The Role of Irisin in Cancer Disease
Abstract
1. Introduction
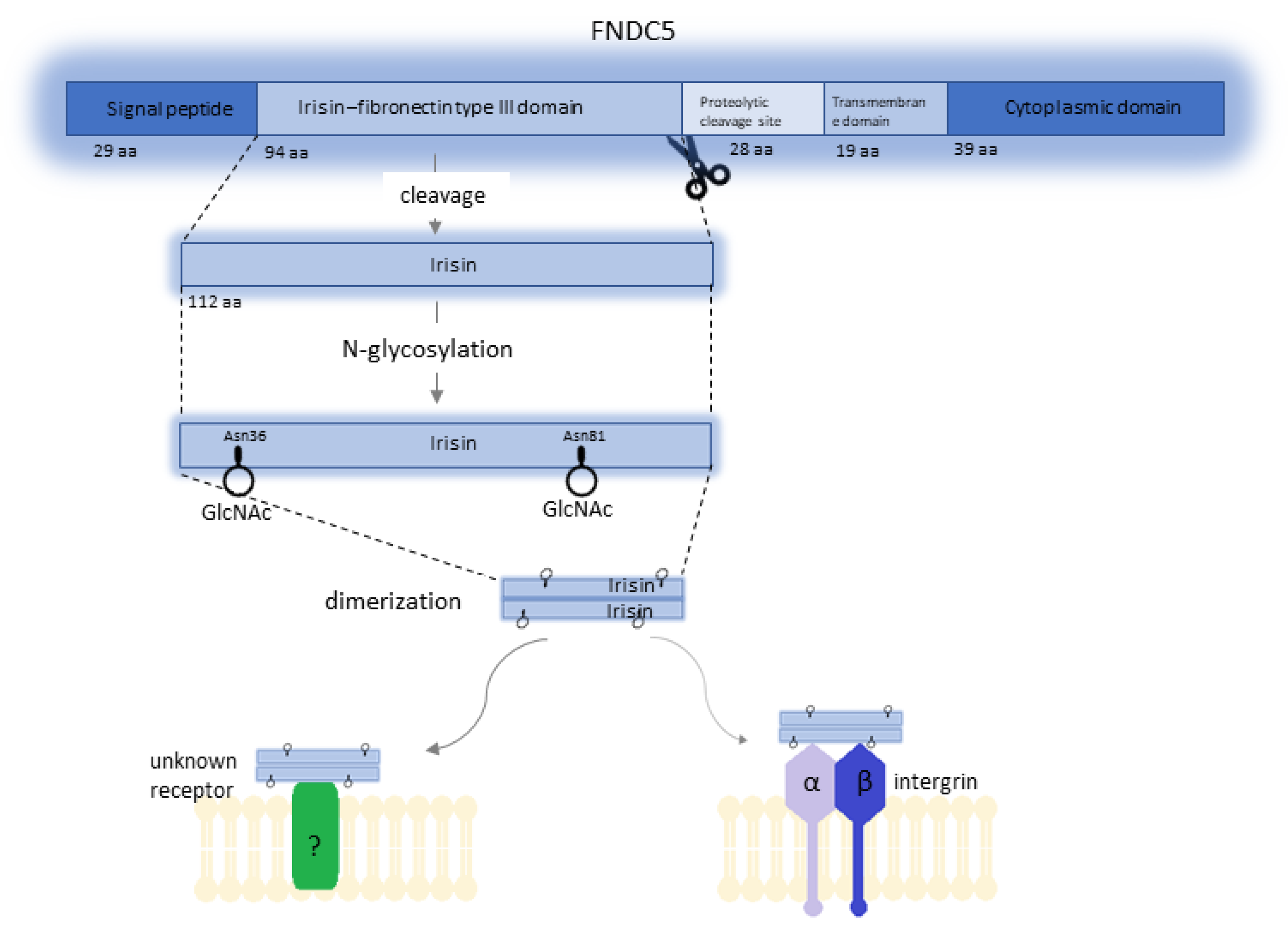
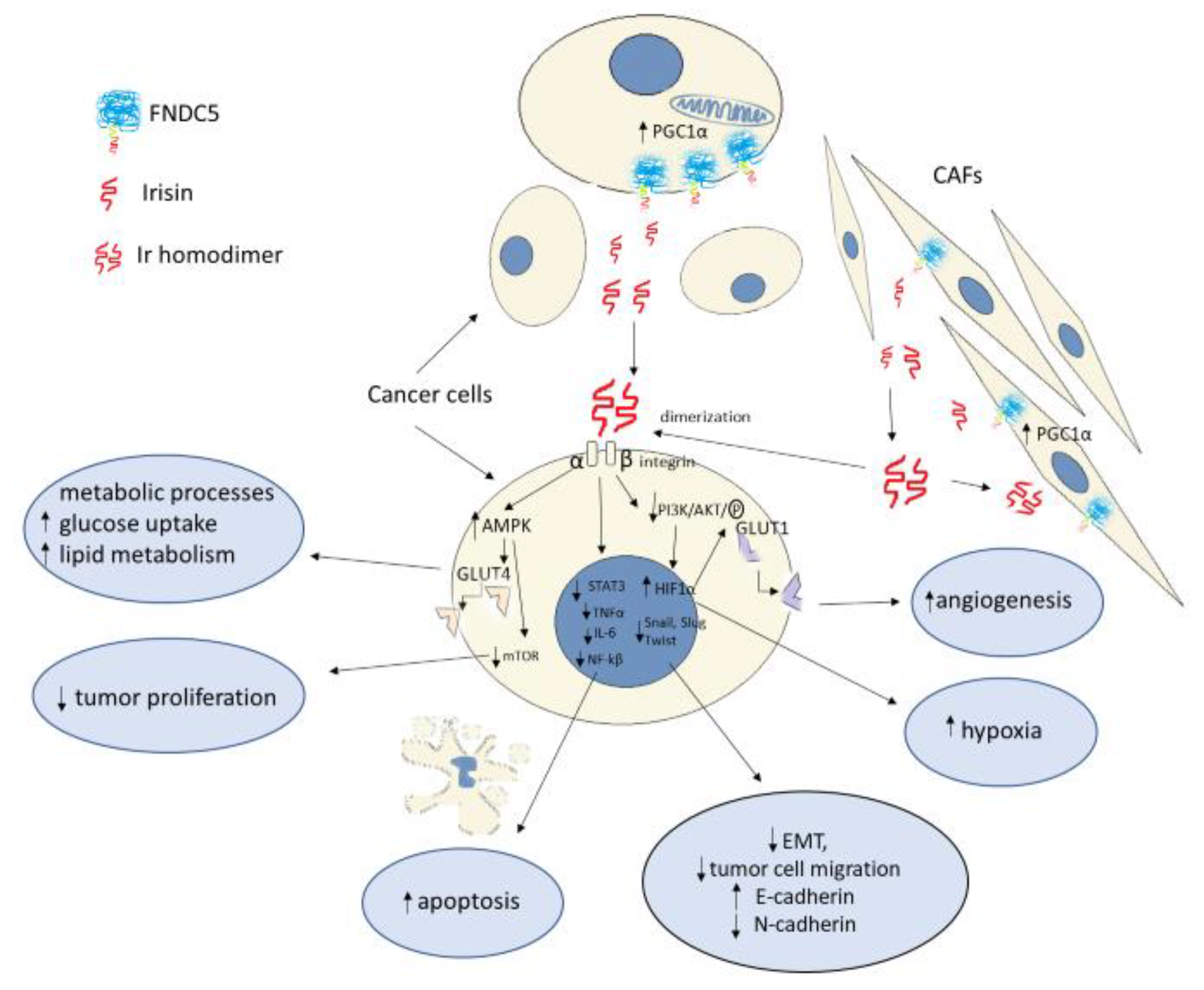
2. Irisin as a Ligand for Integrins
3. Irisin as a Coordinator of Metabolic Processes
4. Irisin in Cancer Proliferation Process
| Research Team | Cancer/Cell Lines | Irisin | Results | Reference Number |
|---|---|---|---|---|
| Moon et al. [63] | Endometrial (KLE, RL95-2) Colon (HT29, MCA38) Thyroid (SW579, BHP7 Esophageal (OE13, OE33) KLE, RL95-2, HT29, SW579 BHP7, OE13, OE33-American Type Culture Collection (ATCC, Manassas, VA, USA) MCA38, National Cancer Institute, National Institute of Health, Dr. Nicholas Restifo | Human recombinant Ir Aviscera Bioscience (Santa Clara, CA, USA) Phoenix Pharmaceuticals (Burlingame, CA, USA) Ir levels: 5–10 nmol/L (physiological) 50–100 nmol/L (pharmacological) | No impact of Ir on tumor cell proliferation, adhesion, or number compared to controls (p < 0.05) | [63] |
| Gannon et al. [30] | Breast MCF-7 MDA-MB-231 MCF-10a- control (American Type Culture Collection; Manassas, VA, USA) | Human recombinant nonmodified Ir-INM Cayman Chemical (Ann Arbor, MI, USA) Human recombinant modified and active (glycosylated) Ir-IM PlexBio (San Francisco, CA, USA) Ir levels: 0.625–20 nM | Reduced number of cancer cells (INM), and migration (INM, IM) Induction of tumor cell apoptosis (INM) Inhibition of NF-κB activity (INM) Enhancement of the effect of Dox on cancer cells by Ir (INM at all tested concentrations; IM at 1.0 μgM) | [30] |
| Tekin et al. [58] | Prostate cancer LNCaP DU-145 PC3 | Ir (Phoenix peptide, Burlingame, CA, USA) Ir levels: 0.1–100 nM | Antiproliferative effect Decreased survival time of LNCaP cells at higher Ir levels (10–100 nM; p < 0.05; p < 0.01) | [58] |
| Shi et al. [31] | Hepatocellular carcinoma HepG2 SMMC7721 | Human recombinant modified and active (glycosylated) Ir-IM PlexBio (San Francisco, CA, USA) Human recombinant non-modified Ir-INM CaymanChemical (Ann Arbor, MI, USA) Ir levels: 0.625–20 nM | Ir increased liver cancer cell viability in all cell lines (IM, INM) The Ir-IM-level of 2.5 nM stimulated an increase in migration and invasiveness of HepG2 cells compared to controls. This increase was statistically significant The level of modified Ir-IM of 2.5 nM significantly inhibited the cytotoxicity of Dox | [31] |
| Shao et al. [59] | Lung cancer A549 (NSCLC) NCl-H446 (SCLC) Institute of Biochemistry and Cell Biology, Chinese Academy of Science, China | Ir levels: 0–50 nM | Ir at levels of 20–50 nM significantly inhibited A549 cell proliferation Ir at levels >20 nM inhibited migration and invasiveness of A549 cells | [59] |
| Kong et al. [61] | Osteosarcoma U2OS MG-63 American Type Culture Collection (ATCC, Manassas, VA, USA) | Ir levels: 0–200 ng/mL | Ir inhibited proliferation, migration, and invasiveness of U2OS and MG-63 cells in a dose- and time-dependent manner | [61] |
| Liu et al. [62] | Pancreatic cancer MIA PaCa-2 Panc03.27 ATCC (Manassas, VA, USA) | Human recombinant glycosylated E-Ir Human nonrecombinant P-Ir Sangon Biotech, Shanghai, China Ir levels: 0–100 nM | Both Ir forms inhibited the growth, migration, and invasiveness of pancreatic cancer cells | [62] |
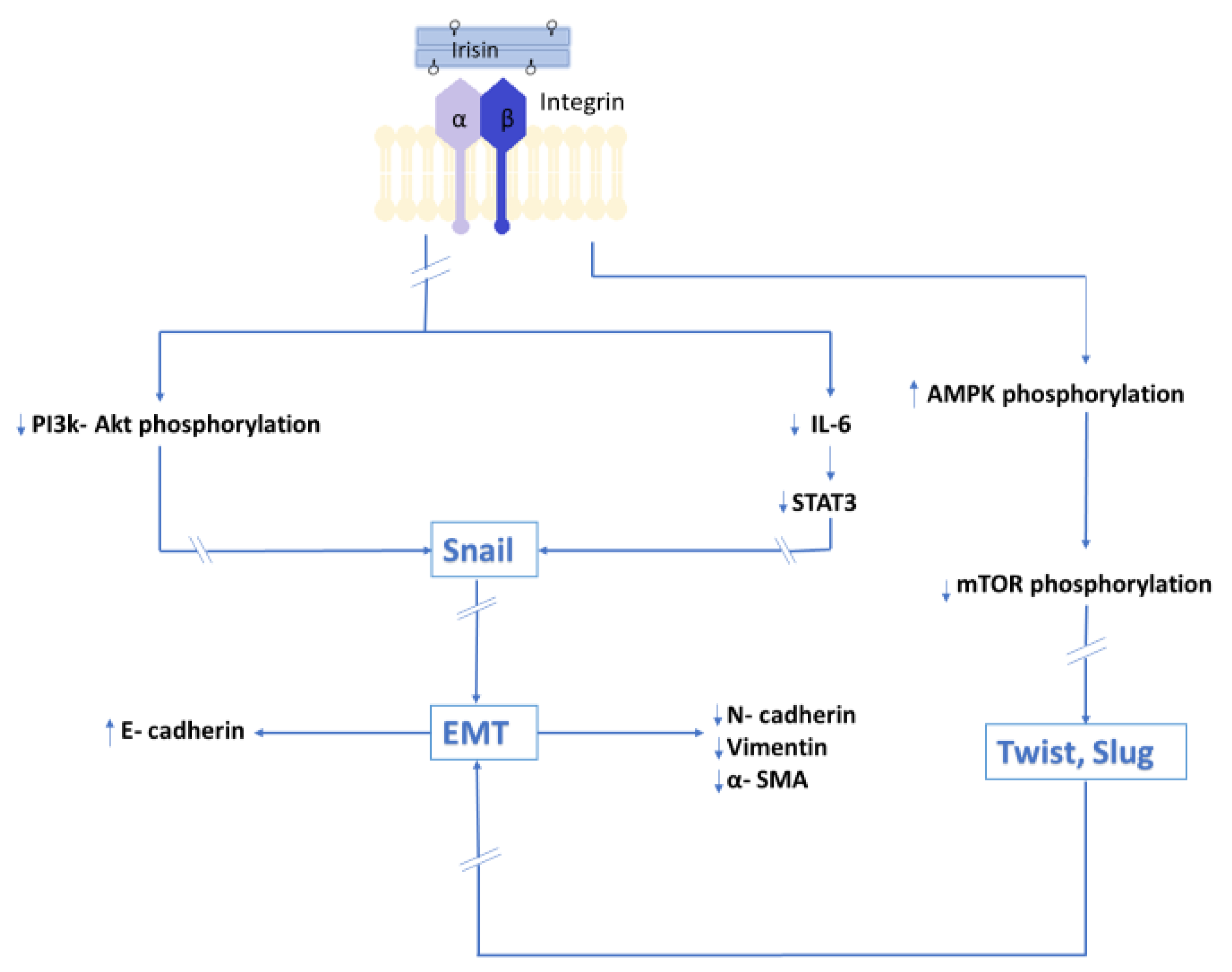
5. Impact of Irisin on Epithelial–Mesenchymal Transition
5.1. Epithelial–Mesenchymal Transition as the Background of Metastatic Processes
5.2. Results of the In Vitro Model Indicating the Impact of Irisin on Epithelial–Mesenchymal Transition
6. Irisin and Its Potential Role in Cancer Therapy
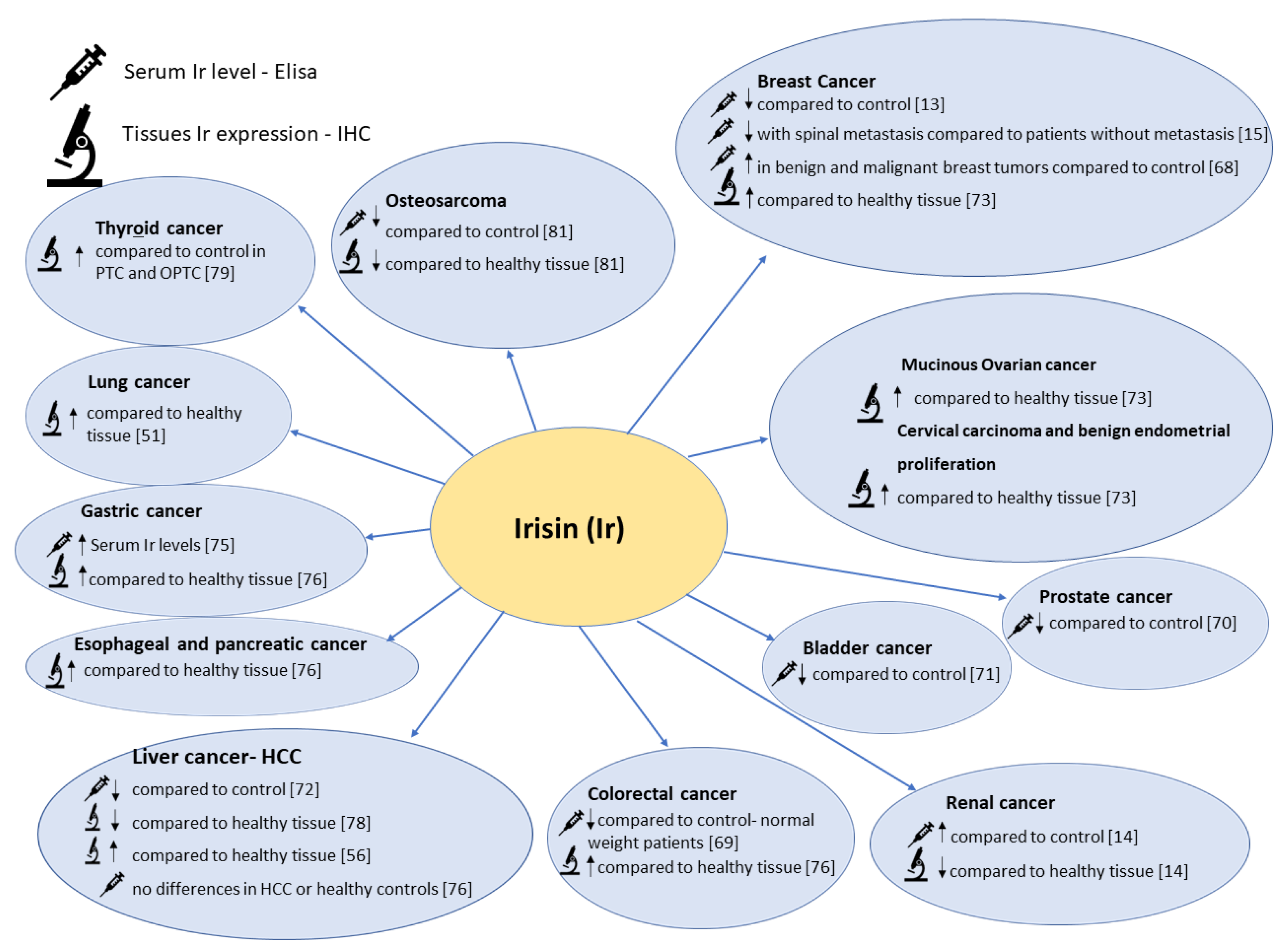
7. The Role of Irisin in Selected Cancer Diseases
7.1. Breast Cancer and Reproductive Tract Cancer
7.1.1. Serum Irisin Level in Patients with Breast Cancer
7.1.2. Irisin Tissue Expression Levels in Patients with Breast Cancer and Reproductive Tract Cancer
7.2. Irisin in Prostate, Kidney, and Bladder Cancer
7.3. Irisin in Gastrointestinal Cancers
7.4. Irisin in Lung Cancer
7.5. Irisin in Thyroid Cancer
7.6. Osteosarcoma
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ir | Irisin |
| FNDC5 | fibronectin type III domain-containing protein 5 |
| WAT | white adipose tissue |
| BAT | brown adipose tissue |
| UCP | uncoupling protein |
| UCP1 | uncoupling protein 1 |
| PPAR | peroxisome proliferator-activated receptor |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1 alpha |
| FAK | focal adhesion kinase; |
| BMP-7 | bone morphogenetic protein 7 |
| CAFs | cancer-associated fibroblasts |
| GLUT | glucose transporters; |
| HIFs | hypoxia-inducible factors |
| VEGF | vascular endothelial growth factor |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Akt | protein kinase B (PKB) |
| PI3K-3 | phosphatidylinositol 3-kinase; |
| mTOR | mammalian target of rapamycin kinase |
| AMPK | 5’AMP-activated protein kinase |
| MMP | matrix metalloproteinase |
| EMT | epithelial–mesenchymal transition |
| α-SMA | alpha-smooth muscle actin |
| NSCLC | non-small-cell lung carcinoma |
| SCLC | small-cell lung carcinoma |
| DOX | doxorubicin |
| INM | human recombinant nonmodified irisin |
| IM | human recombinant modified and active (glycosylated irisin) |
| HCC | human hepatocellular carcinoma; |
| DCIS | ductal carcinoma in situ |
| CEA | carcinoembryonic antigen |
| RCC | renal cell carcinoma |
| ccRCC | clear cell renal cell carcinoma |
| CRC | colorectal cancer |
| OS | overall survival |
| PTC | papillary thyroid carcinoma |
| FTC | follicular thyroid carcinoma |
| OPTC | oncocytic variant of papillary carcinoma of the thyroid |
| OFTC | oncocytic variant of follicular carcinoma of the thyroid; |
| BC | bladder cancer |
| GC | gastric cancer |
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, İ.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef]
- Dun, S.L.; Lyu, R.-M.; Chen, Y.-H.; Chang, J.-K.; Luo, J.J.; Dun, N.J. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 2013, 240, 155–162. [Google Scholar] [CrossRef]
- Pukajło, K.; Kolackov, K.; Łaczmański, Ł.; Daroszewski, J. Iryzyna—Nowy mediator homeostazy energetycznej. Postepy Hig. Med. Dosw. 2015, 69, 233–242. [Google Scholar] [CrossRef]
- Erickson, H.P. Irisin and FNDC5 in retrospect. Adipocyte 2013, 2, 289–293. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprot/Q8NAU1 (accessed on 11 May 2021).
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum irisin levels are lower in patients with breast cancer: Association with disease diagnosis and tumor characteristics. BMC Cancer 2015, 15, 898. [Google Scholar] [CrossRef]
- Altay, D.U.; Keha, E.E.; Karagüzel, E.; Menteşe, A.; Yaman, S.O.; Alver, A. The diagnostic value of FNDC5/Irisin in renal Cell Cancer. Int. Braz. J. Urol. 2018, 44, 734–739. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Li, H.; Liu, T.; Zhao, Q.; Huang, L.; Cao, Z.; He, L.; Hao, D. Serum irisin associates with breast cancer to spinal metastasis. Medicine 2018, 97, e0524. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Kraemer, R.; Shockett, P.; Webb, N.; Shah, U.; Castracane, V. A Transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2013, 46, 150–154. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Goto, K.; Kiuchi, M.; Yamakita, M.; Koyama, K. High-Intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans—Results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Scharhag-Rosenberger, F.; Meyer, T.; Wegmann, M.; Ruppenthal, S.; Kaestner, L.; Morsch, A.; Hecksteden, A. Irisin does not mediate resistance training–Induced alterations in resting metabolic rate. Med. Sci. Sport. Exerc. 2014, 46, 1736–1743. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Schumann, U.; Zügel, M.; Steinacker, J.M. Chronic exercise training and circulating irisin in adults: A meta-analysis. Sport. Med. 2015, 45, 1577–1588. [Google Scholar] [CrossRef]
- Fatouros, I.G. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clin. Chem. Lab. Med. 2018, 56, 525–548. [Google Scholar] [CrossRef]
- Ząbczyńska, M.P.E. The role of protein glycosylation in immune system. Postepy Biochem. 2015, 61, 129–137. [Google Scholar] [PubMed]
- Kozłowska, K.; Rydlewska, M.; Ząbczyńska, M.P.E. IgG glycosylation in autoimmune diseases. Postepy Hig. Med. Dosw. 2018, 72, 975–990. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a multifunctional protein: Implications for health and certain diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef]
- Nie, Y.; Liu, D. N-Glycosylation is required for FDNC5 stabilization and irisin secretion. Biochem. J. 2017, 474, 3167–3177. [Google Scholar] [CrossRef]
- Maak, S.; Norheim, F.; Drevon, A.C.; Erickson, H.P. Progress and challenges in the biology of FNDC5 and irisin. Endocr. Rev. 2021. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Shi, G.; Tang, N.; Qiu, J.; Zhang, D.; Huang, F.; Cheng, Y.; Ding, K.; Li, W.; Zhang, P.; Tan, X. Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 493, 585–591. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef]
- Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Darkwah, S.; Kawamoto, E.; Shimaoka, M. Integrin-ligand interactions in inflammation, cancer, and metabolic disease: Insights into the multifaceted roles of an emerging ligand irisin. Front. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via αV integrin receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. eLife 2020, 9. [Google Scholar] [CrossRef]
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell 2020, 182, 563–577.e20. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin reverses intestinal epithelial barrier dysfunction during intestinal injury via binding to the integrin αVβ5 receptor. J. Cell. Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef]
- Jarmuszkiewicz, W.; Woyda-Płoszczyca, A. Mitochondrial uncoupling proteins: Regulation and physiological role. Postepy Biochem. 2008, 54, 179–187. [Google Scholar] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Smitka, K.; Marešová, D. Adipose tissue as an endocrine organ: An update on pro-inflammatory and anti-inflammatory microenvironment. Prague Med. Rep. 2015, 116, 87–111. [Google Scholar] [CrossRef]
- Xu, B. BDNF (I)rising from exercise. Cell Metab. 2013, 18, 612–614. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Rui, L. Brown and beige adipose tissues in health and disease. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1281–1306. [Google Scholar]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Ścibior-Bentkowska, D.; Czeczot, H. Cancer cells and oxidative stress. Postepy Hig. Med. Dosw. 2009, 63, 58–72. [Google Scholar]
- Liberti, M.V.; Locasale, J.W. The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gasińska, A.; Janecka, A.; Adamczyk, A.; Słonina, D. How tumour cells respirate? Nowotw. J. Oncol. 2013, 63, 124–131. [Google Scholar]
- Nowinska, K.; Jablonska, K.; Pawelczyk, K.; Piotrowska, A.; Partynska, A.; Gomulkiewicz, A.; Ciesielska, U.; Katnik, E.; Grzegrzolka, J.; Glatzel-Plucinska, N.; et al. Expression of irisin/FNDC5 in cancer cells and stromal fibroblasts of non-small cell lung cancer. Cancers 2019, 11, 1538. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, P.; Lipińska, A. The role of glucose transporter 1 (GLUT1) in the diagnosis and therapy of tumors. Postepy Hig. Med. Dosw. 2012, 66, 165–174. [Google Scholar]
- Krześlak, A. Akt kinase: A key regulator of metabolism and progression of tumors. Postepy Hig. Med. Dosw. 2010, 64, 490–503. [Google Scholar]
- Xin, C.; Liu, J.; Zhang, J.; Zhu, D.; Wang, H.; Xiong, L.; Lee, Y.; Ye, J.; Lian, K.; Xu, C.; et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. 2016, 40, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Höpfl, G.; Ogunshola, O.; Gassmann, M. HIFs and tumors—Causes and consequences. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R608–R623. [Google Scholar] [CrossRef]
- Gaggini, M.; Cabiati, M.; Del Turco, S.; Navarra, T.; De Simone, P.; Filipponi, F.; Del Ry, S.; Gastaldelli, A.; Basta, G. Increased FNDC5/Irisin expression in human hepatocellular carcinoma. Peptides 2017, 88, 62–66. [Google Scholar] [CrossRef]
- Us Altay, D.; Keha, E.E.; Ozer Yaman, S.; Ince, I.; Alver, A.; Erdogan, B.; Canpolat, S.; Cobanoglu, U.; Mentese, A. Investigation of the expression of irisin and some cachectic factors in mice with experimentally induced gastric cancer. QJM 2016, 109, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Erden, Y.; Sanda, S.; Yilmaz, B. Is irisin an anticarcinogenic peptide? Med. Sci. 2015, 4, 2172–2180. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.-H.; Zhu, T.-Y.; Huang, J. FNDC5 promotes paclitaxel sensitivity of non-small cell lung cancers via inhibiting MDR1. Cell. Signal. 2020, 72, 109665. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin reverses the IL-6 induced epithelial-mesenchymal transition in osteosarcoma cell migration and invasion through the STAT3/Snail signaling pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef]
- Moon, H.-S.; Mantzoros, C.S. Regulation of cell proliferation and malignant potential by irisin in endometrial, colon, thyroid and esophageal cancer cell lines. Metabolism 2014, 63, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Balcerak, A.; Wakuła, M.; Trębińska, A.; Grzybowska, E.A. Migracja i inwazyjność komórek nowotworowych; rola plastyczności komórek i udział macierzy zewnątrzkomórkowej w tworzeniu przerzutów. Nowotw. J. Oncol. 2016, 66, 45–52. [Google Scholar] [CrossRef][Green Version]
- Park, J.-H.; Lee, J.-Y.; Shin, D.-H.; Jang, K.-S.; Kim, H.-J.; Kong, G. Loss of Mel-18 induces tumor angiogenesis through enhancing the activity and expression of HIF-1α mediated by the PTEN/PI3K/Akt pathway. Oncogene 2011, 30, 4578–4589. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef]
- Gos, M.; Miloszewska, J.; Przybyszewska, M. Epithelial-mesenchymal transition in cancer progression. Postepy Biochem. 2009, 55, 121–128. [Google Scholar] [PubMed]
- Panagiotou, G.; Triantafyllidou, S.; Tarlatzis, B.C.; Papakonstantinou, E. Serum levels of irisin and omentin-1 in breast neoplasms and their association with tumor histology. Int. J. Endocrinol. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Serum and adipose tissue mRNA levels of ATF3 and FNDC5/irisin in colorectal cancer patients with or without obesity. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Aslan, R.; Alp, H.H.; Eryılmaz, R.; Huyut, Z.; Sevim, M.; Araz, Ş.; Ertas, K.; Taken, K. Can the irisin be a biomarker for prostate cancer? A case control study. Asian Pac. J. Cancer Prev. 2020, 21, 505–509. [Google Scholar] [CrossRef]
- Esawy, M.M.; Abdel-Samd, K.M. The diagnostic and prognostic roles of serum irisin in bladder cancer. Curr. Probl. Cancer 2020, 44, 100529. [Google Scholar] [CrossRef]
- Pazgan-Simon, M.; J Zuwala-Jagiello, J.; Menzyk, T.; Bator, M.; Derra, A.; Lekstan, A.; Grzebyk, E.; Simon, K.M. Serum betatrophin and irisin levels in hepatocellular carcinoma. J. Physiol. Pharm. 2020, 71, 113–123. [Google Scholar] [CrossRef]
- Kuloglu, T.; Celik, O.; Aydin, S.; Ozercan, I.H.; Acet, M.; Aydin, Y.; Artas, G.; Turk, A.; Yardim, M.; Ozan, G.; et al. Irisin immunostaining characteristics of breast and ovarian cancer cells. Cell. Mol. Biol. 2016, 62, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kuloğlu, T.; Artaş, G.; Yardim, M.; Sahin, I.; Aydin, Y.; Beyoğlu, N.; Özercan, İ.H.; Yalcin, M.H.; Ugur, K.; Aydin, S. Immunostaining characteristics of irisin in benign and malignant renal cancers. Biotech. Histochem. 2019, 94, 435–441. [Google Scholar] [CrossRef]
- Shahidi, S.; Hejazi, J.; Moghimi, M.; Borji, S.; Zabihian, S.; Fathi, M. Circulating irisin levels and redox status markers in patients with gastric cancer: A case-control study. Asian Pac. J. Cancer Prev. 2020, 21, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Ozercan, M.; Albayrak, S.; Aydin, S.; Bakal, U.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Sarac, M.; et al. Irisin immunohistochemistry in gastrointestinal system cancers. Biotech. Histochem. 2016, 91, 242–250. [Google Scholar] [CrossRef]
- Pernot, S. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 2015, 21, 11428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ke, M.; Ren, Y.; Bi, J.; Du, Z.; Zhang, M.; Wang, Y.; Zhang, L.; Wu, Z.; Lv, Y.; et al. Serum irisin predicts posthepatectomy complications in patients with hepatocellular carcinoma. Dis. Markers 2019, 2019. [Google Scholar] [CrossRef]
- Ugur, K.; Aydin, S.; Kuloglu, T.; Artas, G.; Kocdor, M.A.; Sahin, İ.; Yardim, M.; Hanifi Ozercan, İ. Comparison of irisin hormone expression between thyroid cancer tissues and oncocytic variant cells. Cancer Manag. Res. 2019, 11, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K. Hürthle cell metaplasia in chronic lymphocytic thyroiditis: Role of age factor and review of literature on its molecular pathogenesis. Diagn. Cytopathol. 2019, 47, 475–481. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, D.; Chu, K.; Cao, Z.; Sun, X.; Yang, Y. The Effects of MiR-214-3p and irisin/FNDC5 on the biological behavior of osteosarcoma cells. Cancer Biother. Radiopharm. 2020, 35, 92–100. [Google Scholar] [CrossRef]
- Cai, H.; Miao, M.; Wang, Z. miR-214-3p promotes the proliferation, migration and invasion of osteosarcoma cells by targeting CADM1. Oncol. Lett. 2018, 16, 2620–2628. [Google Scholar] [CrossRef]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The osteosarcoma microenvironment: A complex but targetable ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed]
| Research Team | Study Method | Results | Study Group | Reference Number |
|---|---|---|---|---|
| Provatopoulou et al. [13] | ELISA (AdipoGen International, Liestal, SW); results expressed as μg/mL | Lower serum levels of Ir in patients compared to the control group (2.47 ± 0.57 (mean ± SD) vs. 3.24 ± 0.66 (mean ± SD)) p < 0.001 | 101 female patients with invasive ductal breast cancer 51 healthy women (the control group) | [13] |
| Gaggini et al. [56] | ELISA (Adipogen AG, Liestal, Switzerland); results expressed as μg/mL | Plasma Ir levels did not differ between HCC patients and controls (3.56 ± 0.2 (mean ± SEM) vs. 4.4 ± 0.15 (mean ± SEM)) p = 0.749 | 18 patients with HCC 18 deceased donors | [56] |
| Shi et al. [31] | ELISA (USCN life Science, Wuhan, China); results expressed as μg/mL | Plasma Ir levels were not different between HCC patients and controls | 20 patients with HCC | [31] |
| Altay et al. [14] | ELISA (USCN, Life Science Inc., Catalog No. USCN-E82576Hu, P.R. China); results expressed as pg/mL | Higher FNDC5/Ir levels in renal tumor patients compared to the control group (208 ± 97 (mean ± SD) vs. 110 ± 79 [mean ± SD) p = 0.0001 | 23 patients with renal tumor 25 healthy individuals | [14] |
| Zhang et al. [15] | ELISA (Aviscera Biosciences, Santa Clara, CA, USA); results expressed as ng/mL | Higher Ir levels in patients without spinal metastases (7.60 ± 3.80 (mean ± SD) vs. 6.10 ± 2.62 (mean ± SD)) p = 0.012 | 148 patients with breast cancer, including 53 subjects with spinal metastasis | [15] |
| Zhu et al. [69] | ELISA (USCN Life Science Inc., Wuhan, China); results expressed as μg/mL | Lower Ir levels in patients with colorectal cancer and normal weight compared to controls (0.17 ± 0.01 (mean ± SD) vs. 0.22 ± 0.01 (mean ± SD)) p < 0.05) | 76 patients—38 patients with colon cancer and 38 subjects with rectal cancer 40 healthy controls | [69] |
| Aslan et al. [70] | ELISA (Yl Biont Biotech Co. Shanghai, China); results expressed as pg/mL | Mean Ir level was lower in prostate cancer patients compared to controls (6.92 ± 2.44 (mean ± SD) and 13.5 ± 6.21 (mean ± SD)) p < 0.05 | 50 patients with primary prostate cancer 30 healthy male subjects | [70] |
| Esawy and Abel [71] | ELISA (Bio Vendor Laboratory Medicine, Brno, Czech Republic) [Catalog No. RAG018R]; results expressed as μg/mL | Lower Ir levels in patients with bladder cancer compared to controls (1.07 (0.51–1.96) (mean ± SD) vs. 1.8 (0.5–2.44) (mean ± SD)) p < 0.001 | 75 patients with bladder cancer 75 healthy subjects | [71] |
| Pazgan-Simon et al. [72] | ELISA (Bio Vendor- Laboratorini Medicina a.s. catalog No. RAG018R); results expressed as μg/mL | Lower Ir levels in HCC patients compared to controls (2.52 ± 1.14 (median ± SD) vs. 4.46 ± 1.34 (median ± SD)) p = 0.02 | 69 patients with cirrhosis and hepatocellular carcinoma 24 patients with non-viral cirrhosis 20 healthy volunteers | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinkowska, A.; Podhorska-Okołów, M.; Dzięgiel, P.; Nowińska, K. The Role of Irisin in Cancer Disease. Cells 2021, 10, 1479. https://doi.org/10.3390/cells10061479
Pinkowska A, Podhorska-Okołów M, Dzięgiel P, Nowińska K. The Role of Irisin in Cancer Disease. Cells. 2021; 10(6):1479. https://doi.org/10.3390/cells10061479
Chicago/Turabian StylePinkowska, Agnieszka, Marzenna Podhorska-Okołów, Piotr Dzięgiel, and Katarzyna Nowińska. 2021. "The Role of Irisin in Cancer Disease" Cells 10, no. 6: 1479. https://doi.org/10.3390/cells10061479
APA StylePinkowska, A., Podhorska-Okołów, M., Dzięgiel, P., & Nowińska, K. (2021). The Role of Irisin in Cancer Disease. Cells, 10(6), 1479. https://doi.org/10.3390/cells10061479







