Abstract
Pulmonary fibroelastotic remodelling occurs within a broad spectrum of diseases with vastly divergent outcomes. So far, no comprehensive terminology has been established to adequately address and distinguish histomorphological and clinical entities. We aimed to describe the range of fibroelastotic changes and define stringent histological criteria. Furthermore, we wanted to clarify the corresponding terminology in order to distinguish clinically relevant variants of pulmonary fibroelastotic remodelling. We revisited pulmonary specimens with fibroelastotic remodelling sampled during the last ten years at a large European lung transplant centre. Consensus-based definitions of specific variants of fibroelastotic changes were developed on the basis of well-defined cases and applied. Systematic evaluation was performed in a steps-wise algorithm, first identifying the fulcrum of the respective lesions, and then assessing the morphological changes, their distribution and the features of the adjacent parenchyma. We defined typical alveolar fibro-elastosis as collagenous effacement of the alveolar spaces with accompanying hyper-elastosis of the remodelled and paucicellular alveolar walls, independent of the underlying disease in 45 cases. Clinically, this pattern could be seen in (idiopathic) pleuroparenchymal fibro-elastosis, interstitial lung disease with concomitant alveolar fibro-elastosis, following hematopoietic stem cell and lung transplantation, autoimmune disease, radio-/chemotherapy, and pulmonary apical caps. Novel in-transit and activity stages of fibroelastotic remodelling were identified. For the first time, we present a comprehensive definition of fibroelastotic remodelling, its anatomic distribution, and clinical associations, thereby providing a basis for stringent patient stratification and prediction of outcome.
1. Introduction
Fibroelastotic remodelling (FER) is a common morphological injury pattern occurring in a number of different clinical settings [1,2,3]. Within the broad scope of FER, distinct patterns with slightly diverging histopathological features and clinical phenotypes are recognized. A rare entity of interstitial lung disease (ILD) with a predominant pattern of FER, Pleuroparenchymal fibro-elastosis (PPFE), was first implemented by Frankel et al. in 2004 [4] and was officially recognized by the American Thoracic Society in 2013 [5]. PPFE often occurs idiopathically without a known trigger (iPPFE), but there are also cases of secondary PPFE linked to autoimmune disorders (AID). Moreover, PPFE-like patterns of FER have also been observed following radio- and/or chemotherapy [6] as well as after lung (LTX) and hematopoietic stem cell transplantation (HSCT) [7,8,9,10,11]. All these manifestations share a rather poor prognosis and similar histological features—a fibrous obliteration of the alveolar airspaces associated with preservation and hyper-elastosis of the embedded alveolar septa [6,12]. This histologic pattern of remodelling has been addressed by a variety of terms, including airway-cantered fibro-elastosis [13], intra-alveolar fibrosis with elastosis [14] or (intra)alveolar fibro-elastosis (IAFE, AFE) [15,16]. Moreover, it has also been recognized that the so-called “pulmonary apical caps” (PAC) of the upper lobes share strikingly similar histologic features with PPFE, but usually remain asymptomatic and are often discovered incidentally in resection or autopsy specimens [17]. A comprehensive review of the clinical manifestations of FER and the associated clinical settings is given by Chua et al. (2019) [18] which also attempts to separate clinical and pathological terminology; however, no clearly applicable minimum requirements for the histopathological diagnosis of AFE have been defined thus far. This lack of clear separation and of clinical features and nomenclature on the one hand and morphological terminology, on the other hand hampers the scientific and clinical dialogue. For instance, the clinical term PPFE is still used widely in current studies to describe the histologic pattern of FER [3,19]. In this study, we have reviewed cases with well-defined AFE pattern from the archive of Europe’s largest lung-transplant centre and systematically analysed the histological features and distribution of the FER in order to i. define stringent histological criteria, ii. clarify the corresponding terminology and iii. to distinguish relevant variants of FER.
2. Materials and Methods
We identified pulmonary specimens with FER in the archives of the Institute of Pathology at Hannover Medical School, sampled within the last ten years. To avoid conflicting terms we used stringent definitions for clinical and histological nomenclature (see Table 1).

Table 1.
Terminology on fibroelastotic remodelling.
For histologic evaluation, sections with approximately 1 µm thickness were cut from the formalin-fixed and paraffin-embedded archival tissue blocks and stained with haematoxylin and eosin (HE), periodic acid-Schiff (PAS) and elastic van Gieson (EvG) stains.
To define a basis for systematic evaluation, 14 PPFE and PPFE-like as well as 12 PAC cases were systematically reviewed to develop a reference catalogue of morphological features. Subsequently, all included cases included were systematically evaluated and the observed morphological features methodically catalogued.
We defined typical AFE as i. collagenous effacement of the alveolar spaces with ii. accompanying hyperelastosis of the remodelled and paucicellular alveolar walls characterized by fourfold thickening of the elastic layer when compared with normal alveoli and iii. at least 4 connected alveoli showing these changes.
The systematic evaluation was performed by two trained pulmonary pathologists (P.B. and C.W. or I.M.) in four steps on a dual-observer transmitted light microscope (Olympus BX43) equipped with 2×–40× objective lenses. First, areas of typical AFE were identified, their size (in n alveoli) estimated and the morphological features within the typical AFE regions assessed. In steps two and three, morphological features directly adjacent to the typical AFE and their respective distribution in the anatomical compartments of the lung were evaluated. Finally, the cases were re-evaluated for pathological changes in the lung but not in direct spatial association with the AFE. The consensus of both observers was recorded for future analysis. In case of disagreement cases were discussed with two consulting pulmonary pathologists (D.J. and F.L.). The study was in accordance with the regulation of the ethics committee of the Hannover Medical School (ethics vote no. 2050−2013).
The last available in vivo CT before resection was assessed by a single trained thoracic radiologist to assess if radiologic changes were completely conclusive with the histological changes, were partly conclusive or inconclusive.
3. Results
We evaluated a total of 45 cases. In detail, the PPFE collective consisted of 31 cases. Of these, 6 were iPPFE and 25 had PPFE-like pulmonary fibrosis due to other causes: with underlying AID (n = 2), after radio-/chemotherapy (n = 2), chronic allograft dysfunction (CLAD) after LTX (n = 12) and following HSCT (n = 7). Two cases had AFE pattern as a minor companion pattern in other ILD.
The PAC collective consisted of samples from a total of 14 patients; of these, 12 were incidental PAC (surgery due to unrelated indications) and 2 patients had undergone primary resection due to an unclear pulmonary apical mass.
The patients were between 9 and 61 years old (See Table 2). Detailed patient information and the diagnoses of our collective are shown in Supplementary Table S1.

Table 2.
Patient collective.
After a review of representative AFE cases, we could identify different patterns within typical AFE and a range of common changes in direct proximity to the AFE lesions (Table 3).

Table 3.
Histological patterns.
Within areas of typical AFE, the pattern of intra-alveolar fibrosis/fibrotic obliteration was classified as coarse fibrillary if broad hyalinised bundles of collagenous fibres (usually ~2 µm in diameter) were present and as fine fibrillary if delicate, mostly curled fibres were demonstrable. The presence of anthracophages and lymphoid aggregates was noted as well as an increase in cellularity with diffuse infiltration of lymphocytes or the presence of an increased number of mesenchymal cells such as (myo) fibroblasts in the obliterated alveolar lumen (see Figure 1).
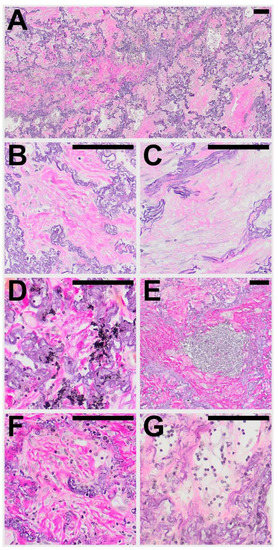
Figure 1.
Typical histological patterns of alveolar fibroelastosis (AFE). (A) Typical AFE is characterized by a complete obliteration of the alveolar lumen with collagenous material with formation of either coarse (B) or fine (C) fibrils. In some cases, aggregates of macrophages containing anthracotic pigment can be observed (D). Lymphoid aggregates are a common finding in or at the border of AFE lesions (E). Increased cellularity with presence of mesenchymal cells (F) or lymphocytes (G) can be observed in the fibrotic areas to a variable degree. All images are elastic van Gieson stainings. Scale bars are 100 µm each.
In the majority of cases (67%) both coarse and fine fibrillary fibrosis could be detected, in the other cases either only fine fibrillary or only coarse fibrillary fibrosis (20% and 13% respectively) were detectable. Features found regularly in areas of AFE were aggregates of lymphatic cells (73%), often at the leading edge of the remodelling process (see Table 4). These appear well circumscribed, organized in an organoid manner, sometimes contain specialized vessels with the appearance of highly endothelialised venules (HEV) and can be distinguished from a diffuse infiltration of the AFE lesion by lymphatic cells which can be observed in approximately 30−40% of cases. Macrophages containing phagocytosed anthracotic pigment can be detected in 53% of total cases and appear less frequently in patients of the HSCT group (14%). Typical fibroblastic foci (FF) could be detected in 11% of cases. Overall, the areas of AFE showed similar morphological characteristics in all investigated groups. PAC showed overall less cellular mesenchymal (0%) and lymphatic (7%) infiltration when compared to the PPFE and PPFE-like cases (45% and 45% respectively).

Table 4.
Histological characteristics of alveolar fibroelastosis, its surroundings and compartmental distribution.
We observed and catalogued several morphological patterns in direct spatial association with regions of typical AFE. Besides structurally intact lung parenchyma, various forms of FER with either fibro-elastic expansion of alveolar septa, incomplete alveolar fibrosis, or irregularly distributed collagenous and elastic fibers could be observed (See Figure 2 and Table 3 for a comprehensive list of catalogued features).
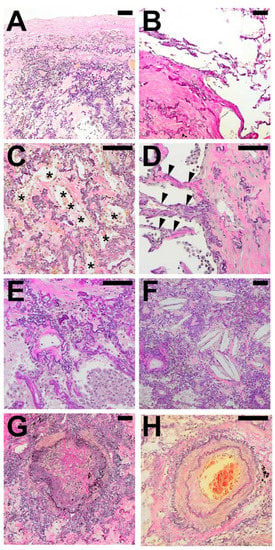
Figure 2.
Typical histological patterns in special association with alveolar fibroelastosis (AFE). A set of typical features regularly found in spatial association with AFE: (A) Pronounced fibrosis of the visceral pleura. (B) Emphysema with an irreversible loss of alveolar septa. (C) Elastosis of the alveolar wall with incomplete alveolar fibrosis of the alveolar lumen with residual airspaces lined by cuboidal epithelium (*). (D) Fibroelastic interstitial expansion of the alveolar septa adjacent to the AFE lesion. (E) Aggregates of intraalveolar macrophages. (F) Cholesterol granulomas with multinucleated giant cells with clefts of cholesterol crystals. (G) Bronchiolitis obliterans with fibrous obliteration of small airways and (H) sclerosis of pulmonary arteries with hypertrophy of the media and intimal hyperplasia. Images are elastic van Gieson stains. Scale bars are 100 µm each.
In the majority of cases (93%) fibroelastic expansion of alveolar septa could be observed at the border of typical AFE besides a direct and abrupt transition to structurally intact alveolar parenchyma (91%). In 84% of cases, areas of incomplete fibroelastosis could be detected. Frequently, pleural fibrosis could be observed adjacent to typical AFE (79%).
Patterns spatially associated with AFE were mostly similar in all cases examined, with the exception of obliterative airway remodelling (bronchiolitis obliterans; BO), which was observed in all cases of CLAD after LTX, and fibrotic pulmonary remodelling following HSCT. BO was also present in half of the PPFE cases but not present in APC.
The compartmental anatomical distribution of the delimitable changes was categorized as subpleural, parabronchial, para-arterial and paraseptal, when AFE was found in association with the respective anatomical structures. Areas of AFE in the parenchyma not associated with the anatomical structures lined out above were classified as “centrolobular” (see Figure 3).
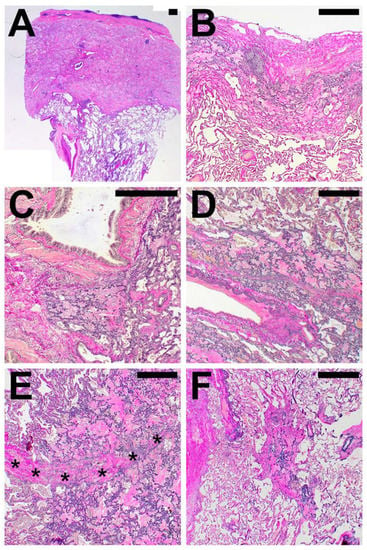
Figure 3.
Compartmental distribution of alveolar fibroelastosis (AFE). AFE is commonly found in the subpleural parenchyma (A,B), in parabronchial (C) and paravascular (D) distribution and along interlobular septa (*, E). When not in association with these structures, we classified the localization as centrolobular (F). Images are haematoxylin and eosin (A) and elastic van Gieson (B–F) stains. Scale bars are 500 µm each.
In PAC, the AFE pattern was always found in direct spatial association with the visceral pleura (in not-PAC cases in 93%) and only extended to other compartments in a minority of cases. In contrast to PAC, PPFE and PPFE-like disease showed AFE affection of the para-arterial (97%), paraseptal (85%) and parabronchial (74%) compartments.
Other typical histologic features of ILD such as architectural distortion, myogenic metaplasia or an NSIP pattern are rarely found in direct association with AFE lesions, even if present within the same lung.
Further radiological information was available in 38 (84%) patients. Of these 38 CT scans, 26 (68%) confirmed a main pattern of alveolar fibro-elastosis, in 10 (26%) patients this was a minor pattern and in 2 (5%) remaining patients, there was no radiologic evidence for AFE (see Figure 4 for representative images)
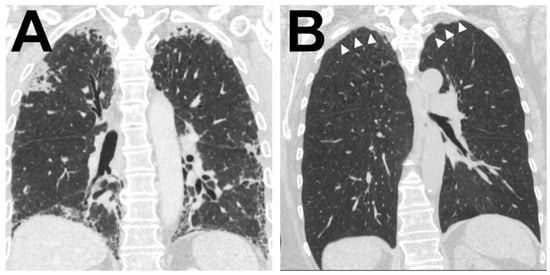
Figure 4.
Radiological correlate of AFE Illustration of the two typical radiological patterns accompanying a histologic AFE diagnosis. (A). Radiological PPFE pattern with typical (sub)pleural distribution of fibrosis. (B) Apical cap.
4. Discussion
FER is has been considered a rather unspecific process in a multitude of diseases for over a century, until Frankel defined FER as the morphological component of a specific form of ILD termed iPPFE. However, some of the patients investigated for their study had received chemotherapy [4] and in the following years we and others identified AFE pattern as sequelae of—amongst other injuries—radiotherapy, LTX and HSCT and also concomitant with other ILD.
In their initial study, Frankel and colleagues used a rather descriptive approach and classified histological features of PPFE without establishing formal criteria. Kusagaya et al. went on to develop criteria, which were then adopted and refined by Thüsen and colleagues in 2013. These not only include intra-alveolar fibrosis and septal elastosis but also comprise a subpleural distribution in the upper lobes with concomitant pleural fibrosis.Therefore, a clear separation of the clinical and histological entities in FER is still lacking and authors often utilize “PPFE” to describe clinical, radiological and histological presentations indiscriminately [3,19]. In addition, authors consistently point out that due to the striking differences in prognosis, PPFE and PAC have to be distinguished, even though i. the histologic patterns of both are very similar and ii. both affect the upper pulmonary compartment and iii. both differ only in some aspects of spatial distribution [16]. Depending on clinical context, manifestations of AFE areassociated with different clinical outcomes (see Figure 5).
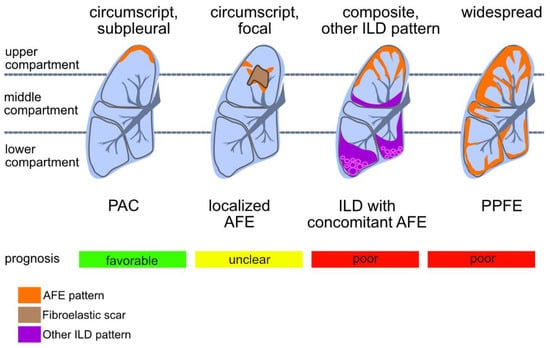
Figure 5.
Patterns of compartmental distribution of alveolar fibroelastosis (AFE): AFE is a pattern defined by the typical fibrous obliteration of the alveolar airspace with hyper-elastosis of the preserved alveolar structure. Similar histologic patterns can be observed in a variety of diseases which are distinguishable by a characteristic distribution of the AFE pattern. When AFE is found circumscript in the subpleural parenchyma of the upper lobe without any other indication of interstitial lung disease (ILD), a prognostic favourable pulmonal apical cap (PAC) is the most likely diagnosis. Further, circumscript focal AFE can be found e.g., after radiotherapy and around (unspecific) scars of the lung parenchyma. When found in association with other ILD patterns (e.g., usual interstitial pneumonia, UIP) the prognosis of the patient may be worse than when concomitant AFE is not found. AFE as dominant pattern with subpleural, parabronchial and paraseptal distribution and accentuation in the upper compartments of the lung is indicative of pleuroparenchymal fibroelastosis (PPFE), a rare ILD with poor prognosis.
PAC are regarded as typically benign lesions in contrast to PPFE with a mean survival time of approximately 24 months [18]. Survival in patients with ILD and concomitant PPFE varies considerably depending on the cohort reported either following the disease trajectory of the underlying ILD or of iPPFE [20,21].
To separate the clinical from the histological presentation, the term AFE was coined, describing the typical histological pattern of collagenous effacement of the alveolar spaces with accompanying hyperelastosis of the remodelled alveolar walls [15,16]. In our present study, we provide a systematic review of cases with AFE pattern histology to comprehensively document the features, distribution and pulmonary surroundings of AFE. To this end, we have employed a pattern-based approach, assessing AFE indiscriminately of its respective manifestation.
4.1. Features in AFE
The AFE-defining features of intra-alveolar fibrosis and septal elastosis were present in all patient groups. However, we noticed a difference in the cellular composition with an increase of lymphatic and mesenchymal cells embedded in the AFE of approximately half PPFE and PPFE-like cases, a feature we could not observe in PAC. This might point towards different states of activity within AFE lesions. Further studies are required to determine if cellularity can be used as prognostic marker. The low cellularity of AFE lesions in ILD cases with concomitant AFE is likely due to the low number of cases included in this study and typical lymphocytic inflammation could be observed in fibrotic (non-AFE) remodelled parenchyma (see Supplemental Figure S1).
Anthracophages, however, were frequently encountered in PAC, PPFE and PPFE-like cases, especially in older patients, pointing towards entrapment of otherwise innocent bystanders in the fibrotic process, unlike in other ILD, where exposure to small particles is known to be a causative agent.
The presence of FF in AFE has been pointed out in several studies. Frankel et al. reported them to be rare (4). Kusagaya et al. [22] described them as appearing in “small numbers” and Von der Thüsen et al. finally as “at most in small numbers” [23]. In our study, FF can be observed in 13% of cases. Increased detection of FF in other studies could be explained by a systematic bias of the respective authors in what they consider to qualify as FF. Unlike in UIP, groups of (myo) fibroblasts observed in AFE do not readily form classical FF with perpendicularly aligned (myo) fibroblasts, embedded in an immature, myxoid extracellular matrix and accompanied by hyperplastic type 2 pneumocytes. The presence of fully formed, typical FF should, therefore, prompt the pathologist to consider AFE concomitant to another ILD.
4.2. Features Surrounding AFE
Incomplete AFE is very common in close spatial proximity to AFE lesions and should possibly be interpreted as an equivalent to typical AFE in the context of the clinical setting. So far it is unclear whether incomplete AFE represents an incomplete transitional state or a premature consolidation. Emphysematous and even inconspicuous alveolar parenchyma can be often observed in direct proximity to AFE lesions, which typically expand with a “pushing border” aspect into the adjacent lung. Pleural fibrosis is common and can easily be recognized. However, it is present in only about 79% of cases and should not be considered as a mandatory criterion for establishing the diagnosis.
Pulmonary arterial sclerosis is common in AFE lesions or in their close proximity both in patients with PPFE, PPFE-like disease and PAC. Some authors have suggested pulmonary arteriolosclerosis and the resulting ventilation-perfusion disparity as a trigger of AFE-type fibrosis [24].
BO is commonly found in close proximity to AFE lesions in CLAD and following HSCT, where it has long been recognized as a defining feature of the disease. In the context of PPFE-like disease, our recent study on fibrotic airway remodelling points towards shared pathways in BO and AFE development [15].
AFOP and intraalveolar macrophage aggregates can be observed in some cases. These features have been proposed to represent a transitory step in the formation of AFE [15]. The rather infrequent detection in our cohort may be explained by a temporal bias with the majority of cases being end-stage lung disease, in which AFE lesions have already consolidated. This is in agreement with a report by Von der Thüsen et al. which described AFOP in 38% of their explanted lungs following redo transplantation [23].
4.3. Compartmental Distribution of AFE
In our study, AFE pattern fibrosis was most commonly found in the subpleural compartment, compatible with current literature [14]. However, when excluding PAC from the analysis, AFE pattern fibrosis is also found para-arterial and para-septal in 80% of patients and para-bronchial in two-thirds of cases. This indicates that these compartments are also commonly affected in PPFE (and PPFE-like) ILD, which has a significant impact on patient survival when compared to PAC. These findings are relevant as they indicate that AFE found in other than subpleural compartments should not preclude the diagnosis of PPFE or PPFE-like disease. Moreover, the subpleural parenchyma can only be accessed by open, but not by conventional transbronchial lung biopsy. However, relevant AFE can be detected in a sufficiently large transbronchial cryobiopsy specimen [13] because fibroelastic changes extend along bronchovascular bundles and interlobular septae. Nonetheless, data regarding the sensitivity of transbronchial biopsies for diagnosing AFE is currently not available for larger patient collectives.
5. Conclusions
i. Cardinal features of AFE are collagenous effacement of the alveolar spaces with accompanying hyperelastosis of remodelled alveolar walls and ii. pleural fibrosis does not represent a condition sine qua non for the diagnosis. iii. Incomplete AFE has to be considered an equivalent lesion to typical AFE, provided an appropriate clinical setting. iv. FF are not a typical feature of AFE and should raise suspicion of a concomitant lung injury pattern, such as UIP. AFE is commonly distributed along the visceral pleura, the bronchovascular bundles and the paraseptal parenchyma, and compartimental involvement can give an indication towards the identification of the underlying disease. vi. The previously proposed, step-wise progression model of AFE from initial fibrinous exudation, over macrophage-rich, insufficient resolution to fully developed AFE has possibly to be complemented by active and inactive AFE, according to the intra-alveolar cellularity in the remodelled alveolar spaces.
Outlook
The exact definition of what makes up AFE is important, not only to help to identify the underlying diseases and therefore specific treatment options, but also to stratify patients and predict their individual outcome. Moreover, the systematic application of exact histological criteria is needed as a basis for all morpho-molecular studies in order to gain further insights into the mechanisms of pulmonary FER.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cells10061362/s1, Figure S1: ILD with concomitant alveolar fibroelastosis, Table S1: Detailed patient information.
Author Contributions
P.B. and D.J. conceptualized the Study. P.B., C.W. and I.M. systematically assessed histology cases. P.B., C.W., I.M., S.E.V., F.L. and D.J. reviewed the histology scoring. S.D. reviewed radiological images. J.G. and G.W. treated the patients. All authors gave critical feedback on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Sonderforschungsbereich, “SFB” 738 (Projekt B9) of the German Research Foundation to Danny Jonigk. The grants of the European Research Council (ERC); European Consolidator Grant, XHale to Danny Jonigk (ref. no. 771883).
Institutional Review Board Statement
The study was approved by the local ethics committee. See materials and methods.
Informed Consent Statement
Patients provided informed consent for scientific investigation.
Data Availability Statement
Datasets are freely available upon request.
Acknowledgments
The authors thank Regina Engelhardt, Annette Mueller Brechlin and Christina Petzold for their excellent technical support and Harshit Shah and Mark Kühnel for editing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parra, E.R.; Kairalla, R.A.; De Carvalho, C.R.R.; Capelozzi, V.L. Abnormal deposition of collagen/elastic vascular fibres and prognostic significance in idiopathic interstitial pneumonias. Thorax 2007, 62, 428–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. Nat. Publ. Group 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.N.; Butt, Y.M.; Johnson, K.A.; Meyer, K.; Batra, K.; Kanne, J.P.; Torrealba, J.R. Pleuroparenchymal fibroelastosis: A pattern of chronic lung injury. Hum. Pathol. 2015, 46, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Frankel, S.K.; Cool, C.D.; Lynch, D.A.; Brown, K.K. Idiopathic Pleuroparenchymal Fibroelastosis. Chest 2004, 126, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Khiroya, R.; Macaluso, C.; Montero, M.A.; Wells, A.U.; Chua, F.; Kokosi, M.; Devaraj, A.; Rice, A.; Renzoni, E.A.; Nicholson, A.G. Pleuroparenchymal Fibroelastosis Between Histologic Parameters and Survival. Am. J. Surg. Pathol. 2017, 41, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Ofek, E.; Sato, M.; Saito, T.; Wagnetz, U.; Roberts, H.C.; Chaparro, C.; Waddell, T.K.; Singer, L.G.; Hutcheon, M.A.; Keshavjee, S.; et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod. Pathol. Nat. Publ. Group 2013, 26, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, Y.; Kanoh, S.; Kouzaki, Y.; Hara, Y.; Matsubara, O.; Kawana, A. Pleuroparenchymal Fibroelastosis as a Series of Airway Complications Associated with Chronic Graft-versus-host Disease following Allogeneic Bone Marrow Transplantation. Intern. Med. 2014, 53, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, T.; Tsubata, Y.; Hamaguchi, M.; Sutani, A.; Hamaguchi, S.; Isobe, T. Pleuroparenchymal fibroelastosis after haematopoietic stem cell transplantation without graft-versus-host disease findings. Respirol. Case Rep. 2018, 6, e00298. [Google Scholar] [CrossRef] [PubMed]
- Greer, M.; Riise, G.C.; Hansson, L.; Perch, M.; Hämmäinen, P.; Roux, A.; Hirschi, S.; Lhuillier, E.; Reynaud-Gaubert, M.; Philit, F.; et al. Dichotomy in pulmonary graft-versus-host disease evident among allogeneic stem-cell transplant recipients undergoing lung transplantation. Eur. Respir. J. 2016, 48, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Bandoh, S.; Kanaji, N.; Tadokoro, A.; Watanabe, N.; Imataki, O.; Dobashi, H.; Kushida, Y.; Haba, R.; Yokomise, H. Air-leak Syndrome by Pleuroparenchymal Fibroelastosis after Bone Marrow Transplantation. Intern. Med. 2016, 55, 105–111. [Google Scholar] [CrossRef][Green Version]
- Watanabe, K. Pleuroparenchymal Fibroelastosis: Its Clinical Characteristics. Curr. Respir. Med. Rev. 2013, 9, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kronborg-White, S.; Ravaglia, C.; Dubini, A.; Piciucchi, S.; Tomassetti, S.; Bendstrup, E.; Poletti, V. Cryobiopsies are diagnostic in Pleuroparenchymal and Airway-centered Fibroelastosis. Respir. Res. 2018, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.K.H.; Chuah, K.L. Pleuroparenchymal fibroelastosis of the lung: A review. Arch. Pathol. Lab. Med. 2016, 140, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Rath, B.; Borchert, P.; Braubach, P.; Maegel, L.; Izykowski, N.; Warnecke, G.; Sommer, W.; Kreipe, H.; Blach, R.; et al. Comparative analysis of morphological and molecular motifs in bronchiolitis obliterans and alveolar fibroelastosis after lung and stem cell transplantation. J. Pathol. Clin. Res. 2016, 3, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.L.; Tominaga, M.; Hansell, D.M.; Von Der Thusen, J.; Rassl, D.; Parfrey, H.; Guy, S.; Twentyman, O.; Rice, A.; Maher, T.M.; et al. Pleuroparenchymal fibroelastosis: A spectrum of histopathological and imaging phenotypes. Eur. Respir. J. 2012, 40, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Lagstein, A. Pulmonary apical cap-what’s old is new again. Arch. Pathol. Lab. Med. 2015, 139, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Chua, F.; Desai, S.R.; Nicholson, A.G.; Renzoni, E.; Rice, A.; Wells, A.U. Pleuroparenchymal Fibroelastosis. A Review of Clinical, Radiological, and Pathological Characteristics. Ann. Ats 2019, 16, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Watanabe, K.; Ishii, H.; Kushima, H.; Hamasaki, M.; Fujita, M.; Nabeshima, K. Pleuroparenchymal fibroelastosis as a histological background of autoimmune diseases. Virchows Arch. 2018, 474, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Ogura, T.; Kiatamura, H.; Hagiware, E.; Baba, T.; Enomoto, Y.; Iwasawa, T.; Okudela, K.; Takemura, T.; Sakai, F.; et al. Distinct Characteristics of Pleuroparenchymal Fibroelastosis With Usual Interstitial Pneumonia Compared with Idiopathic Pulmonary Fibrosis. Chest 2014, 146, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, K.; Handa, T.; Kubo, T.; Chen-Yoshikawa, T.F.; Aoyama, A.; Motoyama, H.; Hijiya, K.; Yoshizawa, A.; Oshima, Y.; Ikezoe, K.; et al. Clinical significance of radiological pleuroparenchymal fibroelastosis pattern in interstitial lung disease patients registered for lung transplantation: A retrospective cohort study. Respir. Res. 2018, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Kusagaya, H.; Nakamura, Y.; Kono, M.; Kaida, Y.; Kuroishi, S.; Enomoto, N.; Fujisawa, T.; Koshimizu, N.; Yokomura, K.; Inui, N.; et al. Idiopathic pleuroparenchymal fibroelastosis: Consideration of a clinicopathological entity in a series of Japanese patients. BMC Pulm Med. 2012, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Von der Thüsen, J.H. Pleuroparenchymal Fibroelastosis: Its Pathological Characteristics. Curr. Respir. Med. Rev. 2013, 9, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, W.; Wang, Z.; Tian, Y.; Da, J.; Zhai, Z. Pleuroparenchymal fibroelastosis secondary to autologous hematopoietic stem cell transplantation: A case report. Exp. Ther. Med. 2019, 17, 2557–2560. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).