Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of Ovaries and COC Retrieval
2.3. In Vitro Maturation (IVM) Medium
2.4. Alginate COC-Microbead Fabrication
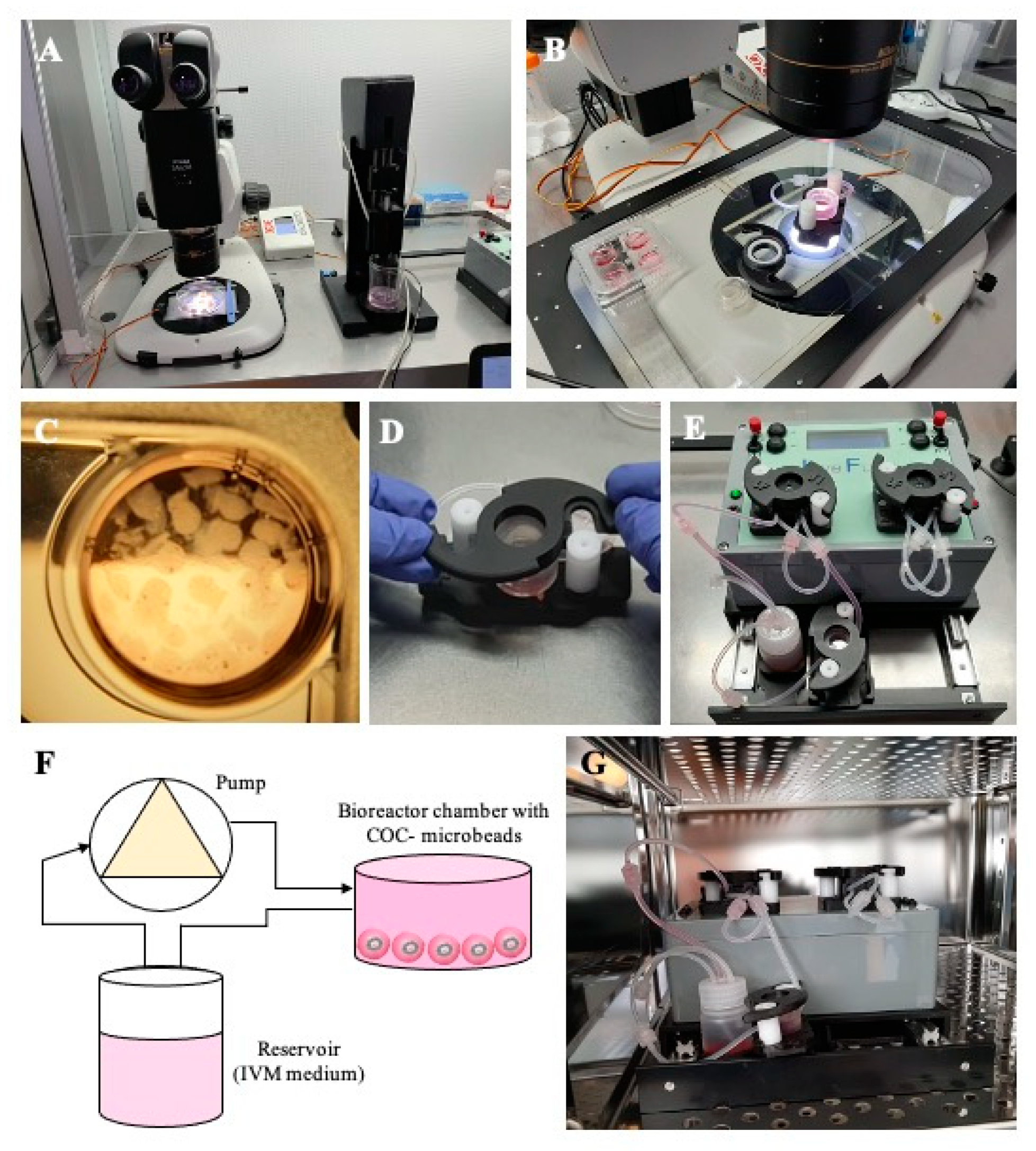
2.5. Three-Dimensional In Vitro Maturation (3D-IVM)
2.6. In Vitro Fertilization (IVF) and In Vitro Embryo Culture (IVEC)
2.7. Three-Dimensional Millifluidic In Vitro Maturation (3D mIVM)
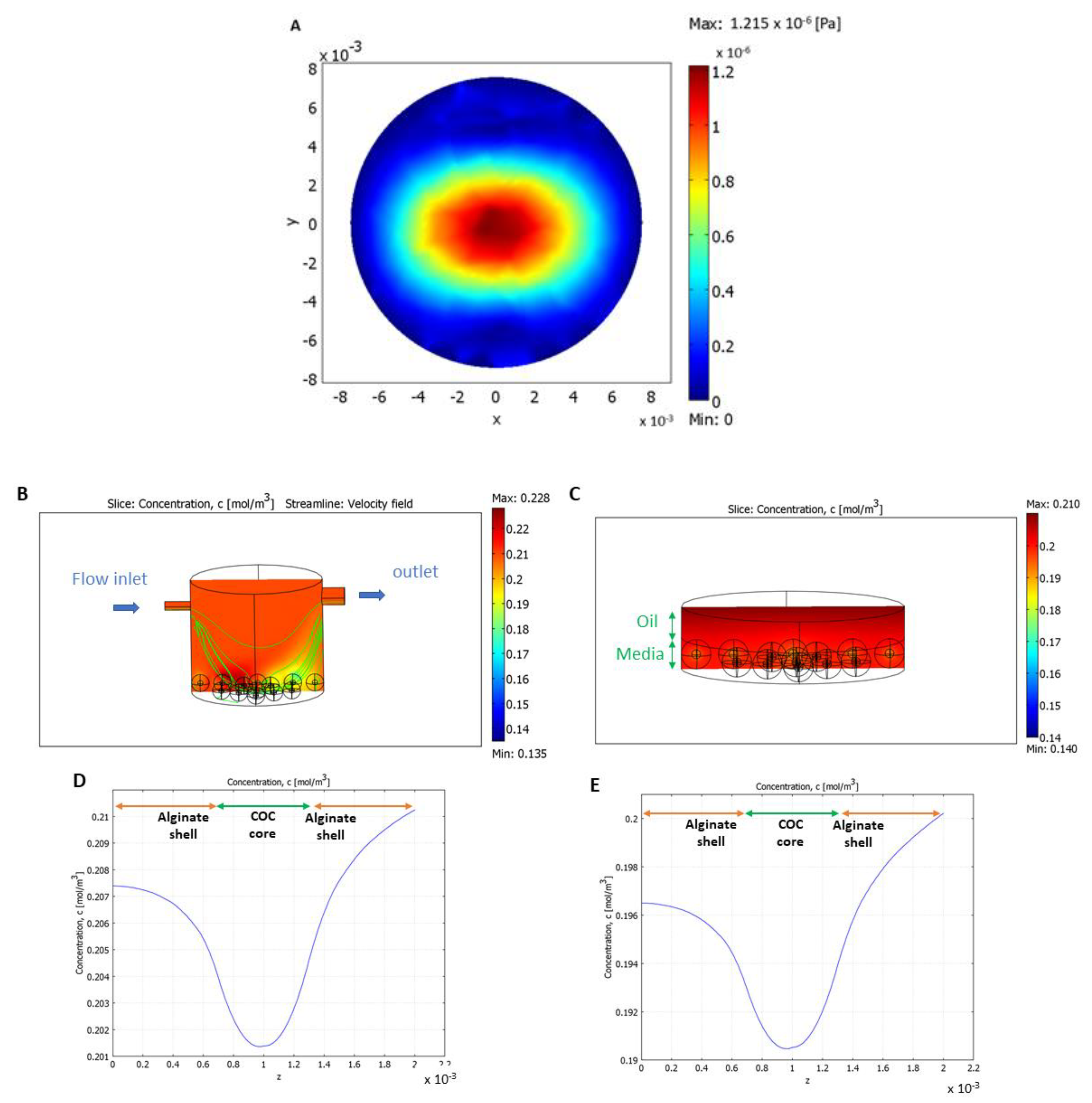
2.8. Computational Models
2.9. Granulosa Cell Isolation
2.10. Type I Collagen Preparation
2.11. COC-GC Co-Cultures
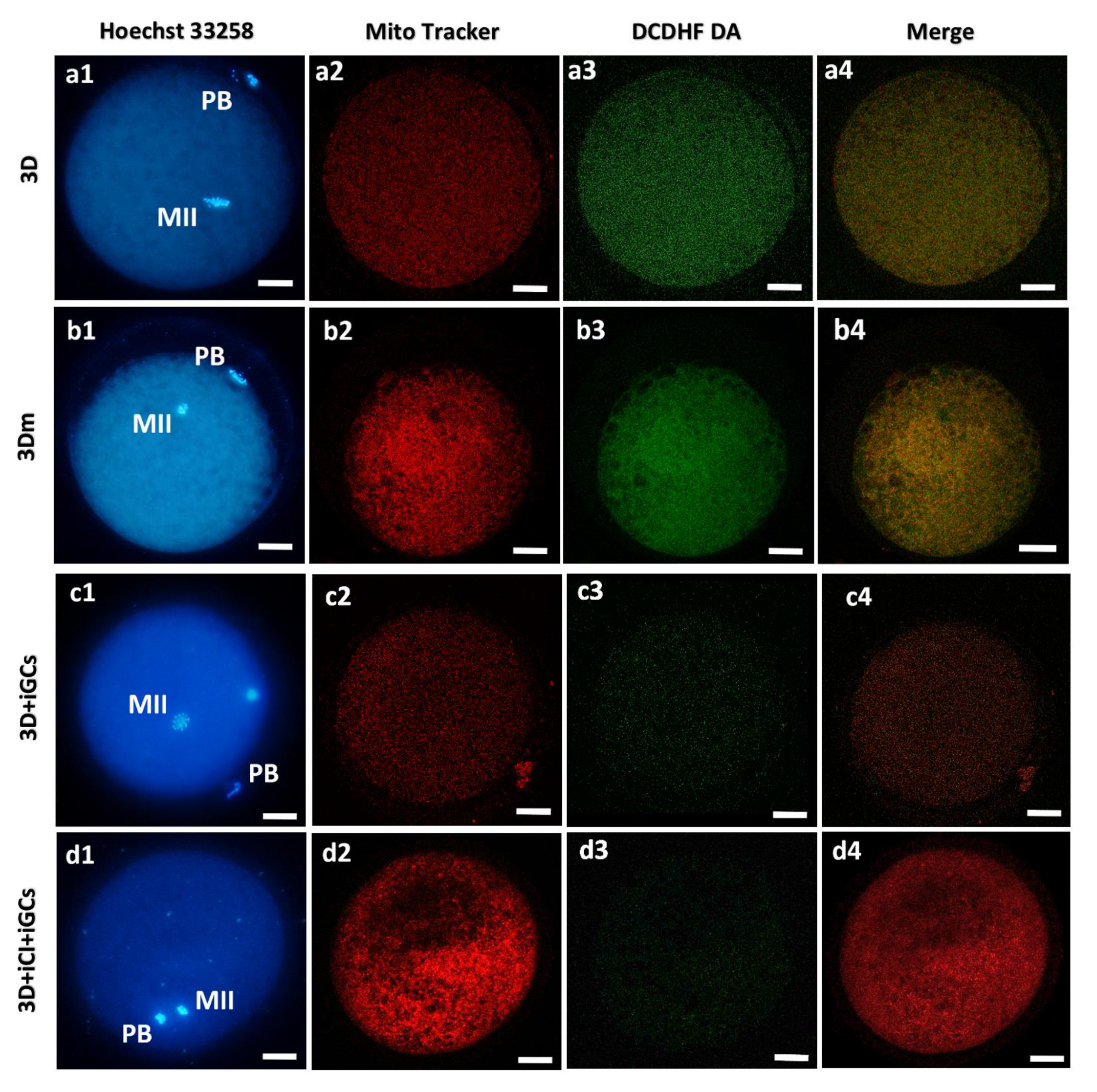
2.12. Oocyte and Blastocyst Mitochondria and ROS Staining
2.13. Nuclear Chromatin Evaluation of Oocytes and Embryos
2.14. Assessment of Mitochondrial Distribution Pattern and Intracellular ROS Localization
2.15. Quantification of MitoTracker Orange CMTMRos and H2DCF-DA Fluorescence Intensity
2.16. Mitochondria/ROS Colocalization Analysis
2.17. Statistical Analysis
3. Results
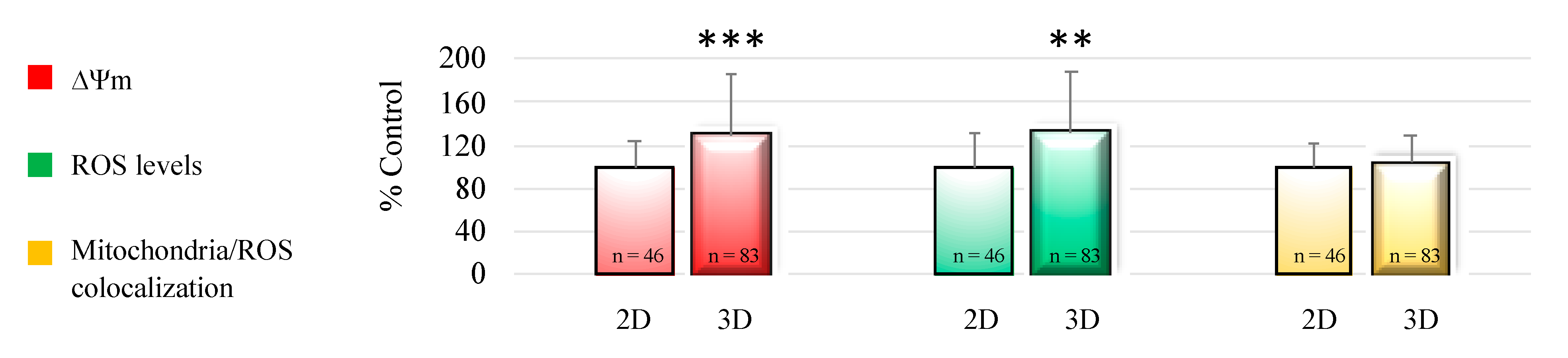
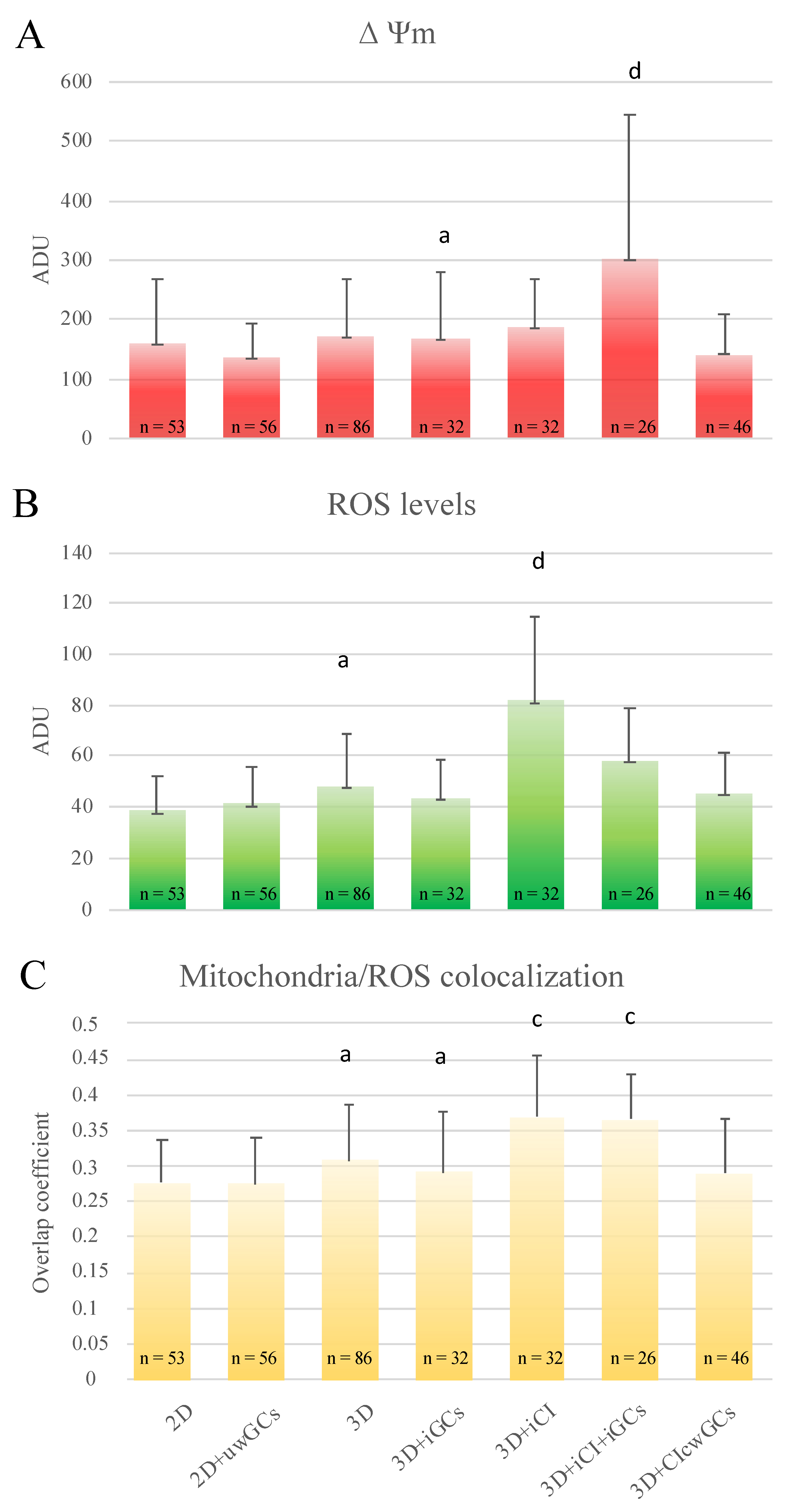
3.1. 3D-IVM Improved Prepubertal Oocyte Maturation Rate and Bioenergetic/Oxidative Status
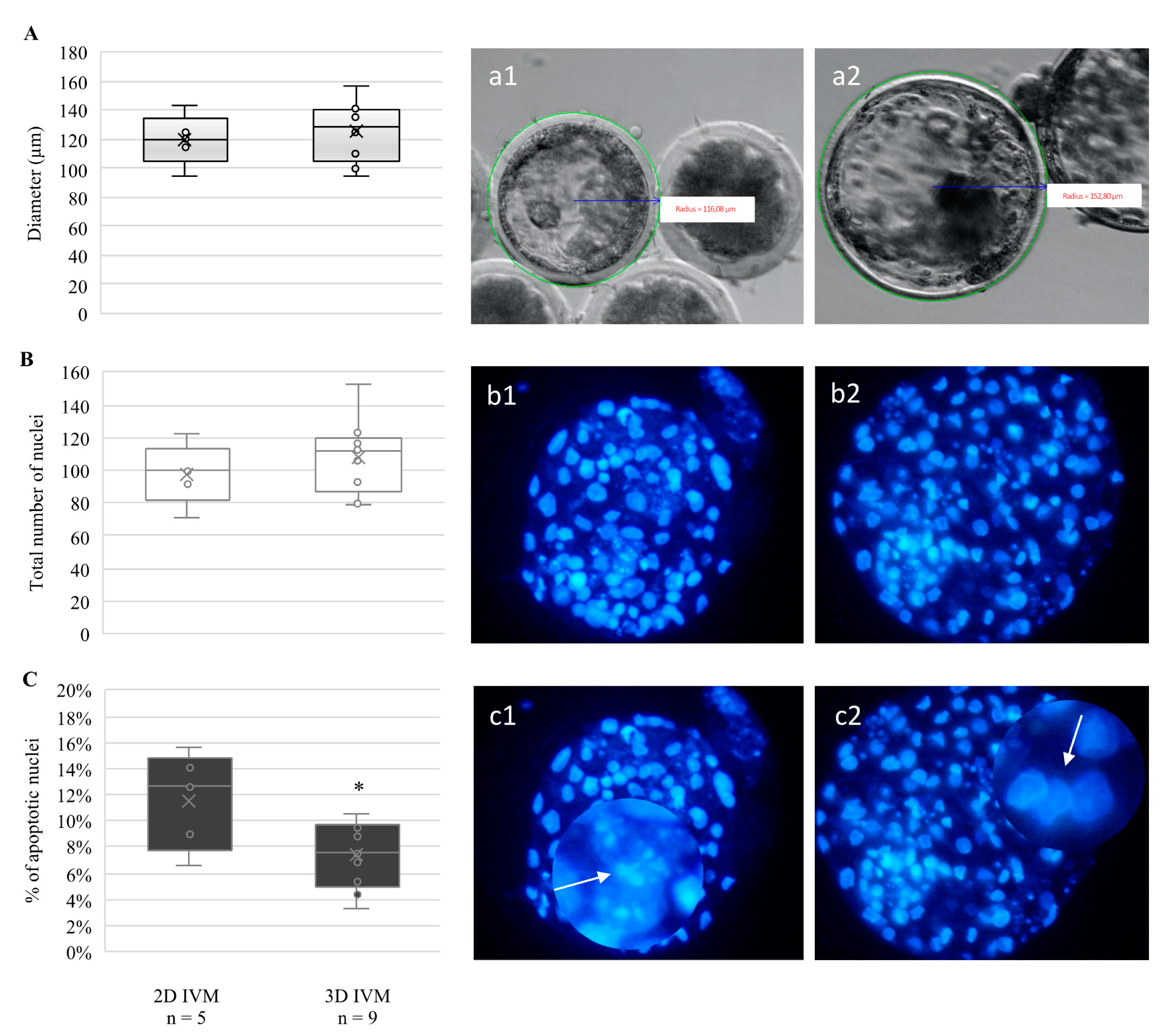
3.2. 3D-IVM Improved Prepubertal Embryo Cleavage and Blastocyst Quality
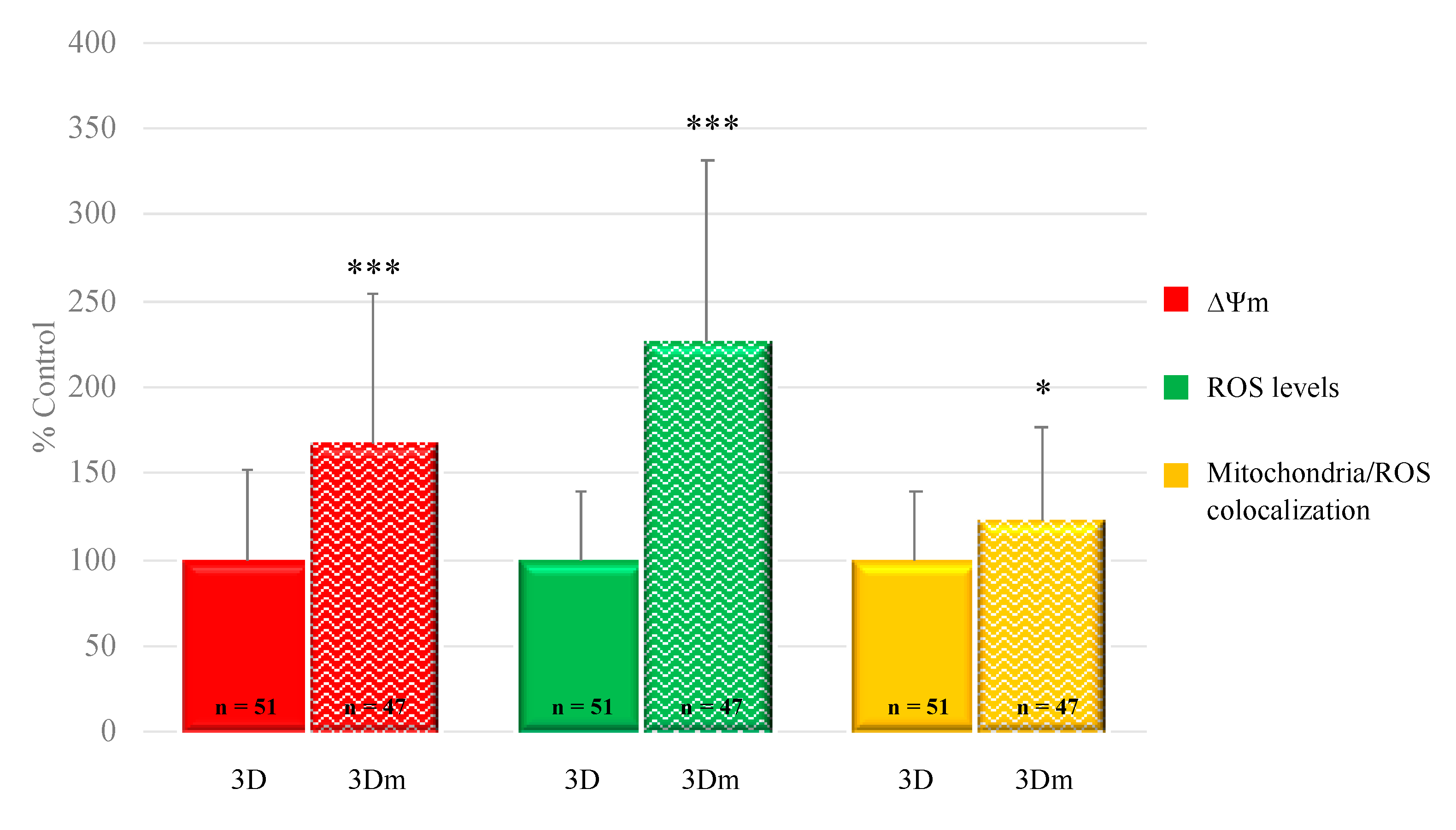
3.3. 3D Millifluidic IVM (3D-mIVM) Boosted Oocyte Bioenergetic/Oxidative Status
3.4. Shear Stress and Nutrient Supply Were Enhanced with Flow
3.5. Granulosa Cells and Type I Collagen Improved Oocyte Maturation and Bioenergetic/Oxidative Status
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, K.M.; Catt, S.L.; Chis Maxwell, W.M.; Evans, G. In vitro and in vivo developmental capabilities and kinetics of in vitro development of in vitro matured oocytes from adult, unstimulated and hormone-stimulated prepubertal ewes. Theriogenology 2005, 64, 1320–1332. [Google Scholar] [CrossRef]
- Morton, K.M. Developmental capabilities of embryos produced in vitro from prepubertal lamb oocytes. Reprod. Domest. Anim. 2008, 43, 137–143. [Google Scholar] [CrossRef]
- Paramio, M.T.; Izquierdo, D. Current status of in vitro embryo production in sheep and goats. Reprod. Domest. 2014, 49, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, D.; Catalá, M.G.; Paramio, M.T. Small ruminants: Prepubertal oocyte donors. Methods Mol. Biol. 2019, 2006, 155–163. [Google Scholar] [PubMed]
- Baldassarre, H. Laparoscopic ovum pick-up followed by in vitro embryo production and transfer in assisted breeding programs for ruminants. Animals 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rovira, L.; Succu, S.; Pasciu, V.; Manca, M.E.; Gonzalez-Bulnes, A.; Leoni, G.G.; Pennino, M.G.; Spezzigu, A.; Gallus, M.; Dattena, M.; et al. Postnatal pituitary and follicular activation: A revisited hypothesis in a sheep model. Reproduction 2016, 151, 215–225. [Google Scholar] [CrossRef]
- O’Brien, J.K.; Beck, N.F.G.; Maxwell, W.M.C.; Evans, G. Effect of hormone pre-treatment of prepubertal sheep on the production and developmental capacity of oocytes in vitro and in vivo. Reprod. Fertil. Dev. 1997, 9, 625–631. [Google Scholar] [CrossRef]
- Ledda, S.; Bogliolo, L.; Leoni, G.; Naitana, S. Production and lambing rate of blastocysts derived from in vitro matured oocytes after gonadotropin treatment of prepubertal ewes. J. Anim. Sci. 1999, 77, 2234–2239. [Google Scholar] [CrossRef]
- Kochhar, H.P.; Wu, B.; Morris, L.H.; Buckrell, B.C.; Pollard, J.W.; Basrur, P.K.; King, W.A. Maturation status, protein synthesis and developmental competence of oocytes derived from lambs and ewes. Reprod. Domest. Anim. 2002, 37, 19–25. [Google Scholar] [CrossRef]
- Ptak, G.; Loi, P.; Dattena, M.; Tischner, M.; Cappai, P. Offspring from one-month-old lambs: Studies on the developmental capability of prepubertal oocytes. Biol. Reprod. 1999, 61, 1568–1574. [Google Scholar] [CrossRef]
- Kelly, J.M.; Kleeman, D.O.; Walker, S.K. The effect of nutrition during pregnancy on the in vitro production of embryos from resulting lambs. Theriogenology 2005, 63, 2020–2031. [Google Scholar] [CrossRef]
- Kelly, J.M.; Kleeman, D.O.; Walker, S.K. Enhanced efficiency in the production of offspring from 4- to 8-weekold lambs. Theriogenology 2005, 63, 1876–1890. [Google Scholar] [CrossRef]
- Leoni, G.G.; Palmerini, M.G.; Satta, V.; Succu, S.; Pasciu, V.; Zinellu, A.; Carru, C.; Macchiarelli, G.; Nottola, S.A.; Naitana, S.; et al. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS ONE 2015, 10, e0124911. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.Z.; Cao, X.Y.; Cui, W.; Lian, H.Y.; Miao, Y.L.; Wu, X.F.; Han, D.; Tan, J.H. Developmental potential of prepubertal mouse oocytes is compromised due mainly to heir impaired synthesis of glutathione. PLoS ONE 2013, 8, e58018. [Google Scholar] [CrossRef]
- Leoni, G.G.; Succu, S.; Satta, V.; Paolo, M.; Bogliolo, L.; Bebbere, D.; Spezzigu, A.; Madeddu, M.; Berlinguer, F.; Ledda, S.; et al. In vitro production and cryotolerance of prepubertal and adult goat blastocysts obtained from oocytes collected by laparoscopic oocyte-pick-up (LOPU) after FSH treatment. Reprod. Fertil. Dev. 2009, 21, 901–908. [Google Scholar] [CrossRef]
- Marchal, R.; Feugang, J.M.; Perreau, C.; Venturi, E.; Terqui, M.; Mermillod, P. Meiotic and developmental competence of prepubertal and adult swine oocytes. Theriogenology 2001, 56, 17–29. [Google Scholar] [CrossRef]
- Palma, G.A.; Clement-Sengewald, A.; Krefft, H. In vitro production of cattle embryos from calf oocytes. Theriogenology 1993, 39, 278. [Google Scholar] [CrossRef]
- Masala, L.; Ariu, F.; Bogliolo, L.; Bellu, E.; Ledda, S.; Bebbere, D. Delay in maternal transcript degradation in ovine embryos derived from low competence oocytes. Mol. Reprod. Dev. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Bebbere, D.; Ariu, F.; Bogliolo, L.; Masala, L.; Murrone, O.; Fattorini, M.; Falchi, L.; Ledda, S. Expression of maternally derived KHDC3, NLRP5, OOEP and TLE6 is associated with oocyte developmental competence in the ovine species. BMC Dev. Biol. 2014, 14, 40. [Google Scholar] [CrossRef]
- Romar, R.; De Santis, T.; Papillier, P.; Perreau, C.; Thélie, A.; Dell’Aquila, M.E.; Mermillod, P.; Dalbiès-Tran, R. Expression of maternal transcripts during bovine oocyte in vitro maturation is affected by donor age. Reprod. Domest. Anim. 2011, 46, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.G.; Bebbere, D.; Succu, S.; Berlinguer, F.; Mossa, F.; Galioto, M.; Bogliolo, L.; Ledda, S.; Naitana, S. Relations between relative mRNA abundance and developmental competence of ovine oocytes. Mol. Reprod. Dev. 2007, 74, 249–257. [Google Scholar] [CrossRef]
- Anguita, B.; Jimenez-Macedo, A.; Izquierdo, D.; Mogas, T.; Paramio, M. Effect of oocyte diameter on meiotic competence, embryo development, p34 (cdc2) expression and MPF activity in prepubertal goat oocytes. Theriogenology 2007, 67, 526–536. [Google Scholar] [CrossRef]
- Masala, L.; Burrai, G.P.; Bellu, E.; Ariu, F.; Bogliolo, L.; Ledda, S.; Bebbere, D. Methylation dynamics during folliculogenesis and early embryo development in sheep. Reproduction 2017, 153, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Ptak, G.; Matsukawa, K.; Palmieri, C.; Salda, L.D.; Scapolo, P.A.; Loi, P. Developmental and functional evidence of nuclear immaturity in prepubertal oocytes. Hum. Reprod. 2006, 21, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Velilla, E.; Izquierdo, D.; Rodríguez-González, E.; López-Béjar, M.; Vidal, F.; Paramio, M.T. Distribution of prepubertal and adult goat oocyte cortical granules during meiotic maturation and fertilisation: Ultrastructural and cytochemical study. Mol. Reprod. Dev. 2004, 68, 507–514. [Google Scholar] [CrossRef]
- Velilla, E.; Rodriguez-Gonzalez, E.; Vidal, F.; Izquierdo, D.; Paramio, M.T. Mitochondrial organization in prepubertal goat oocytes during in vitro maturation and fertilization. Mol. Reprod. Dev. 2006, 73, 617–626. [Google Scholar] [CrossRef]
- Reader, K.L.; Cox, N.R.; Stanton, J.L.; Juengel, J.L. Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM. Reprod. Fertil. Dev. 2015, 27, 513–522. [Google Scholar] [CrossRef]
- Velilla, E.; Rodriguez-Gonzalez, E.; Vidal, F.; Paramio, M.T. Microtubule and microfilament organization in immature, in vitro matured and in vitro fertilized prepubertal goat oocytes. Zygote 2005, 13, 155–165. [Google Scholar] [CrossRef]
- Serra, E.; Succu, S.; Berlinguer, F.; Porcu, C.; Leoni, G.G.; Naitana, S.; Gadau, S.D. Tubulin posttranslational modifications in in vitro matured prepubertal and adult ovine oocytes. Theriogenology 2018, 114, 237–243. [Google Scholar] [CrossRef]
- Dorji; Ohkubo, Y.; Miyoshi, K.; Yoshida, M. Gene expression differences in oocytes derived from adult and prepubertal Japanese black cattle during in vitro maturation. Reprod. Domest. Anim. 2011, 47, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, M.G.; Nottola, S.A.; Leoni, G.G.; Succu, S.; Borshi, X.; Berlinguer, F.; Naitana, S.; Bekmukhambetov, Y.; Macchiarelli, G. In vitro maturation is slowed in prepubertal lamb oocytes: Ultrastructural evidences. Reprod. Biol. Endocrinol. 2014, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Qi, Q.; Yan, F.; Wang, C.; Hou, F.; Ren, W.; Zhang, L.; Hou, J. Enhancing the developmental competence of prepubertal lamb oocytes by supplementing the in vitro maturation medium with sericin and the fibroblast growth factor 2—Leukemia inhibitory factor—Insulin-like growth factor 1 combination. Theriogenology 2021, 159, 13–19. [Google Scholar] [CrossRef]
- Tian, H.; Liu, K.; Zhang, Y.; Qi, Q.; Wang, C.; Guan, H.; Yan, F.; Hou, J. Adult follicular fluid supplementation during in vitro maturation improves the developmental competence of prepubertal lamb oocytes. Theriogenology 2019, 130, 157–162. [Google Scholar] [CrossRef] [PubMed]
- McGrice, H.; Kelly, J.M.; Kleemann, D.O.; Kind, K.L.; Hampton, A.J.; Hannemann, P.; Walker, S.K.; van Wettere, W.H.E.J. Plasma anti-Müllerian hormone concentration as a predictive endocrine marker for selection of donor lambs to improve success in juvenile in vitro embryo transfer programs. Reprod. Fertil Dev. 2020, 32, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Mastrorocco, A.; Cacopardo, L.; Martino, N.A.; Fanelli, D.; Camillo, F.; Ciani, E.; Roelen, B.A.J.; Ahluwalia, A.; Dell’Aquila, M.E. One-step automated bioprinting-based method for cumulus-oocyte complex microencapsulation for 3D in vitro maturation. PLoS ONE 2020, 15, e0238812. [Google Scholar] [CrossRef]
- Martino, N.A.; Marzano, G.; Mangiacotti, M.; Miedico, O.; Sardanelli, A.M.; Gnoni, A.; Lacalandra, G.M.; Chiaravalle, A.E.; Ciani, E.; Bogliolo, L.; et al. Exposure to cadmium during in vitro maturation at environmental nanomolar levels impairs oocyte fertilization through oxidative damage: A large animal model study. Reprod. Toxicol. 2017, 69, 132–145. [Google Scholar] [CrossRef]
- de Oliveira Santos, M.V.; de Queiroz Neta, L.B.; Azevedo Borges, A.; Fernandez Pereira, A. Influence of commercially available follicle stimulating hormone on the in vitro maturation of bovine oocytes. In Semina: Ciencias Agrarias; Garcia, J.L., Lopera Barrero, N.M., Eds.; Universidade Estadual de Londrina: Londrina, Brazil, 2017; Volume 38, n. 3; pp. 1393–1402. [Google Scholar]
- Tirella, A.; Magliaro, C.; Penta, M.; Troncone, M.; Pimentel, R.; Ahluwalia, A. Sphyga: A multiparameter open source tool for fabricating smart and tunable hydrogel microbeads. Biofabrication 2014, 6, 025009. [Google Scholar] [CrossRef]
- Tervit, H.R.; Whittingham, D.G.; Rowson, L.E.A. Successful culture in vitro of sheep and cattle ova. Reproduction 1972, 30, 493–497. [Google Scholar] [CrossRef]
- Martino, N.A.; Ariu, F.; Bebbere, D.; Uranio, M.F.; Chirico, A.; Marzano, G.; Sardanelli, A.M.; Cardinali, A.; Minervini, F.; Bogliolo, L.; et al. Supplementation with nanomolar concentrations of verbascoside during in vitro maturation improves embryo development by protecting the oocyte against oxidative stress: A large animal model study. Reprod. Toxicol. 2016, 65, 204–211. [Google Scholar] [CrossRef]
- Walker, S.K.; Hill, J.L.; Kleemann, D.O.; Nancarrow, C.D. Development of ovine embryos in synthetic oviductal fluid containing amino acids at oviductal fluid concentrations. Biol. Reprod. 1996, 55, 703–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berger, E.; Magliaro, C.; Paczia, N.; Monzel, A.S.; Antony, P.; Linster, C.L.; Bolognin, S.; Ahluwalia, A.; Schwamborn, J.C. Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip 2018, 18, 3172. [Google Scholar] [CrossRef] [PubMed]
- Mattei, G.; Magliaro, C.; Giusti, S.; Ramachandran, S.D.; Heinz, S.; Braspenning, J.; Ahluwalia, A. On the adhesion-cohesion balance and oxygen consumption characteristics of liver organoids. PLoS ONE 2017, 12, e0173206. [Google Scholar] [CrossRef]
- Mehmetaglu, U.; Ates, S.; Berber, S. Oxygen diffusivity in calcium alginate gel beads containing Gluconobacter suboxydans. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996, 24, 91–106. [Google Scholar] [CrossRef]
- Muller, B.; Lewis, N.; Adeniyi, T.; Leese, H.J.; Brison, D.R.; Sturmey, R.G. Application of extracellular flux analysis for determining mitochondrial function in mammalian oocytes and early embryos. Sci. Rep. 2019, 9, 16778. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.G.; Rosati, I.; Succu, S.; Bogliolo, L.; Bebbere, D.; Berlinguer, F.; Ledda, S.; Naitana, S. A low oxygen atmosphere during IVF accelerates the kinetic of formation of in vitro produced ovine blastocysts. Reprod. Domest. Anim. 2007, 42, 299–304. [Google Scholar] [CrossRef]
- Kovacic, B.; Vlaisavljević, V. Influence of atmospheric versus reduced oxygen concentration on development of human blastocysts in vitro: A prospective study on sibling oocytes. Reprod. Biomed. Online 2008, 17, 229–236. [Google Scholar] [CrossRef]
- Van Montfoort, A.P.A.; Arts, E.G.J.M.; Wijnandts, L.; Sluijmer, A.; Pelinck, M.J.; Land, J.A.; Van Echten-Arends, J. Reduced oxygen concentration during human IVF culture improves embryo utilization and cumulative pregnancy rates per cycle. Hum. Reprod. Open 2020, 2020, hoz036. [Google Scholar] [CrossRef] [PubMed]
- Stokes, Y.M. Quantifying oxygen diffusion in paraffin oil used in oocyte and embryo culture. Mol. Reprod. Dev. 2009, 76, 1178–1187. [Google Scholar] [CrossRef]
- Faustini, M.; Curone, G.; Torre, M.L.; Vigo, D. Bioencapsulation of oocytes and granulosa cells. Methods Mol. Biol. 2018, 1817, 89–93. [Google Scholar]
- Mastrorocco, A.; Martino, N.A.; Marzano, G.; Lacalandra, G.M.; Ciani, E.; Roelen, B.A.J.; Dell’Aquila, M.E.; Minervini, F. The mycotoxin beauvericin induces oocyte mitochondrial dysfunction and affects embryo development in the juvenile sheep. Mol. Reprod. Dev. 2019, 86, 1430–1443. [Google Scholar] [CrossRef] [PubMed]
- Somoskoi, B.; Martino, N.A.; Cardone, R.A.; Lacalandra, G.M.; Dell’Aquila, M.E.; Cseh, S. Different chromatin and energy/redox responses of mouse morulae and blastocysts to slow freezing and vitrification. Reprod. Biol. Endocrinol. 2015, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Hwang, K.J.; Kwon, H.C.; Kim, H.S.; Choi, K.W.; Oh, K.S. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum. Reprod. 1998, 13, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Ariu, F.; Bogliolo, L.; Leoni, G.; Falchi, L.; Bebbere, D.; Nieddu, S.M.; Zedda, M.T.; Pau, S.; Ledda, S. Effects of caffeine treatment before vitrification on MPF and MAPK activity and spontaneous parthenogenetic activation of in vitro matured ovine oocytes. Cryo. Lett. 2014, 35, 530–536. [Google Scholar]
- Martino, N.A.; Dell’Aquila, M.E.; Cardone, R.A.; Somoskoi, B.; Lacalandra, G.M.; Cseh, S. Vitrification preserves chromatin integrity, bioenergy potential and oxidative parameters in mouse embryos. Reprod. Biol. Endocrinol. 2013, 11, 27. [Google Scholar] [CrossRef]
- Dell’Aquila, M.E.; Bogliolo, L.; Russo, R.; Martino, N.A.; Filioli Uranio, M.; Ariu, F.; Amati, F.; Sardanelli, A.M.; Linsalata, V.; Ferruzzi, M.G.; et al. Prooxidant effects of verbascoside, a bioactive compound from olive oil mill wastewater, on in vitro developmental potential of ovine prepubertal oocytes and bioenergetic/oxidative stress parameters of fresh and vitrified oocytes. Biomed. Res. Int. 2014, 2014, 878062. [Google Scholar]
- Dorati, R.; Genta, I.; Ferrari, M.; Vigone, G.; Merico, V.; Garagna, S.; Zuccotti, M.; Conti, B. Formulation and stability evaluation of 3D alginate beads potentially useful for cumulus-oocyte complexes culture. J. Microencapsul. 2016, 33, 137–145. [Google Scholar] [CrossRef]
- Morselli, M.G.; Canziani, S.; Vigo, D.; Luvoni, G.C. A three-dimensional alginate system for in vitro culture of cumulus-denuded feline oocytes. Reprod. Domest. Anim. 2017, 52, 83–88. [Google Scholar] [CrossRef]
- Morselli, M.G.; Luvoni, G.C.; Comizzoli, P. The nuclear and developmental competence of cumulus–oocyte complexes is enhanced by three-dimensional coculture with conspecific denuded oocytes during in vitro maturation in the domestic cat model. Reprod. Domest. Anim. 2017, 2, 82–87. [Google Scholar] [CrossRef]
- Morselli, M.G.; Loiacono, M.; Colombo, M.; Mortarino, M.; Luvoni, G.C. Nuclear competence and genetic expression of growth differentiation factor-9 (GDF-9) of canine oocytes in 3D culture. Reprod. Domest. Anim. 2018, 3, 117–124. [Google Scholar] [CrossRef]
- Colombo, M.; Morselli, M.G.; Tavares, M.R.; Apparicio, M.; Luvoni, G.C. Developmental competence of domestic cat vitrified oocytes in 3D enriched culture conditions. Animals 2019, 9, 329. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obs. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kreeger, P.K.; Shea, L.D.; Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006, 12, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, D.; Giusti, S.; Sbrana, T.; Ahluwalia, A. A multi-compartmental modular bioreactor as innovative system for dynamic cell cultures and co-cultures. In Bioreactors: Design, Properties and Applications; Antolli, P.G., Liu, Z., Eds.; Nova Science Inc.: Hauppauge, NY, USA, 2012; pp. 159–178. [Google Scholar]
- Giusti, S.; Mazzei, D.; Cacopardo, L.; Mattei, G.; Domenici, C.; Ahluwalia, A. Environmental control in flow bioreactors. Processes 2017, 5, 16. [Google Scholar] [CrossRef]
- Colombo, R.; Paolillo, M.; Papetti, A. A new millifluidic-based gastrointestinal platform to evaluate the effect of simulated dietary methylglyoxal intakes. Food Funct. 2019, 10, 4330–4338. [Google Scholar] [CrossRef] [PubMed]
- Ledda, S.; Idda, A.; Ariu, F.; Bogliolo, L.; Bebbere, D. A novel technique for in vitro maturation of sheep oocytes in a liquid marble microbioreactor. J. Assist. Reprod. Genet. 2016, 33, 513–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carnegie, J.A.; Byard, R.; Dardick, I.; Tsang, B.K. Culture of granulosa cells in collagen gels: The influence of cell shape on steroidogenesis. Biol. Reprod. 1988, 38, 881–890. [Google Scholar] [CrossRef]
- Kreeger, P.K.; Woodruff, T.K.; Shea, L.D. Murine granulosa cell morphology and function are regulated by a synthetic Arg–Gly–Asp matrix. Mol. Cell Endocrinol. 2003, 205, 1–10. [Google Scholar] [CrossRef]
- Sites, C.K.; Kessel, B.; LaBarbera, A.R. Adhesion proteins increase cellular attachment, follicle-stimulating hormone receptors, and progesterone production in cultured porcine granulosa cells. Exp. Biol. Med. 1996, 212, 78–83. [Google Scholar] [CrossRef]
- Vigo, D.; Villani, S.; Faustini, M.; Accorsi, P.A.; Galeati, G.; Spinaci, M.; Munari, E.; Russo, V.; Asti, A.; Conte, U.; et al. Follicle-like model by granulosa cell encapsulation in a barium alginate–protamine membrane. Tissue Eng. 2005, 11, 709–714. [Google Scholar] [CrossRef]
- Gutiérrez, C.G.; Glazyrin, A.L.; Robertson, G.W.; Campbell, B.K.; Gong, J.G.; Bramley, T.A.; Webb, R. Ultra-structural characteristics of bovine granulosa cells associated with maintenance of oestradiol production in vitro. Mol. Cell Endocrinol. 1997, 134, 51–58. [Google Scholar] [CrossRef]
- Bussenot, I.; Ferre, G.; Azoulay-Barjonet, C.; Murgo, C.; Vieitez, G.; Parinaud, J. Culture of human preovulatory granulosa cells: Effect of extracellular matrix on steroidogenesis. Biol. Cell 1993, 77, 181–186. [Google Scholar] [CrossRef]
- Huet, C.; Pisselet, C.; Mandon-Pépin, B.; Monget, P.; Monniaux, D. Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: Relationships between cell shape and function. J. Endocrinol. 2001, 169, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Berkholtz, C.B.; Shea, L.D.; Woodruff, T.K. Extracellular matrix functions in follicle maturation. Semin. Reprod. Med. 2006, 24, 262–269. [Google Scholar] [CrossRef]
- Liu, W.; Xin, Q.; Wang, X.; Wang, S.; Wang, H.; Zhang, W.; Yang, Y.; Zhang, Y.; Zhang, Z.; Wang, C.; et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis. 2017, 8, e2662. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Powell, C.; Ahmed, S.M.; Alsberg, E. Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng. Part A 2010, 16, 2915–2925. [Google Scholar] [CrossRef]


| Culture Condition | No. of Cultured COCs | No. of Analyzed COCs | Nuclear Chromatin Configurations Number (%) | P/S Mitochondrial Pattern (*) | |||
|---|---|---|---|---|---|---|---|
| GV | MI to TI | MII | Abnormal | ||||
| 2D | 172 | 163 | 30 (18.4) | 32 (19.6) | 74 (45.4) a | 27 (16.6) a | 26/46 (57) a |
| 3D | 181 | 175 | 30 (17.1) | 24 (13.7) | 106 (60.6) c | 15 (8.6) b | 58/83 (70) b |
| Culture Condition | No. of Inseminated Oocytes | No. of Zygotes Evaluated after IVF | Embryo Developmental Stages Number (%) | Total Cleavage Number (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2- to 3- Cell | 4- to 7- Cell | 8- to 15- Cell | 16- to 31- Cell | Morula (>32 Cells) | Blastocyst (>64 Cells) | ||||
| 2D | 163 | 157 | 4 (2.5) a | 22 (14.0) | 37 (23.6) | 19 (12.1) | 4 (2.5) | 5 (3.2) | 91 (57.9) a |
| 3D | 175 | 172 | 25 (14.5) c | 34 (19.8) | 30 (17.5) | 16 (9.3) | 4 (2.3) | 9 (5.2) | 118 (68.6) b |
| Culture Condition | No. of Cultured COCs | No. of Analyzed COCs | Nuclear Chromatin Configurations Number (%) | P/S Mitochondrial Pattern (*) | |||
|---|---|---|---|---|---|---|---|
| GV | MI to TI | MII | Abnormal | ||||
| 3D | 180 | 171 | 35 (20.5) | 23 (13.5) | 99 (57.9) | 14 (8.2) | 26/51 (51) |
| 3D Millifluidic | 177 | 170 | 50 (29.4) | 15 (8.8) | 91 (53.5) | 14 (8.2) | 32/47 (68.1) |
| IVM Method | GC Culture Condition | Collagen I | No. of Cultured COCs (*) | No. of Analyzed COCs | CC Apoptotic Index (%) | Nuclear Chromatin Configurations Number (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GV | MI to TI | MII | Activated | Abnormal | P/S Mitochondrial Pattern | ||||||
| 2D | - | - | 178 (7) | 123 | 24 a | 19 (15) a | 10 (8) | 61 (50) a | 3 (2) | 30 (25) c | 16/53 (30) a |
| Monolayer on uncoated wells | - | 182 (7) | 139 | 37 b | 36 (26) b | 10 (7) | 62 (45) | 3 (2) | 28 (20) | 13/56 (24) | |
| 3D | - | - | 166 (7) | 148 | 24 a | 29 (20) x | 15 (10) x | 93 (63) b,c | 2 (1) | 9 (6) c,d | 44/86 (51) b |
| Included in microbeads | - | 136 (8) | 126 | 37 b | 45 (36) y | 3 (2) y | 32 (25) d,x | 4 (3) | 42 (33) x,d | 12/32 (38) a | |
| - | In microbeads | 59 (3) | 59 | 43 b, e | 16 (27) | 0 (0) | 32 (54) | 4 (7) | 7 (12) | 18/32 (56) | |
| Included in microbeads | In microbeads | 46 (3) | 45 | 29 f | 15 (33) | 0 (0) | 29 (64) y | 0 (0) | 1 (2) y | 18/26 (69) b | |
| Monolayer on CI-coated wells | As plate coating | 70 (3) | 69 | U.D. | 17 (25) | 0 (0) | 46 (66) y | 2 (3) | 4 (6) y | 19/46 (41) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrorocco, A.; Cacopardo, L.; Lamanna, D.; Temerario, L.; Brunetti, G.; Carluccio, A.; Robbe, D.; Dell’Aquila, M.E. Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes. Cells 2021, 10, 1458. https://doi.org/10.3390/cells10061458
Mastrorocco A, Cacopardo L, Lamanna D, Temerario L, Brunetti G, Carluccio A, Robbe D, Dell’Aquila ME. Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes. Cells. 2021; 10(6):1458. https://doi.org/10.3390/cells10061458
Chicago/Turabian StyleMastrorocco, Antonella, Ludovica Cacopardo, Daniela Lamanna, Letizia Temerario, Giacomina Brunetti, Augusto Carluccio, Domenico Robbe, and Maria Elena Dell’Aquila. 2021. "Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes" Cells 10, no. 6: 1458. https://doi.org/10.3390/cells10061458
APA StyleMastrorocco, A., Cacopardo, L., Lamanna, D., Temerario, L., Brunetti, G., Carluccio, A., Robbe, D., & Dell’Aquila, M. E. (2021). Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes. Cells, 10(6), 1458. https://doi.org/10.3390/cells10061458








