] [CrossRef]
- Llopis, M.; Cassard, A.M.; Wrzosek, L.; Boschat, L.; Bruneau, A.; Ferrere, G.; Puchois, V.; Martin, J.C.; Lepage, P.; Le Roy, T.; et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016, 65, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Trinchet, J.-C.; Gerhardt, M.-F.; Balkau, B.; Munz, C.; Poupon, R.E. Serum bile acids and cholestasis in alcoholic hepatitis. Relationship with usual liver tests and histological features. J. Hepatol. 1994, 21, 235–240. [Google Scholar] [CrossRef]
- Beigel, F.; Teich, N.; Howaldt, S.; Lammert, F.; Maul, J.; Breiteneicher, S.; Rust, C.; Göke, B.; Brand, S.; Ochsenkühn, T. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn’s disease: A randomized, double-blind, placebo-controlled study. J. Crohns Colitis 2014, 8, 1471–1479. [Google Scholar] [CrossRef]
- Kuiper, E.M.M.; Van Erpecum, K.J.; Beuers, U.; Hansen, B.; Thio, H.B.; De Man, R.A.; Janssen, H.L.A.; Van Buuren, H.R. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: Results of a double-blind, randomized, placebo-controlled trial. Hepatology 2010, 52, 1334–1340. [Google Scholar] [CrossRef]
- Blahová, T.; Peterková, L.; Leníček, M.; Vlachová, M.; Zemankova, K.; Adámková, V.; Vitek, L.; Kovar, J. The Effect of Colesevelam Treatment on Bile Acid and Lipid Metabolism and Glycemic Control in Healthy Men. Physiol. Res. 2016, 65, 995–1003. [Google Scholar] [CrossRef]
- Dayer-Berenson, L.; Finckenor, M. Expanded Colesevelam Administration Options with Oral Suspension Formulation for Patients with Diabetes and Hypercholesterolemia. Postgrad. Med. 2014, 126, 126–134. [Google Scholar] [CrossRef]
- Bajaj, H.S.; Brown, R.E.; Jiandani, D.; Venn, K.; Al-Asaad, H.; Khandwala, H.; Steen, O.; Abdel-Salam, S.; Aronson, R. Goal achievement of HbA1c and LDL- cholesterol in a randomized trial comparing colesevelam with ezetimibe: GOAL-RCT. Diabetes Obes. Metab. 2020, 22, 1722–1728. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Paumgartner, G.; Mlitz, V.; Kunczer, V.; Halilbasic, E.; Leditznig, N.; Wahlström, A.; Ståhlman, M.; Thüringer, A.; Kashofer, K.; et al. Colesevelam attenuates cholestatic liver and bile duct injury in Mdr2(-/-) mice by modulating composition, signalling and excretion of faecal bile acids. Gut 2018, 67, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Out, C.; Groen, A.K.; Brufau, G. Bile acid sequestrants: More than simple resins. Curr. Opin. Lipidol. 2012, 23, 43–55. [Google Scholar] [CrossRef]
- Handelsman, Y.; Goldberg, R.B.; Garvey, W.T.; Fonseca, V.A.; Rosenstock, J.; Jones, M.R.; Lai, Y.-L.; Jin, X.; Misir, S.; Nagendran, S.; et al. Colesevelam Hydrochloride to Treat Hypercholesterolemia and Improve Glycemia in Prediabetes: A Randomized, Prospective Study. Endocr. Pr. 2010, 16, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Brufau, G.; Stellaard, F.; Prado, K.; Bloks, V.W.; Jonkers, E.; Boverhof, R.; Kuipers, F.; Murphy, E.J. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology 2010, 52, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.; Potts, A.; He, T.; Duarte, J.A.G.; Taussig, R.; Mangelsdorf, D.J.; Kliewer, S.A.; Burgess, S.C. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am. J. Physiol. Liver Physiol. 2013, 304, G371–G380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Hoeke, G.; Li, Z.; Eibergen, A.C.; Schonk, A.W.; Koehorst, M.; Boverhof, R.; Havinga, R.; Kuipers, F.; Coskun, T.; et al. Colesevelam enhances the beneficial effects of brown fat activation on hyperlipidaemia and atherosclerosis development. Cardiovasc. Res. 2020, 116, 1710–1720. [Google Scholar] [CrossRef]
- Le, T.; Chen, J.; Changchien, C.; Peterson, M.R.; Kono, Y.; Patton, H.; Cohen, B.L.; Brenner, D.; Sirlin, C.; Loomba, R.; et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: A randomized controlled trial. Hepatology 2012, 56, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miyamoto, Y.; Mazagova, M.; Lee, K.-C.; Eckmann, L.; Schnabl, B. Microbiota Protects Mice Against Acute Alcohol-Induced Liver Injury. Alcohol. Clin. Exp. Res. 2015, 39, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Matye, D.; Zhang, Y.; Dennis, K.; Ding, W.-X.; Li, T. Bile acids regulate cysteine catabolism and glutathione regeneration to modulate hepatic sensitivity to oxidative injury. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandak, W.M.; Hylemon, P.B. LXR alpha is the dominant regulator of CYP7A1 transcription. Biochem. Biophys. Res. Commun. 2002, 293, 338–343. [Google Scholar] [CrossRef]

| Variables | Alcoholic Hepatitis | |
|---|---|---|
| Patient 1 | Patient 2 | |
| Sex (male) | Male | Male |
| BMI (kg/m2) | 26.7 | 27.5 |
| Age (years) | 53 | 35 |
| ALT (IU/L) | 24.0 | 49.0 |
| AST (IU/L) | 84 | 170 |
| ALP (IU/L) | 231 | 116 |
| Bilirubin (mg/dL) | 14.1 | 8.0 |
| Creatinine (μmol/L) | 3.1 | 3.3 |
| Albumin (g/dL) | 2.4 | 1.8 |
| INR | 1.6 | 1.8 |
| Platelet count (109/L) | 161.0 | 73.0 |
| Stage of fibrosis | 2 | 2 |
| MELD | 33 | 33 |
| Child–Pugh stage | C | C |
| Gene | Primer | Sequence |
|---|---|---|
| Mouse 18S | F | 5′-AGTCCCTGCCCTTTGTACACA-3′ |
| R | 5′-CGATCCCAGGGCCTCACTA-3′ | |
| Mouse Acc1 | F | 5′-TGGAGAGCCCAACACACA-3′ |
| R | 5′-GGACAGACTGATCGCAGAGAA-3′ | |
| Mouse Adh1 | F | 5′-GGGTTCTCAACTGGCTATGG-3′ |
| R | 5′-ACAGACAGACCGACACCTCC-3′ | |
| Mouse Cxcl1 | F | 5′-TGCACCCAAACCGAAGTC-3′ |
| R | 5′-GTCAGAAGCCAGCGTTCACC-3′ | |
| Mouse Cxcl2 | F | 5′-AAAGTTTGCCTTGACCCTGAA-3′ |
| R | 5′-CTCAGACAGCGAGGCACATC-3′ | |
| Mouse Ccl2 | F | 5′-ATTGGGATCATCTTGCTGGT-3′ |
| R | 5′-CCTGCTGTTCACAGTTGCC-3′ | |
| Mouse Cyp2e1 | F | 5′-GGGACATTCCTGTGTTCCAG-3′ |
| R | 5′-CTTAGGGAAAACCTCCGCAC-3′ | |
| Mouse Cyp7b1 | F | 5′-AGGCATGACGATCCTGAAATAG-3′ |
| R | 5′-CAGCCTCAGAACCTCAAGAATAG-3′ | |
| Mouse Cyp8b1 | F | 5′-AGGGTGGTACAGGAGGATTAT-3′ |
| R | 5′-GAGTCTGAGCTGGTAGGATTTG-3′ | |
| Mouse Cyp27a1 | F | 5′-GGAGGGCAAGTACCCAATAAG-3′ |
| R | 5′-GCAAGGTGGTAGAGAAGATGAG-3′ | |
| Mouse Fasn | F | 5′-GTCGTCTGCCTCCAGAGC-3′ |
| R | 5′-GTTGGCCCAGAACTCCTGTA-3′ | |
| Mouse Hmgcr | F | 5′-GGCCTCCATTGAGATCCG-3′ |
| R | 5′-CACAATAACTTCCCAGGGGT-3′ | |
| Mouse Mpo | F | 5′-GATGACCCCTGCCTCCTC-3′ |
| R | 5′-GCTCTCGAACAAAGAGGGTG-3′ | |
| Mouse Srebp1c | F | 5′-GGAGCCATGGATTGCACATT-3′ |
| R | 5′-GCTTCCAGAGAGGAGGCCAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabré, N.; Duan, Y.; Llorente, C.; Conrad, M.; Stern, P.; Yamashita, D.; Schnabl, B. Colesevelam Reduces Ethanol-Induced Liver Steatosis in Humanized Gnotobiotic Mice. Cells 2021, 10, 1496. https://doi.org/10.3390/cells10061496
Cabré N, Duan Y, Llorente C, Conrad M, Stern P, Yamashita D, Schnabl B. Colesevelam Reduces Ethanol-Induced Liver Steatosis in Humanized Gnotobiotic Mice. Cells. 2021; 10(6):1496. https://doi.org/10.3390/cells10061496
Chicago/Turabian StyleCabré, Noemí, Yi Duan, Cristina Llorente, Mary Conrad, Patrick Stern, Dennis Yamashita, and Bernd Schnabl. 2021. "Colesevelam Reduces Ethanol-Induced Liver Steatosis in Humanized Gnotobiotic Mice" Cells 10, no. 6: 1496. https://doi.org/10.3390/cells10061496
APA StyleCabré, N., Duan, Y., Llorente, C., Conrad, M., Stern, P., Yamashita, D., & Schnabl, B. (2021). Colesevelam Reduces Ethanol-Induced Liver Steatosis in Humanized Gnotobiotic Mice. Cells, 10(6), 1496. https://doi.org/10.3390/cells10061496





