Hydrogen Sulfide Metabolism and Pulmonary Hypertension
Abstract
1. Introduction
2. H2S Metabolism in the Pulmonary Vasculature
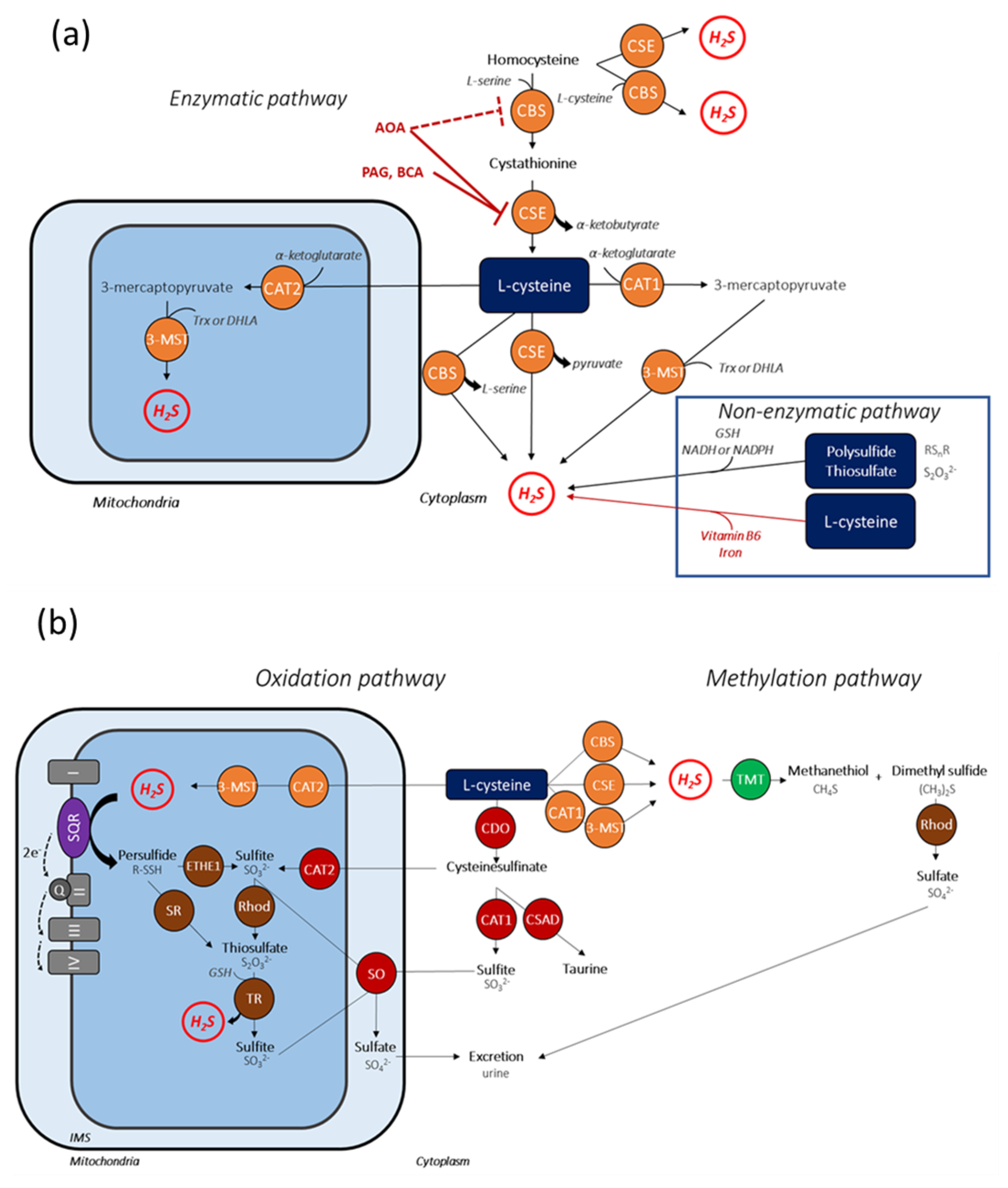
2.1. Anabolic Pathways of H2S
2.2. Catabolic Pathways of H2S
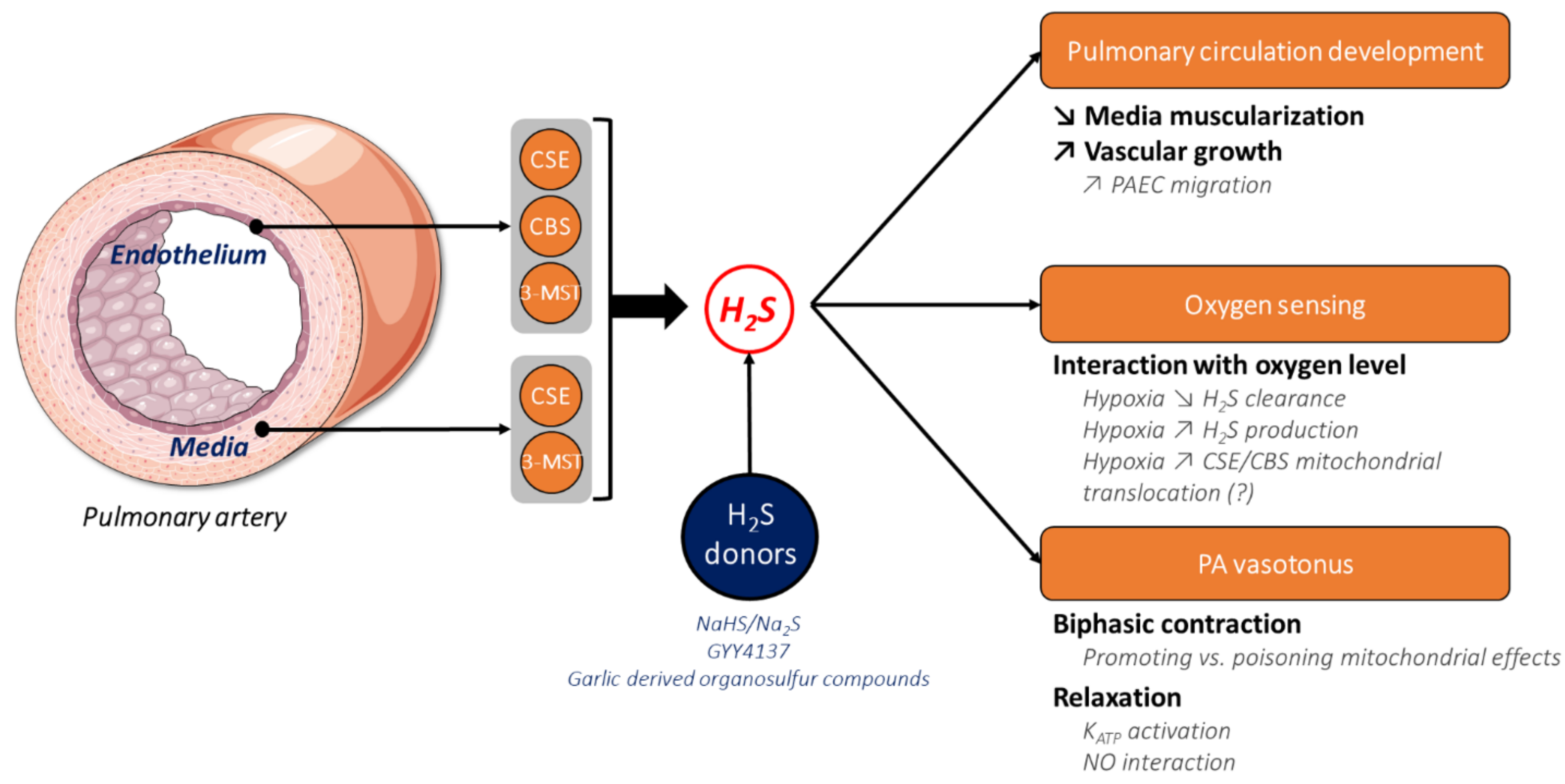
2.3. H2S in the Pulmonary Circulation
3. The Physiological Role of H2S in Pulmonary Circulation
3.1. The Role of H2S in Lung and Pulmonary Circulation Development
3.2. The Role of H2S in Oxygen Sensing and Hypoxic Pulmonary Vasocontriction
3.3. The Role of H2S in Pulmonary Artery Relaxation
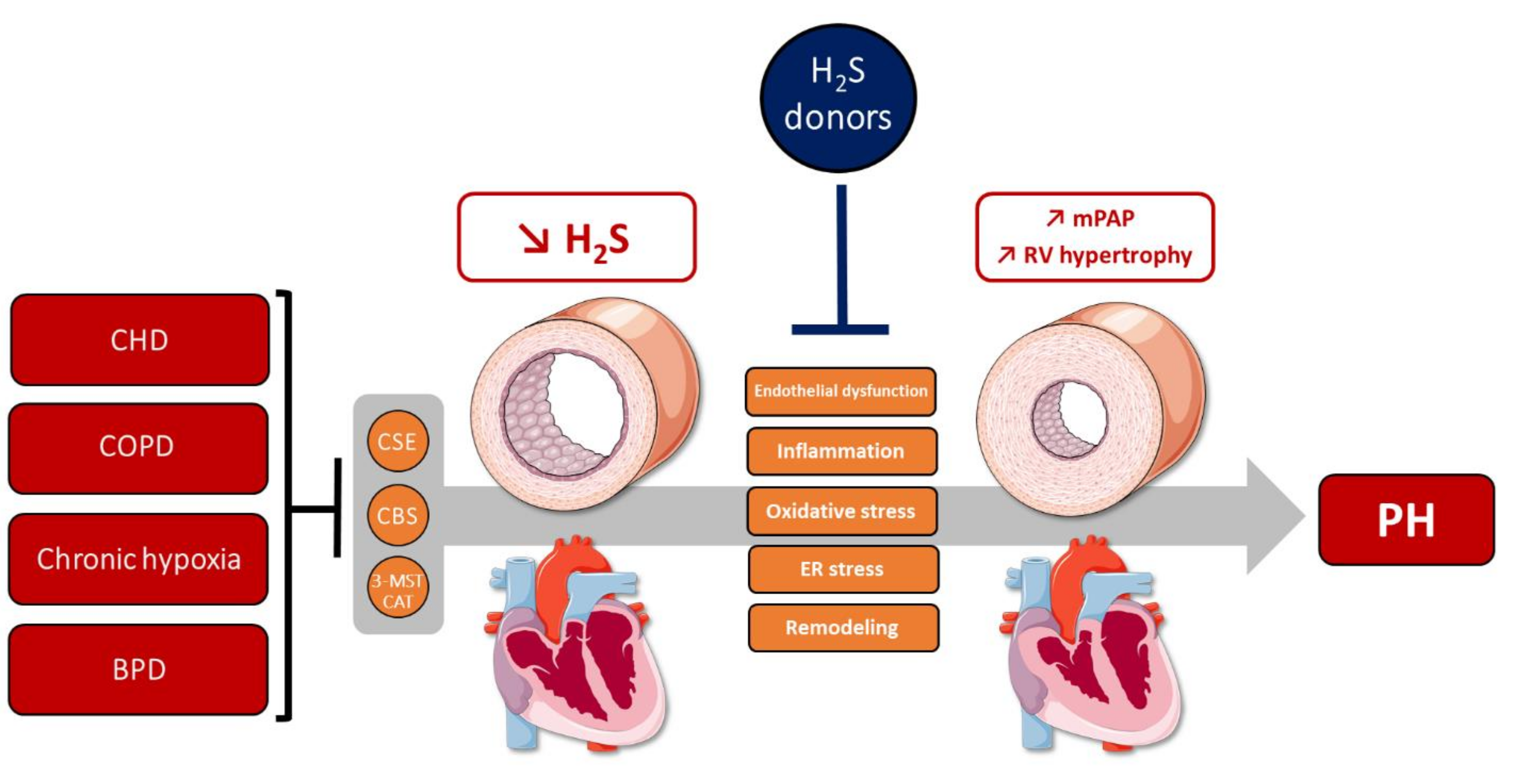
4. The Role of H2S in Hallmarks of PH
4.1. H2S Metabolism Alterations in PH
4.1.1. H2S Metabolism in Human PH
4.1.2. H2S Metabolism in Experimental PH Models
4.2. H2S Exerts Protective Effects against PH
5. Conclusions and Clinical Perspectives
5.1. H2S Significance and Perspectives in PH
5.2. H2S-Releasing Molecules as a New Therapeutic Strategy for PH?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An insider view on the World Symposium on Pulmonary Hypertension. Lancet Respir. Med. 2019, 7, 484–485. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.M.; Kaminski, K.A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2015, 37, 67–119. [Google Scholar] [CrossRef]
- Noordegraaf, A.V.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- De La Roque, E.D.; Smeralda, G.; Quignard, J.-F.; Freund-Michel, V.; Courtois, A.; Marthan, R.; Muller, B.; Guibert, C.; Dubois, M. Altered vasoreactivity in neonatal rats with pulmonary hypertension associated with bronchopulmonary dysplasia: Implication of both eNOS phosphorylation and calcium signaling. PLoS ONE 2017, 12, e0173044. [Google Scholar] [CrossRef] [PubMed]
- Huertas, A.; Guignabert, C.; Barberà, J.A.; Bärtsch, P.; Bhattacharya, J.; Bhattacharya, S.; Bonsignore, M.R.; Dewachter, L.; Dinh-Xuan, A.T.; Dorfmüller, P.; et al. Pulmonary vascular endothelium: The orchestra conductor in respiratory diseases. Eur. Respir. J. 2018, 51, 1700745. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Yun, X.; Kliment, C.; Huetsch, J.; Damarla, M.; Shimoda, L.A. Reactive oxygen species induced Ca2+ influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L893–L907. [Google Scholar] [CrossRef]
- Rodat, L.; Savineau, J.-P.; Marthan, R.; Guibert, C. Effect of chronic hypoxia on voltage-independent calcium influx activated by 5-HT in rat intrapulmonary arteries. Pflügers Arch. Eur. J. Physiol. 2006, 454, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, T.; Zhang, H.; Yan, Y.; Wang, D.; Fang, L.; Lu, Y.; Du, G. Activation of Nrf2 Attenuates Pulmonary Vascular Remodeling via Inhibiting Endothelial-to-Mesenchymal Transition: An Insight from a Plant Polyphenol. Int. J. Biol. Sci. 2017, 13, 1067–1081. [Google Scholar] [CrossRef]
- Tamura, Y.; Phan, C.; Tu, L.; Le Hiress, M.; Thuillet, R.; Jutant, E.-M.; Fadel, E.; Savale, L.; Huertas, A.; Humbert, M.; et al. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J. Clin. Investig. 2018, 128, 1956–1970. [Google Scholar] [CrossRef]
- Pak, O.; Scheibe, S.; Esfandiary, A.; Gierhardt, M.; Sydykov, A.; Logan, A.; Fysikopoulos, A.; Veit, F.; Hecker, M.; Kroschel, F.; et al. Impact of the mitochondria-targeted antioxidant MitoQ on hypoxia-induced pulmonary hypertension. Eur. Respir. J. 2018, 51, 1701024. [Google Scholar] [CrossRef]
- Song, T.; Zheng, Y.-M.; Wang, Y.-X. Cross Talk Between Mitochondrial Reactive Oxygen Species and Sarcoplasmic Reticulum Calcium in Pulmonary Arterial Smooth Muscle Cells. Adv. Exp. Med. Biol. 2017, 967, 289–298. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Zhang, B.; Wang, Y.; Liu, Y.; Luo, Y.; Niu, W.; Dong, M.; Liu, M.; Dong, H.; et al. Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int. J. Med. Sci. 2016, 13, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Hendry-Hofer, T.B.; Witeof, A.E.; Brenner, M.; Mahon, S.B.; Boss, G.R.; Haouzi, P.; Bebarta, V.S. Hydrogen Sulfide Toxicity: Mechanism of Action, Clinical Presentation, and Countermeasure Development. J. Med. Toxicol. 2019, 15, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Wang, R.; Li, K.; Wang, H.; Jiao, H.; Wang, X.; Zhao, J.; Lin, H. Endogenous CSE/Hydrogen Sulfide System Regulates the Effects of Glucocorticoids and Insulin on Muscle Protein Synthesis. Oxidative Med. Cell. Longev. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soriano, R.N.; Braga, S.P.; Breder, J.S.C.; Batalhao, M.E.; Oliveira-Pelegrin, G.R.; Ferreira, L.F.R.; Rocha, M.J.A.; Carnio, E.C.; Branco, L.G.S. Endogenous peripheral hydrogen sulfide is propyretic: Its permissive role in brown adipose tissue thermogenesis in rats. Exp. Physiol. 2018, 103, 397–407. [Google Scholar] [CrossRef]
- Kuo, M.M.; Kim, D.H.; Jandu, S.; Bergman, Y.; Tan, S.; Wang, H.; Pandey, D.R.; Abraham, T.P.; Shoukas, A.A.; Berkowitz, D.E.; et al. MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. Am. J. Physiol. Circ. Physiol. 2016, 310, H71–H79. [Google Scholar] [CrossRef]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative Contributions of Cystathionine β-Synthase and γ-Cystathionase to H2S Biogenesis via Alternative Trans-sulfuration Reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The Quantitative Significance of the Transsulfuration Enzymes for H2S Production in Murine Tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Ogasawara, Y.; Kimura, H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011, 439, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137). Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine -Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, H.; Gao, L.; Gu, T.; Wang, C.; Zhang, H. Hydrogen Sulfide Attenuates Atherosclerosis in a Partially Ligated Carotid Artery Mouse model via Regulating Angiotensin Converting Enzyme 2 Expression. Front. Physiol. 2017, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Bibli, S.-I.; Li, Z.; Chatzianastasiou, A.; Varela, A.; Katsouda, A.; Zukunft, S.; Bucci, M.; Vellecco, V.; Davos, C.H.; et al. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020, 176, 113833. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hamdan, A.; Guedouari-Bounihi, H.; Lenoir, V.; Andriamihaja, M.; Blachier, F.; Bouillaud, F. Oxidation of H2S in Mammalian Cells and Mitochondria. Methods Enzymol. 2015, 554, 201–228. [Google Scholar] [PubMed]
- Zhang, D.; Wang, X.; Tian, X.; Zhang, L.; Yang, G.; Tao, Y.; Liang, C.; Li, K.; Yu, X.; Tang, X.; et al. The Increased Endogenous Sulfur Dioxide Acts as a Compensatory Mechanism for the Downregulated Endogenous Hydrogen Sulfide Pathway in the Endothelial Cell Inflammation. Front. Immunol. 2018, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Chunyu, Z.; Junbao, D.; Dingfang, B.; Hui, Y.; Xiuying, T.; Chaoshu, T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem. Biophys. Res. Commun. 2003, 302, 810–816. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, C. A Novel Mechanism of Sildenafil Improving the Excessive Proliferation and H2S Production in Pulmonary Arterial Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2019, 74, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, X.; Chen, S.; Chen, S.; Yu, W.; Liu, X.; Yang, G.; Tao, Y.; Tang, X.; Bu, D.; et al. Endogenous hydrogen sulfide sulfhydrates IKKβ at cysteine 179 to control pulmonary artery endothelial cell inflammation. Clin. Sci. 2019, 133, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006, 209, 4011–4023. [Google Scholar] [CrossRef]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; Leger, J.S.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R51–R60. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Liu, D.; Nie, W.; Yuan, J.; Wang, Z.; Guo, Y. Sodium hydrosulfide prevents hypoxia-induced pulmonary arterial hypertension in broilers. Br. Poult. Sci. 2012, 53, 608–615. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, R.; Geng, B.; Qi, Y.-F.; Wang, P.-P.; Yao, W.-Z.; Tang, C.-S. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 2009, 45, 117–123. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, C.-S.; Jin, H.-F.; Du, J.-B. The vasorelaxing effect of hydrogen sulfide on isolated rat aortic rings versus pulmonary artery rings. Acta Pharmacol. Sin. 2011, 32, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Lloret, J.; Shaifta, Y.; Ward, J.P.T.; Aaronson, P.I. Hypoxic pulmonary vasoconstriction in isolated rat pulmonary arteries is not inhibited by antagonists of H2S-synthesizing pathways. J. Physiol. 2015, 593, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, D.; Tang, C.; Du, J.; Liu, A.D.; Holmberg, L.; Jin, H. Sulfur dioxide upregulates the inhibited endogenous hydrogen sulfide pathway in rats with pulmonary hypertension induced by high pulmonary blood flow. Biochem. Biophys. Res. Commun. 2013, 433, 519–525. [Google Scholar] [CrossRef]
- Madden, J.A.; Ahlf, S.B.; Dantuma, M.W.; Olson, K.R.; Roerig, D.L. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J. Appl. Physiol. 2012, 112, 411–418. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Markel, T.A.; Drucker, N.; Boone, D.; Whiteman, M.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; et al. Extended hypoxia-mediated H2S production provides for long-term oxygen sensing. Acta Physiol. 2020, 228, e13368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Mainali, P.; Tang, C.-S.; Shi, L.; Zhang, C.-Y.; Yan, H.; Liu, X.-Q.; Du, J.-B. Effects of nitric oxide and hydrogen sulfide on the relaxation of pulmonary arteries in rats. Chin. Med. J. 2008, 121, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, J.; Jin, H.; Geng, B.; Tang, C. Sodium hydrosulfide alleviates pulmonary artery collagen remodeling in rats with high pulmonary blood flow. Heart Vessel. 2008, 23, 409–419. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Jin, H.; Tang, X.; Bu, D.; Tang, C. The regulatory effect of endogenous hydrogen sulfide on pulmonary vascular structure and gasotransmitters in rats with high pulmonary blood flow. Life Sci. 2007, 81, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, Y.; Ma, Y.; Zhang, J.; Wang, C. Protective effect of hydrogen sulfide on monocrotaline-induced pulmonary arterial hypertension via inhibition of the endothelial mesenchymal transition. Int. J. Mol. Med. 2019, 44, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pan, W.; Wang, C.; Dong, H.; Xing, L.; Hou, J.; Fang, S.; Li, H.; Yang, F.; Yu, B. H2S attenuates endoplasmic reticulum stress in hypoxia-induced pulmonary artery hypertension. Biosci. Rep. 2019, 39, 20190304. [Google Scholar] [CrossRef]
- Vadivel, A.; Alphonse, R.S.; Ionescu, L.; Machado, D.S.; O’Reilly, M.; Eaton, F.; Haromy, A.; Michelakis, E.D.; Thébaud, B. Exogenous Hydrogen Sulfide (H2S) Protects Alveolar Growth in Experimental O2-Induced Neonatal Lung Injury. PLoS ONE 2014, 9, e90965. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-L.; Zhang, C.-Y.; Jin, H.-F.; Tang, C.-S.; Du, J.-B. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol. Sin. 2008, 29, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Rose, P.; Dymock, B.W.; Moore, P.K. GYY4137, a Novel Water-Soluble, H2S-Releasing Molecule. Methods Enzymol. 2015, 554, 143–167. [Google Scholar]
- Zhang, H.; Hao, L.-Z.; Pan, J.-A.; Gao, Q.; Zhang, J.-F.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Microfluidic fabrication of inhalable large porous microspheres loaded with H2S-releasing aspirin derivative for pulmonary arterial hypertension therapy. J. Control. Release 2021, 329, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.B.; Abrams, G.A.; Abdel-Razek, T.T.; Dai, J.; Chen, S.-J.; Chen, Y.-F.; Luo, B.; Oparil, S.; Ku, D.D. Garlic prevents hypoxic pulmonary hypertension in rats. Am. J. Physiol. Cell. Mol. Physiol. 1998, 275, L283–L287. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R592–R603. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. H2S and polysulfide metabolism: Conventional and unconventional pathways. Biochem. Pharmacol. 2018, 149, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lancaster, J.R. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Kabil, O.; Banerjee, R. High Turnover Rates for Hydrogen Sulfide Allow for Rapid Regulation of Its Tissue Concentrations. Antioxid. Redox Signal. 2012, 17, 22–31. [Google Scholar] [CrossRef]
- Libiad, M.; Vitvitsky, V.; Bostelaar, T.; Bak, D.W.; Lee, H.-J.; Sakamoto, N.; Fearon, E.; Lyssiotis, C.A.; Weerapana, E.; Banerjee, R. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J. Biol. Chem. 2019, 294, 12077–12090. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, P.K.; Xiong, X.; Dwivedi, S.K.D.; Mustafi, S.B.; Leigh, N.R.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathionine β-synthase regulates endothelial function via protein S -sulfhydration. FASEB J. 2016, 30, 441–456. [Google Scholar] [CrossRef]
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-sensitive mitochondrial accumulation of cystathionine -synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S Biogenesis, Decay and Signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.K.; Murray, T.V.A.; Prysyazhna, O.; Martin, D.; Burgoyne, J.; Santos, C.; Eaton, P.; Shah, A.M.; Brewer, A.C. Transcriptional Regulation of Cystathionine-γ-Lyase in Endothelial Cells by NADPH Oxidase 4-Dependent Signaling. J. Biol. Chem. 2016, 291, 1774–1788. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, J.G.; Carlisle, R.E.; Jerome, D.E.; Mohammed-Ali, Z.; Jiang, H.; Yang, G.; Mani, S.; Garg, S.K.; Banerjee, R.; Kaufman, R.J.; et al. Integrated Stress Response Modulates Cellular Redox State via Induction of Cystathionine γ-Lyase. J. Biol. Chem. 2012, 287, 7603–7614. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Liang, X.; Wu, J.; Dong, S.; Li, H.; Jin, M.; Sun, D.; Zhang, W.; Zhong, X. Inhibition of hydrogen sulfide on the proliferation of vascular smooth muscle cells involved in the modulation of calcium sensing receptor in high homocysteine. Exp. Cell Res. 2016, 347, 184–191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Shi, S.; Gao, F.; Wu, J.; Dong, S.; Zhang, W.; Liu, Y.; Zhong, X. Calcium sensing receptor initiating cystathionine-gamma-lyase/hydrogen sulfide pathway to inhibit platelet activation in hyperhomocysteinemia rat. Exp. Cell Res. 2017, 358, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen Sulfide-Linked Sulfhydration of NF-κB Mediates Its Antiapoptotic Actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Kohl, J.B.; Mellis, A.-T.; Schwarz, G. Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. Br. J. Pharmacol. 2019, 176, 554–570. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide: Its production and functions. Exp. Physiol. 2011, 96, 833–835. [Google Scholar] [CrossRef]
- Módis, K.; Coletta, C.; Erdélyi, K.; Papapetropoulos, A.; Szabo, C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013, 27, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chen, C.-T.; Chen, C.-S.; Wang, Y.-M.; Hsieh, H.-J.; Wang, D.L. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem. Biophys. Res. Commun. 2015, 464, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOxproduction. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Roman, H.B.; Hirschberger, L.L.; Krijt, J.; Valli, A.; Kožich, V.; Stipanuk, M.H. The Cysteine Dioxgenase Knockout Mouse: Altered Cysteine Metabolism in Nonhepatic Tissues Leads to Excess H2S/HS−Production and Evidence of Pancreatic and Lung Toxicity. Antioxid. Redox Signal. 2013, 19, 1321–1336. [Google Scholar] [CrossRef]
- Olson, K.R. Hydrogen Sulfide as an Oxygen Sensor. Antioxid. Redox Signal. 2015, 22, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Toombs, C.F.; Insko, M.A.; Wintner, E.A.; Deckwerth, T.L.; Usansky, H.; Jamil, K.; Goldstein, B.; Cooreman, M.; Szabo, C. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br. J. Clin. Pharmacol. 2010, 69, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Bazhanov, N.; Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Hydrogen Sulfide: A Novel Player in Airway Development, Pathophysiology of Respiratory Diseases, and Antiviral Defenses. Am. J. Respir. Cell Mol. Biol. 2017, 57, 403–410. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, J.; Munakata, M.; Shibata, Y. Hydrogen sulfide as a novel biomarker of asthma and chronic obstructive pulmonary disease. Allergol. Int. 2021, 70, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef]
- Madurga, A.; Golec, A.; Pozarska, A.; Ishii, I.; Mižíková, I.; Nardiello, C.; Vadász, I.; Herold, S.; Mayer, K.; Reichenberger, F.; et al. The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L710–L724. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Wondimu, T.; Ross, B.; Wang, R. Hydrogen sulfide and asthma. Exp. Physiol. 2011, 96, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Yanfei, W.; Lin, S.; Junbao, D.; Chaoshu, T. Impact of l-arginine on hydrogen sulfide/cystathionine-γ-lyase pathway in rats with high blood flow-induced pulmonary hypertension. Biochem. Biophys. Res. Commun. 2006, 345, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Xiaohui, L.; Junbao, D.; Lin, S.; Jian, L.; Xiuying, T.; Jianguang, Q.; Bing, W.; Hongfang, J.; Chaoshu, T. Down-Regulation of Endogenous Hydrogen Sulfide Pathway in Pulmonary Hypertension and Pulmonary Vascular Structural Remodeling Induced by High Pulmonary Blood Flow in Rats. Circ. J. 2005, 69, 1418–1424. [Google Scholar] [CrossRef]
- Han, W.; Dong, Z.; Dimitropoulou, C.; Su, Y. Hydrogen Sulfide Ameliorates Tobacco Smoke-Induced Oxidative Stress and Emphysema in Mice. Antioxid. Redox Signal. 2011, 15, 2121–2134. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Du, S.-X.; Jin, H.-F.; Bu, D.-F.; Zhao, X.; Geng, B.; Tang, C.-S.; Du, J.-B. Endogenously generated sulfur dioxide and its vasorelaxant effect in rats1. Acta Pharmacol. Sin. 2008, 29, 923–930. [Google Scholar] [CrossRef]
- Luo, L.; Chen, S.; Jin, H.; Tang, C.; Du, J. Endogenous generation of sulfur dioxide in rat tissues. Biochem. Biophys. Res. Commun. 2011, 415, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shen, Z.; Chen, Q.; Huang, P.; Zhang, H.; Du, S.; Geng, B.; Zhang, C.; Li, K.; Tang, C.; et al. Endogenous sulfur dioxide alleviates collagen remodeling via inhibiting TGF-β/Smad pathway in vascular smooth muscle cells. Sci. Rep. 2016, 6, 19503. [Google Scholar] [CrossRef]
- Liu, J.; Yu, W.; Liu, Y.; Chen, S.; Huang, Y.; Li, X.; Liu, C.; Zhang, Y.; Li, Z.; Du, J.; et al. Mechanical stretching stimulates collagen synthesis via down-regulating SO2/AAT1 pathway. Sci. Rep. 2016, 6, 21112. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, D.; Liang, C.; Ochs, T.; Chen, S.; Chen, S.; Du, S.; Tang, C.; Huang, Y.; Du, J.; et al. Sulfur Dioxide Protects Against Collagen Accumulation in Pulmonary Artery in Association with Downregulation of the Transforming Growth Factor β1/Smad Pathway in Pulmonary Hypertensive Rats. J. Am. Heart Assoc. 2016, 5, e003910. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Nelin, V.E.; Locy, M.L.; Jin, Y.; Tipple, T.E. Thioredoxin-1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L389–L395. [Google Scholar] [CrossRef]
- Adesina, S.E.; Wade, B.E.; Bijli, K.M.; Kang, B.-Y.; Williams, C.R.; Ma, J.; Go, Y.-M.; Hart, C.M.; Sutliff, R.L. Hypoxia inhibits expression and function of mitochondrial thioredoxin 2 to promote pulmonary hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L599–L608. [Google Scholar] [CrossRef]
- Lignelli, E.; Palumbo, F.; Bayindir, S.G.; Nagahara, N.; Vadász, I.; Herold, S.; Seeger, W.; Morty, R.E. The H2S-generating enzyme 3-mercaptopyruvate sulfurtransferase regulates pulmonary vascular smooth muscle cell migration and proliferation but does not impact normal or aberrant lung development. Nitric Oxide 2021, 107, 31–45. [Google Scholar] [CrossRef]
- Tao, B.; Wang, R.; Sun, C.; Zhu, Y. 3-Mercaptopyruvate Sulfurtransferase, Not Cystathionine β-Synthase Nor Cystathionine γ-Lyase, Mediates Hypoxia-Induced Migration of Vascular Endothelial Cells. Front. Pharmacol. 2017, 8, 657. [Google Scholar] [CrossRef]
- Smith, K.A.; Schumacker, P.T. Sensors and signals: The role of reactive oxygen species in hypoxic pulmonary vasoconstriction. J. Physiol. 2018, 597, 1033–1043. [Google Scholar] [CrossRef]
- Leach, R.M.; Robertson, T.P.; Twort, C.H.; Ward, J.P. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. Am. J. Physiol. Cell. Mol. Physiol. 1994, 266, L223–L231. [Google Scholar] [CrossRef]
- Sommer, N.; Strielkov, I.; Pak, O.; Weissmann, N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur. Respir. J. 2015, 47, 288–303. [Google Scholar] [CrossRef]
- Prieto-Lloret, J.; Snetkov, V.A.; Shaifta, Y.; Docio, I.; Connolly, M.J.; Mackay, C.E.; Knock, G.A.; Ward, J.P.T.; Aaronson, P.I. Role of reactive oxygen species and sulfide-quinone oxoreductase in hydrogen sulfide-induced contraction of rat pulmonary arteries. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L670–L685. [Google Scholar] [CrossRef]
- Szijártó, I.A.; Markó, L.; Filipovic, M.R.; Miljkovic, J.L.; Tabeling, C.; Tsvetkov, D.; Wang, N.; Rabelo, L.A.; Witzenrath, M.; Diedrich, A.; et al. Cystathionine γ-Lyase–Produced Hydrogen Sulfide Controls Endothelial NO Bioavailability and Blood Pressure. Hypertension 2018, 71, 1210–1217. [Google Scholar] [CrossRef]
- Connolly, M.J.; Prieto-Lloret, J.; Becker, S.; Ward, J.P.T.; Aaronson, P.I. Hypoxic pulmonary vasoconstriction in the absence of pretone: Essential role for intracellular Ca2+ release. J. Physiol. 2013, 591, 4473–4498. [Google Scholar] [CrossRef]
- Ariyaratnam, P.; Loubani, M.; Morice, A.H. Hydrogen sulphide vasodilates human pulmonary arteries: A possible role in pulmonary hypertension? Microvasc. Res. 2013, 90, 135–137. [Google Scholar] [CrossRef]
- Cui, T.; Liu, W.; Chen, S.; Yu, C.; Li, Y.; Zhang, J.-Y. Antihypertensive effects of allicin on spontaneously hypertensive rats via vasorelaxation and hydrogen sulfide mechanisms. Biomed. Pharmacother. 2020, 128, 110240. [Google Scholar] [CrossRef]
- Kaye, A.D.; De Witt, B.J.; Anwar, M.; Smith, D.E.; Feng, C.J.; Kadowitz, P.J.; Nossaman, B.D. Analysis of responses of garlic derivatives in the pulmonary vascular bed of the rat. J. Appl. Physiol. 2000, 89, 353–358. [Google Scholar] [CrossRef]
- Ku, D.D.; Abdel-Razek, T.T.; Dai, J.; Kim-Park, S.; Fallon, M.B.; Abrams, G.A. Garlic and its Active Metabolite Allicin Produce Endothelium- and Nitric Oxide-Dependent Relaxation In Rat Pulmonary Arteries. Clin. Exp. Pharmacol. Physiol. 2002, 29, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kim-Park, S.; Ku, D.D. Garlic elicits a nitric oxide-dependent relaxation and inhibits hypoxic pulmonary vasoconstriction in rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen Sulfide as Endothelium-Derived Hyperpolarizing Factor Sulfhydrates Potassium Channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.; Deitch, E.A.; Szabó, C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci. 2008, 83, 589–594. [Google Scholar] [CrossRef]
- Giuffrè, A.; Vicente, J.B. Hydrogen Sulfide Biochemistry and Interplay with Other Gaseous Mediators in Mammalian Physiology. Oxidative Med. Cell. Longev. 2018, 2018, 1–31. [Google Scholar] [CrossRef]
- Dongó, E.; Beliczai-Marosi, G.; Dybvig, A.S.; Kiss, L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide 2018, 81, 75–87. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Yu, W.; Chen, S.; Yao, Q.; Zhang, C.; Bu, D.; Tang, C.; Du, J.; Jin, H. Sulfhydration-associated phosphodiesterase 5A dimerization mediates vasorelaxant effect of hydrogen sulfide. Oncotarget 2017, 8, 31888–31900. [Google Scholar] [CrossRef]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; He, G.-W. Imbalance of Homocysteine and H2S: Significance, Mechanisms, and Therapeutic Promise in Vascular Injury. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Micinski, D.; Lieblong, B.J.; Stapleton, T. Relationship between hydrogen sulfide levels and HDL-cholesterol, adiponectin, and potassium levels in the blood of healthy subjects. Atherosclerosis 2012, 225, 242–245. [Google Scholar] [CrossRef]
- Peter, E.A.; Shen, X.; Shah, S.H.; Pardue, S.; Glawe, J.D.; Zhang, W.W.; Reddy, P.; Akkus, N.I.; Varma, J.; Kevil, C.G. Plasma Free H2S Levels are Elevated in Patients with Cardiovascular Disease. J. Am. Heart Assoc. 2013, 2, e000387. [Google Scholar] [CrossRef]
- Rajpal, S.; Katikaneni, P.; Deshotels, M.; Pardue, S.; Glawe, J.; Shen, X.; Akkus, N.; Modi, K.; Bhandari, R.; Dominic, P.; et al. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biol. 2018, 15, 480–489. [Google Scholar] [CrossRef]
- Sun, L.; Sun, S.; Li, Y.; Pan, W.; Xie, Y.; Wang, S.; Zhang, Z. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: The role of homocysteine and hydrogen sulfide. Chin. Med. J. 2014, 127, 893–899. [Google Scholar]
- Sanli, C.; Oguz, D.; Olguntürk, R.; Tunaoglu, F.S.; Kula, S.; Pasaoglu, H.; Gulbahar, O.; Cevik, A. Elevated Homocysteine and Asymmetric Dimethyl Arginine Levels in Pulmonary Hypertension Associated with Congenital Heart Disease. Pediatr. Cardiol. 2012, 33, 1323–1331. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, S.; Ren, X.; Zhang, C.; Xu, F. The prognostic implications of perioperative endogenous hydrogen sulfide and nitric oxide levels in children with congenital heart disease complicated by pulmonary arterial hypertension. Eur. J. Nucl. Med. Mol. Imaging 2021, 180, 1915–1922. [Google Scholar] [CrossRef]
- Seeger, W.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, S.; et al. Pulmonary Hypertension in Chronic Lung Diseases. J. Am. Coll. Cardiol. 2013, 62, D109–D116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Yao, W.-Z.; Geng, B.; Ding, Y.-L.; Lu, M.; Zhao, M.-W.; Tang, C.-S. Endogenous Hydrogen Sulfide in Patients With COPD. Chest 2005, 128, 3205–3211. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, K.; Li, M.-X.; He, W.; Chang, J.-R.; Liao, C.-C.; Lin, F.; Qi, Y.-F.; Wang, R.; Chen, Y.-H. Metabolic changes of H2S in smokers and patients of COPD which might involve in inflammation, oxidative stress and steroid sensitivity. Sci. Rep. 2015, 5, 14971. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Chen, Y.; Yao, W.; Rosner, E.; Mastropietro, C.W. Exhaled Hydrogen Sulfide Predicts Airway Inflammation Phenotype in COPD. Respir. Care 2014, 60, 251–258. [Google Scholar] [CrossRef]
- Morty, R.E. Recent advances in the pathogenesis of BPD. Semin. Perinatol. 2018, 42, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Kurata, H.; Ochiai, M.; Inoue, H.; Kusuda, T.; Fujiyoshi, J.; Ichiyama, M.; Wakata, Y.; Takada, H. Inflammation in the neonatal period and intrauterine growth restriction aggravate bronchopulmonary dysplasia. Pediatr. Neonatol. 2019, 60, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Donda, K.; Zambrano, R.; Moon, Y.; Percival, J.; Vaidya, R.; Dapaah-Siakwan, F.; Luo, S.; Duncan, M.R.; Bao, Y.; Wang, L.; et al. Riociguat prevents hyperoxia-induced lung injury and pulmonary hypertension in neonatal rats without effects on long bone growth. PLoS ONE 2018, 13, e0199927. [Google Scholar] [CrossRef]
- Baker, C.D.; Alvira, C.M. Disrupted lung development and bronchopulmonary dysplasia: Opportunities for Lung Repair and Regeneration. Curr. Opin. Pediatr. 2014, 26, 306–314. [Google Scholar] [CrossRef]
- Viña, J.; Vento, M.; García-Sala, F.; Puertes, I.R.; Gascó, E.; Sastre, J.; Asensi, M.; Pallardó, F.V. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am. J. Clin. Nutr. 1995, 61, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.H.; Anderson, G.H. The Development of Cystathionase Activity During the First Year of Life. Pediatr. Res. 1982, 16, 65–68. [Google Scholar] [CrossRef]
- Sztuka, K.; Jasińska-Stroschein, M. Animal models of pulmonary arterial hypertension: A systematic review and meta-analysis of data from 6126 animals. Pharmacol. Res. 2017, 125, 201–214. [Google Scholar] [CrossRef]
- Rosenberg, H.C.; Rabinovitch, M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am. J. Physiol. Circ. Physiol. 1988, 255, H1484–H1491. [Google Scholar] [CrossRef]
- Feng, S.; Chen, S.; Yu, W.; Zhang, D.; Zhang, C.; Tang, C.; Du, J.; Jin, H. H2S inhibits pulmonary arterial endothelial cell inflammation in rats with monocrotaline-induced pulmonary hypertension. Lab. Investig. 2017, 97, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Qingyou, Z.; Junbao, D.; Weijin, Z.; Hui, Y.; Chaoshu, T.; Chunyu, Z. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem. Biophys. Res. Commun. 2004, 317, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hongfang, J.; Bailin, C.; Bin, Z.; Chunyu, Z.; Xinmin, L.; Weijin, Z.; Ying, S.; Chaoshu, T.; Junbao, D. Effects of hydrogen sulfide on hypoxic pulmonary vascular structural remodeling. Life Sci. 2006, 78, 1299–1309. [Google Scholar] [CrossRef]
- López, V.; Moraga, F.A.; Llanos, A.J.; Ebensperger, G.; Taborda, M.I.; Uribe, E. Plasmatic Concentrations of ADMA and Homocystein in Llama (Lama glama) and Regulation of Arginase Type II: An Animal Resistent to the Development of Pulmonary Hypertension Induced by Hypoxia. Front. Physiol. 2018, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, H.-F.; Liu, D.; Sun, J.-H.; Jian, P.-J.; Li, X.-H.; Tang, C.-S.; Du, J.-B. Hydrogen sulfide induces apoptosis of pulmonary artery smooth muscle cell in rats with pulmonary hypertension induced by high pulmonary blood flow. Chin. Med. J. 2009, 122, 3032–3038. [Google Scholar]
- Yuan, S.; Yurdagul, A.; Peretik, J.M.; Alfaidi, M.; Al Yafeai, Z.; Pardue, S.; Kevil, C.G.; Orr, A.W.; Pearson, B.H. Cystathionine γ-Lyase Modulates Flow-Dependent Vascular Remodeling. Arter. Thromb. Vasc. Biol. 2018, 38, 2126–2136. [Google Scholar] [CrossRef]
- Fan, J.; Fan, X.; Li, Y.; Ding, L.; Zheng, Q.; Guo, J.; Xia, D.; Xue, F.; Wang, Y.; Liu, S.; et al. Chronic Normobaric Hypoxia Induces Pulmonary Hypertension in Rats: Role of NF-κB. High Alt. Med. Biol. 2016, 17, 43–49. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, Z.; Feng, W.; Hu, T.; Guan, H.; Mao, Y. AOS ameliorates monocrotaline-induced pulmonary hypertension by restraining the activation of P-selectin/p38MAPK/NF-κB pathway in rats. Biomed. Pharmacother. 2019, 109, 1319–1326. [Google Scholar] [CrossRef]
- Giustarini, D.; Del Soldato, P.; Sparatore, A.; Rossi, R. Modulation of thiol homeostasis induced by H2S-releasing aspirin. Free. Radic. Biol. Med. 2010, 48, 1263–1272. [Google Scholar] [CrossRef]
- Ding, H.-B.; Liu, K.-X.; Huang, J.-F.; Wu, D.-W.; Chen, J.-Y.; Chen, Q. Protective effect of exogenous hydrogen sulfide on pulmonary artery endothelial cells by suppressing endoplasmic reticulum stress in a rat model of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2018, 105, 734–741. [Google Scholar] [CrossRef]
- Pan, T.; Zhang, L.; Miao, K.; Wang, Y. A crucial role of endoplasmic reticulum stress in cellular responses during pulmonary arterial hypertension. Am. J. Transl. Res. 2020, 12, 1481–1490. [Google Scholar] [PubMed]
- Rao, G.; Murphy, B.; Dey, A.; Dwivedi, S.K.D.; Zhang, Y.; Roy, R.V.; Chakraborty, P.; Bhattacharya, R.; Mukherjee, P. Cystathionine beta synthase regulates mitochondrial dynamics and function in endothelial cells. FASEB J. 2020, 34, 9372–9392. [Google Scholar] [CrossRef] [PubMed]
- Madurga, A.; Mižíková, I.; Ruiz-Camp, J.; Vadász, I.; Herold, S.; Mayer, K.; Fehrenbach, H.; Seeger, W.; Morty, R.E. Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L684–L697. [Google Scholar] [CrossRef]
- Donnarumma, E.; Trivedi, R.K.; Lefer, D.J. Protective Actions of H2S in Acute Myocardial Infarction and Heart Failure. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 583–602. ISBN 9780470650714. [Google Scholar]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B.; Gojon, G.; Giordano, T.; Krum, H. A Novel Hydrogen Sulfide Prodrug, SG 1002, Promotes Hydrogen Sulfide and Nitric Oxide Bioavailability in Heart Failure Patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef]
- Kondo, K.; Bhushan, S.; King, A.L.; Prabhu, S.D.; Hamid, T.; Koenig, S.; Murohara, T.; Predmore, B.L.; Gojon, G.; Wang, R.; et al. H2S Protects Against Pressure Overload–Induced Heart Failure via Upregulation of Endothelial Nitric Oxide Synthase. Circulation 2013, 127, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.H.; Yu, Y.; Chang, L.; Tan, B.; Jia, W.; Xiong, Y.; Dai, T.; Zhong, R.; Zhang, W.; Le, V.M.; et al. A Novel Liposomal S-Propargyl-Cysteine: A Sustained Release of Hydrogen Sulfide Reducing Myocardial Fibrosis via TGF-β1/Smad Pathway. Int. J. Nanomed. 2019, 14, 10061–10077. [Google Scholar] [CrossRef]
- Culley, M.K.; Chan, S.Y. Mitochondrial metabolism in pulmonary hypertension: Beyond mountains there are mountains. J. Clin. Investig. 2018, 128, 3704–3715. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Liu, J.; Liu, S.; Song, Q.; Liu, L.; Xie, L.; Han, Y.; Ji, Y. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br. J. Pharmacol. 2018, 175, 1126–1145. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Chen, Y.; Liu, L.; Xu, M.; Sun, L.; Luo, H.; Wang, Y.; Meng, G. Exogenous Hydrogen Sulfide Supplement Attenuates Isoproterenol-Induced Myocardial Hypertrophy in a Sirtuin 3-Dependent Manner. Oxidative Med. Cell. Longev. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Murphy, B.; Mustafi, S.B.; Dey, A.; Xiong, X.; Rao, G.; Naz, S.; Zhang, M.; Yang, D.; Dhanasekaran, D.N.; et al. Cystathionine β-synthase regulates mitochondrial morphogenesis in ovarian cancer. FASEB J. 2018, 32, 4145–4157. [Google Scholar] [CrossRef]
- Quatredeniers, M.; Mendes-Ferreira, P.; Santos-Ribeiro, D.; Nakhleh, M.; Ghigna, M.-R.; Cohen-Kaminsky, S.; Perros, F. Iron Deficiency in Pulmonary Arterial Hypertension: A Deep Dive into the Mechanisms. Cells 2021, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Ried, K. Garlic Lowers Blood Pressure in Hypertensive Individuals, Regulates Serum Cholesterol, and Stimulates Immunity: An Updated Meta-analysis and Review. J. Nutr. 2016, 146, 389S–396S. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Muzaffar, S.; Jeremy, J.Y.; Sparatore, A.; Del Soldato, P.; Angelini, G.D.; Shukla, N. H2 S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: Role of protein kinases A and G. Br. J. Pharmacol. 2008, 155, 984–994. [Google Scholar] [CrossRef]
- Verma, R.; Akhtar, Y.; Singh, S. A Review of Patents on Therapeutic Potential and Delivery of Hydroge n Sulfide. Recent Pat. Drug Deliv. Formul. 2017, 11, 114–123. [Google Scholar] [CrossRef] [PubMed]

| Experimental Models | H2S-Releasing Molecules | Animal Species | Summary of Reported Effects on PH | References |
|---|---|---|---|---|
| MCT | NaHS 56 μmol/kg/day (21 days) | Wistar rat | ↘ mPAP ↘ RV hypertrophy ↘ PA remodeling ↘ ICAM-1, TNF-α, IL-6, IL-8 MCP-1 (plasma and lung) | [141] |
| NaHS 56 μmol/kg/day (21 days) | Wistar rat | ↘ mPAP ↘ TNF-α, IL-6 (lung) | [34] | |
| NaHS 1 mg/kg/day (7 days after MCT injection) | Sprague Dawley rat | ↘ mPAP ↘ RV hypertrophy ↘ PA remodeling ↗ RV ejection fraction ↗ VE-cadherin, ↘ α-SMA (PA) | [50] | |
| ACS 14 46.5 mg/kg (7 days after MCT injection) | Sprague Dawley rat | ↘ mPAP ↘ RV hypertrophy ↘ PA remodeling ↗ RV ejection fraction ↗ VE-cadherin, ↘ α-SMA (PA) | [56] | |
| Hypoxia-induced PH | NaHS 14 μmol/kg/day (21 days) | Wistar rat | ↘ mPAP ↘ RV hypertrophy ↘ PA remodeling | [35] |
| NaHS 14 μmol/kg/day (21 days) | Wistar rat | ↘ mPAP ↗ CO (Plasma) ↗ HO-1 (PA media) | [142] | |
| NaHS 14 μmol/kg/day (21 days) | Wistar rat | ↘ mPAP ↘ number of muscularized PA ↘ collagen type I/III, elastin (PA media) | [143] | |
| NaHS 14 μmol/kg/day (21 days) | Wistar rat | ↘ mPAP ↘ RV hypertrophy ↗ total antioxidant capacity (lung) | [53] | |
| NaHS 10 μmol/kg/day (21 days) | Broiler | ↘ mPAP ↘ PA remodeling | [40] | |
| Garlic powder 100 mg/kg/day (5 days) | Sprague Dawley rat | ↘ mPAP ↗ relaxation intralobar PA (90 min hypoxia) | [57] | |
| GYY4137 (concentration not reported, daily, 4 weeks) | Sprague Dawley rat | ↘ mPAP, and PA resistances ↘ RV hypertrophy ↘ PA remodeling ↗ treadmill running distance ↘ RE stress proteins ATF6 and Grp78 (PA) | [51] | |
| COPD models | NaHS 50 μmol/kg/day (12 or 24 weeks) | C57BL/6 mice | ↘ mPAP ↘ RV hypertrophy ↘ TNF-α (BAL) ↘ 8-hydroxyguanine (lung) | [94] |
| NaHS 56 μmol/kg/day (60 days) | Sprague Dawley rat | ↘ apoptosis of PAEC | [150] | |
| High pulmonary blood flow | NaHS 56 μmol/kg/day (11 weeks) | Sprague Dawley rat | ↘ mPAP ↘ PA remodeling ↘ collagen type I/III (PA) ↘ ET-1 (lung) | [48] |
| NaHS 56 μmol/kg/day (11 weeks) | Sprague Dawley rat | ↘ mPAP ↗ PASMC apoptosis | [145] | |
| BDP-PH | GYY4137 37.75 mg/kg/day (10 days) | Newborn rat pups | ↘ PA remodeling ↘ RV afterload and hypertrophy ↗ pulmonary vessels density | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roubenne, L.; Marthan, R.; Le Grand, B.; Guibert, C. Hydrogen Sulfide Metabolism and Pulmonary Hypertension. Cells 2021, 10, 1477. https://doi.org/10.3390/cells10061477
Roubenne L, Marthan R, Le Grand B, Guibert C. Hydrogen Sulfide Metabolism and Pulmonary Hypertension. Cells. 2021; 10(6):1477. https://doi.org/10.3390/cells10061477
Chicago/Turabian StyleRoubenne, Lukas, Roger Marthan, Bruno Le Grand, and Christelle Guibert. 2021. "Hydrogen Sulfide Metabolism and Pulmonary Hypertension" Cells 10, no. 6: 1477. https://doi.org/10.3390/cells10061477
APA StyleRoubenne, L., Marthan, R., Le Grand, B., & Guibert, C. (2021). Hydrogen Sulfide Metabolism and Pulmonary Hypertension. Cells, 10(6), 1477. https://doi.org/10.3390/cells10061477






