The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Pre- and Post-Surgery Procedures
2.3. Spinal Cord Contusion
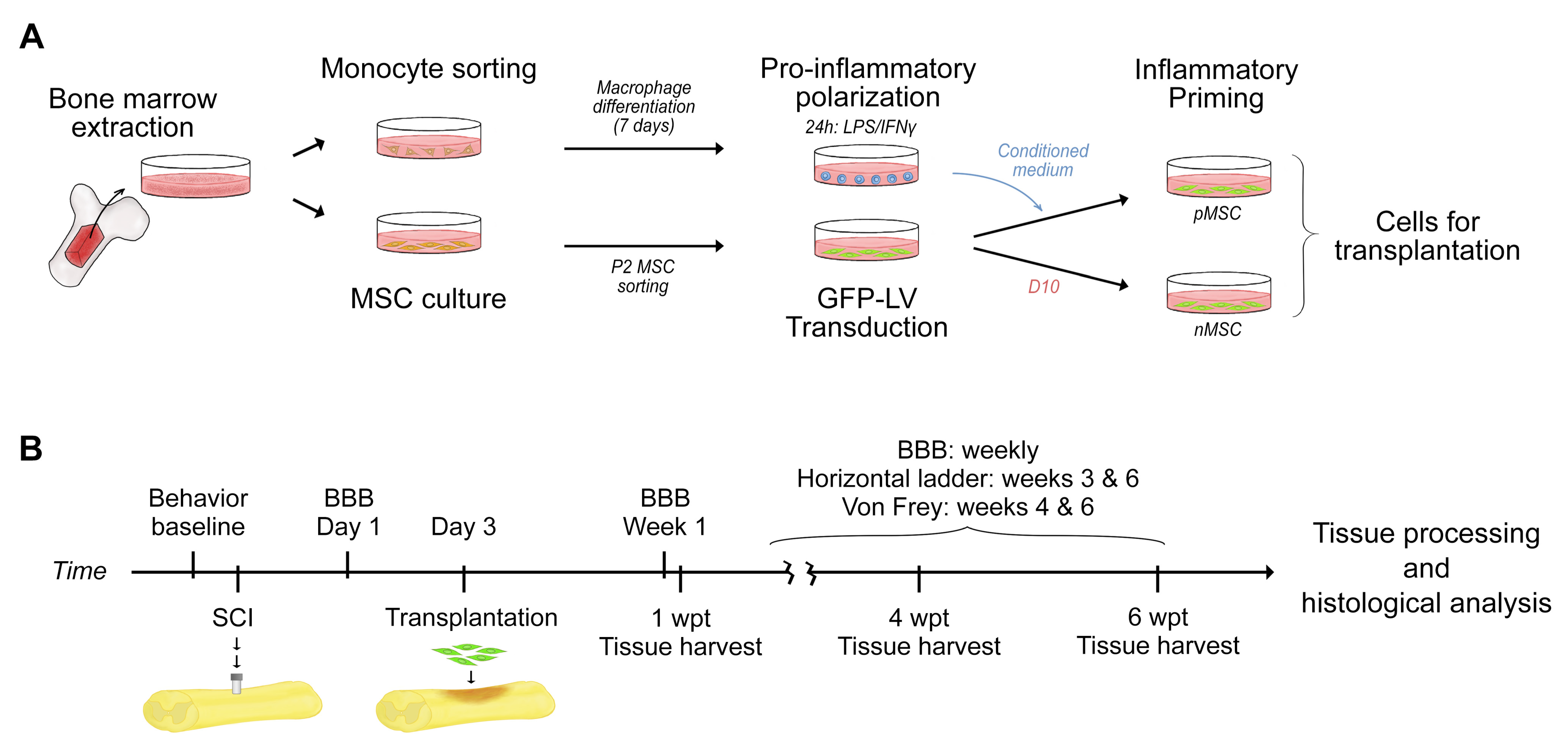
2.4. Cell Preparation
2.5. Cell Transplantation
2.6. Tissue Harvest and Processing
2.7. Immunohistochemistry
2.8. MSC Transplant
2.9. Spinal Cord Nervous Tissue
2.10. Assessment of Macrophage Phenotype
2.11. Assessment of Blood Vessels
2.12. Assessment of Hind Limb Sensorimotor Performance
2.13. Statistical Analysis
3. Results
3.1. Study Design
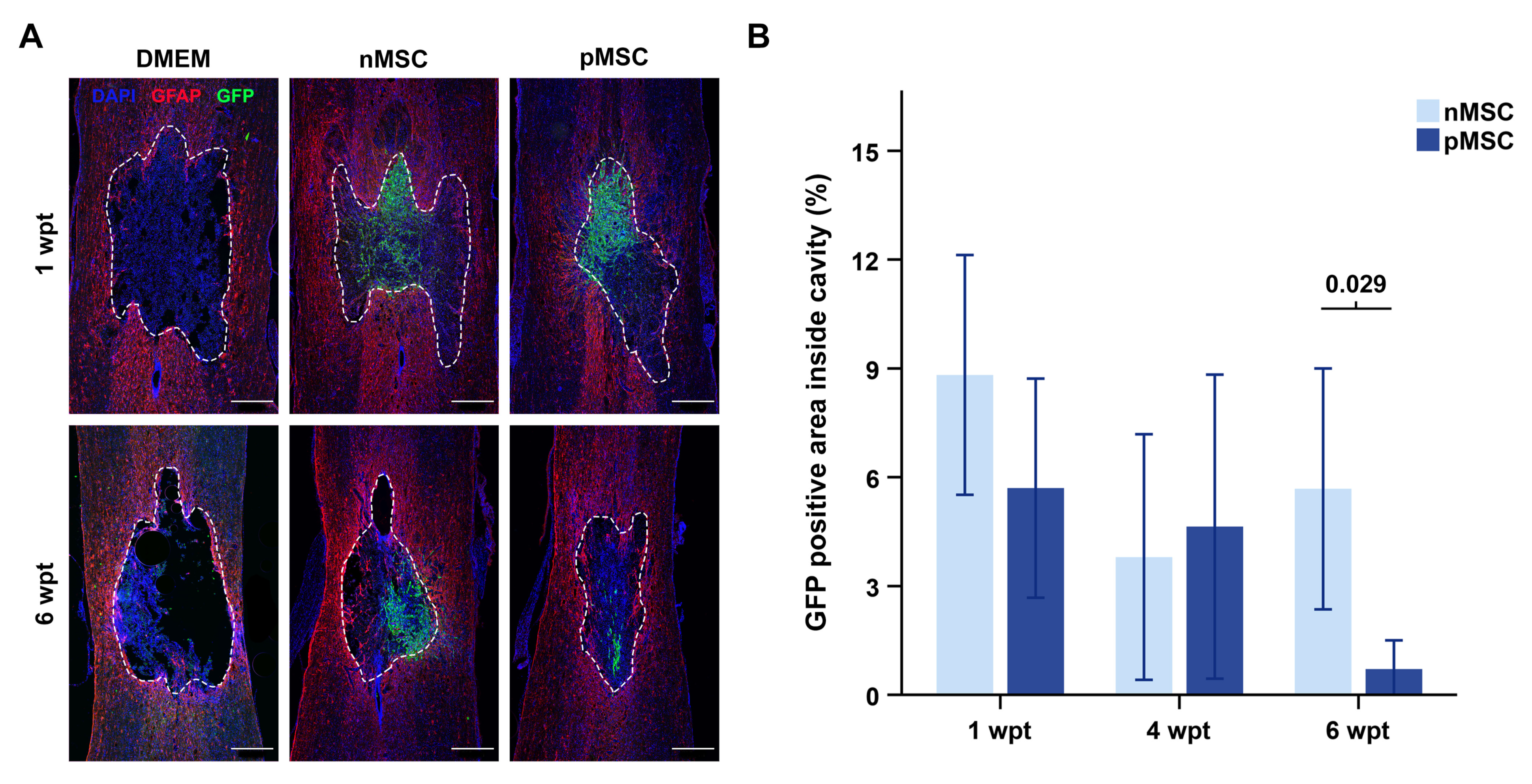
3.2. MSC Transplant Survival
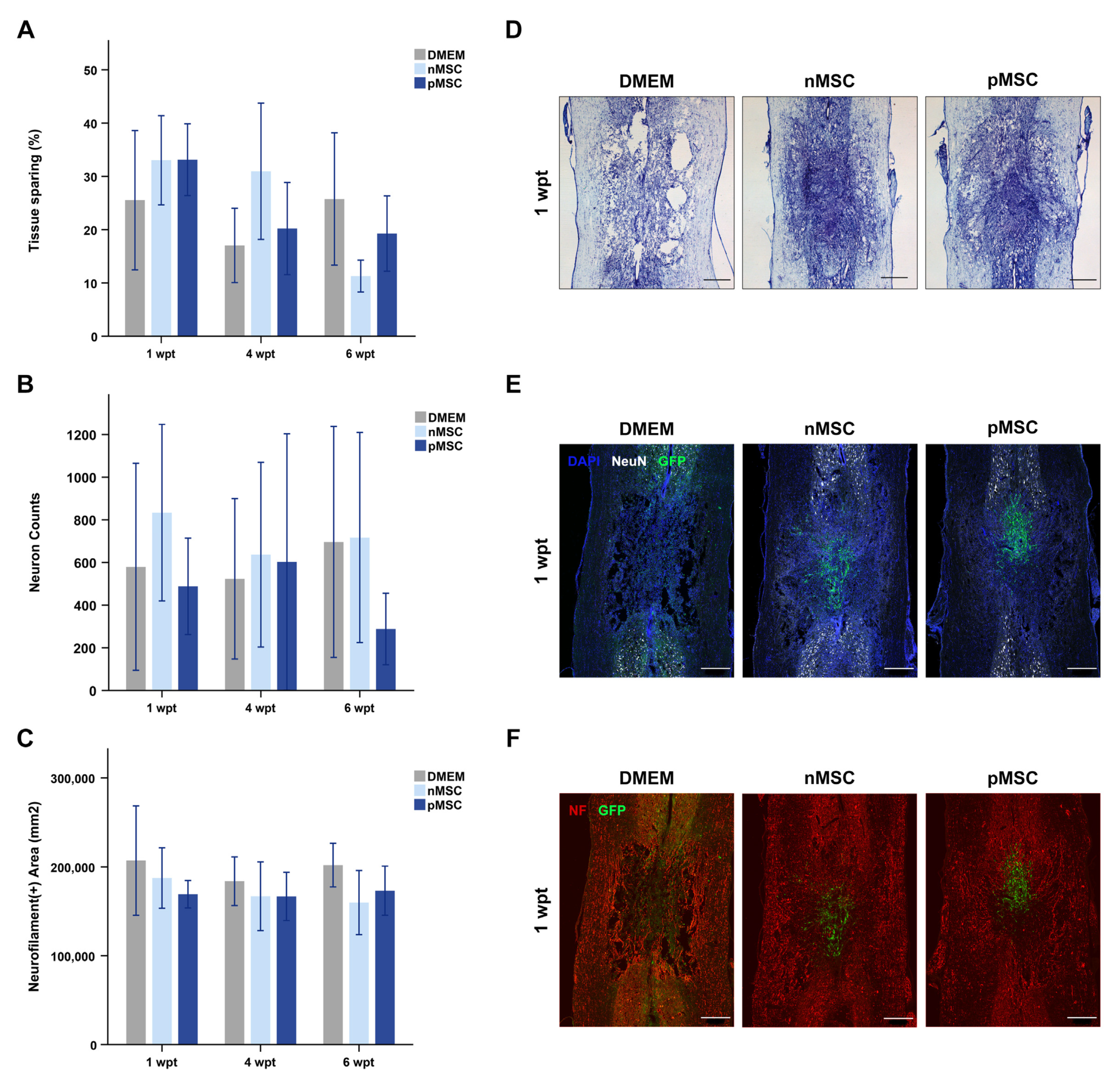
3.3. Effects of pMSC on the Contused Spinal Cord
3.4. Therapeutic Outcomes Associated with Transplant Presence
3.5. Hind Limb Sensorimotor Performance was Unchanged
4. Discussion
4.1. Inflammatory Priming and Transplant Survival in SCI
4.2. The primed Secretome and Nervous Tissue Sparing
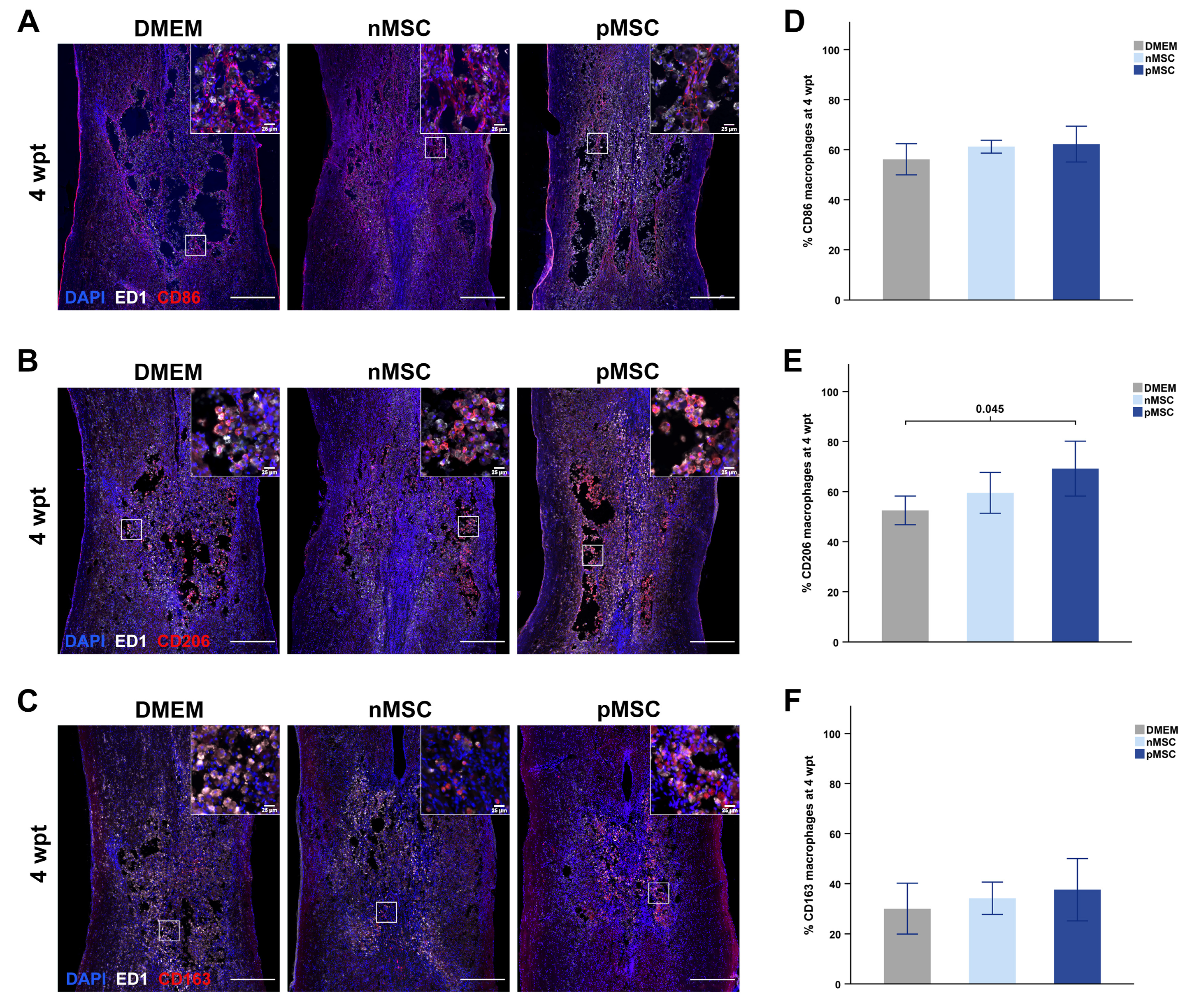
4.3. Immunomodulation by Primed MSC
4.4. The Primed Secretome and Revascularization after SCI
4.5. MSC Priming
4.6. Tackling Long Term Transplant Survival
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bcl2 | B-cell lymphoma 2 gene |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dubbelco’s Modified Eagle Medium |
| EGF | epidermal growth factor |
| FACS | fluorescence-activated cell sorting |
| GDNF | glial derived neurotrophic factor |
| GFAP | glial fibrillary acidic factor |
| GF | growth factors |
| HGF | hepatocyte growth factor |
| IACUC | Institutional Animal Care and Use Committee |
| IFNγ | interferon gamma |
| LPS | lipopolysaccharide |
| NeuN | neuronal nuclei |
| NF | neurofilament |
| NGF | nerve growth factor |
| nMSC | naïve MSC |
| P2 | passage 2 |
| PBS | phosphate-buffered saline |
| PDL | poly-D-lysine |
| PGE2 | prostaglandin-E2 |
| pMSC | primed MSC |
| RECA-1 | rat endothelial cell antigen 1 |
| ROI(s) | region(s) of interest |
| SCI | spinal cord injury |
| SR | slow release |
| T8 | thoracic vertebra/segment 8 |
| TNFα | tumor necrosis factor alpha |
| VEGF | vascular endothelial growth factor |
| wpt | weeks post-transplant |
| ZO-1 | zonula occludens 1 |
References
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2014, 137, 503–519. [Google Scholar] [CrossRef]
- Asami, T.; Ishii, M.; Fujii, H.; Namkoong, H.; Tasaka, S.; Matsushita, K.; Ishii, K.; Yagi, K.; Fujiwara, H.; Funatsu, Y.; et al. Modulation of murine macrophage TLR7/8-mediated cytokine expression by mesenchymal stem cell-conditioned medium. Mediat. Inflamm. 2013, 2013, 264260. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dulchavsky, D.S.; Gao, X.; Kwon, D.; Chopp, M.; Dulchavsky, S.; Gautam, S.C. Wound repair by bone marrow stromal cells through growth factor production. J. Surg. Res. 2006, 136, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ritfeld, G.J.; Rauck, B.M.; Novosat, T.L.; Park, D.; Patel, P.; Roos, R.A.; Wang, Y.; Oudega, M. The effect of a polyurethane-based reverse thermal gel on bone marrow stromal cell transplant survival and spinal cord repair. Biomaterials 2014, 35, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef]
- Noort, W.A.; Feye, D.; Van Den Akker, F.; Stecher, D.; Chamuleau, S.A.; Sluijter, J.P.; Doevendans, P.A. Mesenchymal stromal cells to treat cardiovascular disease: Strategies to improve survival and therapeutic results. Panminerva Med. 2010, 52, 27–40. [Google Scholar]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Q.; Jiang, Y.; Wu, L.; Xu, G.; Liu, X. Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated. Neuroscience 2015, 290, 288–299. [Google Scholar] [CrossRef]
- Xie, N.; Li, Z.; Adesanya, T.M.; Guo, W.; Liu, Y.; Fu, M.; Kilic, A.; Tan, T.; Zhu, H.; Xie, X. Transplantation of placenta-derived mesenchymal stem cells enhances angiogenesis after ischemic limb injury in mice. J. Cell Mol. Med. 2016, 20, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.K.; Popovich, P.G. Neuroinflammation in spinal cord injury: Therapeutic targets for neuroprotection and regeneration. Prog. Brain Res. 2009, 175, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003, 41, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Guan, Z.; McGaughy, V.; Fisher, L.; Hickey, W.F.; Basso, D.M. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002, 61, 623–633. [Google Scholar] [CrossRef]
- Hagg, T.; Oudega, M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 2006, 23, 264–280. [Google Scholar] [CrossRef]
- Nandoe Tewarie, R.D.; Hurtado, A.; Ritfeld, G.J.; Rahiem, S.T.; Wendell, D.F.; Barroso, M.M.; Grotenhuis, J.A.; Oudega, M. Bone marrow stromal cells elicit tissue sparing after acute but not delayed transplantation into the contused adult rat thoracic spinal cord. J. Neurotrauma 2009, 26, 2313–2322. [Google Scholar] [CrossRef]
- Chang, J.; Koh, A.J.; Roca, H.; McCauley, L.K. Juxtacrine interaction of macrophages and bone marrow stromal cells induce interleukin-6 signals and promote cell migration. Bone Res. 2015, 3, 15014. [Google Scholar] [CrossRef] [PubMed]
- Fasslrinner, F.; Wobus, M.; Duryagina, R.; Müller, K.; Stopp, S.; Wehner, R.; Rauner, M.; Hofbauer, L.C.; Schmitz, M.; Bornhäuser, M. Differential effects of mixed lymphocyte reaction supernatant on human mesenchymal stromal cells. Exp. Hematol. 2012, 40, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Kim, B.-S.; Ryu, D.-B.; Kim, T.W.; Park, G.; Min, C.-K. The therapeutic efficacy of mesenchymal stromal cells on experimental colitis was improved by the IFN-γ and poly (I: C) priming through promoting the expression of indoleamine 2, 3-dioxygenase. Stem Cell Res. Ther. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Pulkova, N.V.; Pevzner, I.B.; Zorova, L.D.; Silachev, D.N.; Morosanova, M.A.; Sukhikh, G.T.; Zorov, D.B. Inflammatory pre-conditioning of mesenchymal multipotent stromal cells improves their immunomodulatory potency in acute pyelonephritis in rats. Cytotherapy 2013, 15, 679–689. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; McDevitt, T.C. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy 2014, 16, 331–345. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, N.; Wang, J.A. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J. Endocrinol. Investig. 2008, 31, 103–110. [Google Scholar] [CrossRef]
- Esmaeili, R.; Darbandi-Azar, A.; Sadeghpour, A.; Majidzadeh, A.K.; Eini, L.; Jafarbeik-Iravani, N.; Hoseinpour, P.; Vajhi, A.; Bakhshaiesh, T.O.; Masoudkabir, F.; et al. Mesenchymal stem cells Pretreatment with stromal-derived factor-1 alpha augments cardiac function and angiogenesis in infarcted myocardium. Am. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Carvalho, J.L.; Braga, V.B.; Melo, M.B.; Campos, A.C.; Oliveira, M.S.; Gomes, D.A.; Ferreira, A.J.; Santos, R.A.; Goes, A.M. Priming mesenchymal stem cells boosts stem cell therapy to treat myocardial infarction. J. Cell Mol. Med. 2013, 17, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Lasunción, I.; O’Neill, N.; Umland, O.; Verhaagen, J.; Oudega, M. Macrophage-Derived Inflammation Induces a Transcriptome Makeover in Mesenchymal Stromal Cells Enhancing Their Potential for Tissue Repair. Int. J. Mol. Sci. 2021, 22, 781. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, Y.; Markel, T.A.; Wang, M.; Lahm, T.; Meldrum, D.R. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am. J. Physiol. Cell Physiol. 2008, 294, C675–C682. [Google Scholar] [CrossRef]
- Ritfeld, G.J.; Nandoe Tewarie, R.D.; Vajn, K.; Rahiem, S.T.; Hurtado, A.; Wendell, D.F.; Roos, R.A.; Oudega, M. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. 2012, 21, 1561–1575. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Porcheray, F.; Viaud, S.; Rimaniol, A.C.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dormont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Kunkel-Bagden, E.; Dai, H.-N.; Bregman, B.S. Recovery of function after spinal cord hemisection in newborn and adult rats: Differential effects on reflex and locomotor function. Exp. Neurol. 1992, 116, 40–51. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.; Pogrel, J.; Chung, J.; Yaksh, T. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Crowe, M.J.; Bresnahan, J.C.; Shuman, S.L.; Masters, J.N.; Beattie, M.S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997, 3, 73–76. [Google Scholar] [CrossRef]
- McTigue, D.M.; Tani, M.; Krivacic, K.; Chernosky, A.; Kelner, G.S.; Maciejewski, D.; Maki, R.; Ransohoff, R.M.; Stokes, B.T. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J. Neurosci. Res. 1998, 53, 368–376. [Google Scholar] [CrossRef]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef]
- Neri, S.; Borzi, R.M. Molecular Mechanisms Contributing to Mesenchymal Stromal Cell Aging. Biomolecules 2020, 10, 340. [Google Scholar] [CrossRef]
- Jakovljevic, J.; Harrell, C.R.; Fellabaum, C.; Arsenijevic, A.; Jovicic, N.; Volarevic, V. Modulation of autophagy as new approach in mesenchymal stem cell-based therapy. Biomed. Pharmacother. 2018, 104, 404–410. [Google Scholar] [CrossRef]
- Gray, A.; Schloss, R.S.; Yarmush, M. Donor variability among anti-inflammatory pre-activated mesenchymal stromal cells. Technology 2016, 4, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Jagtap, J.C.; Kim, D.-K.; Shah, R.D.; Das, G.; Shivayogi, M.; Pujari, R.; Shastry, P. TNF-α and IFN-γ together up-regulates par-4 expression and induce apoptosis in human neuroblastomas. Biomedicines 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J. Cytokines and macrophages. Biomed. Pharmacother. 1994, 48, 445–453. [Google Scholar] [CrossRef]
- Darwich, L.; Coma, G.; Peña, R.; Bellido, R.; Blanco, E.J.; Este, J.A.; Borras, F.E.; Clotet, B.; Ruiz, L.; Rosell, A. Secretion of interferon-γ by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology 2009, 126, 386–393. [Google Scholar] [CrossRef]
- Klusch, A.; Gorzelanny, C.; Reeh, P.W.; Schmelz, M.; Petersen, M.; Sauer, S.K. Local NGF and GDNF levels modulate morphology and function of porcine DRG neurites, In Vitro. PLoS ONE 2018, 13, e0203215. [Google Scholar] [CrossRef]
- Fine, E.G.; Decosterd, I.; Papaloïzos, M.; Zurn, A.D.; Aebischer, P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur. J. Neurosci. 2002, 15, 589–601. [Google Scholar] [CrossRef]
- Chen, J.; Chu, Y.; Chen, J.; Li, B. Synergistic effects of NGF, CNTF and GDNF on functional recovery following sciatic nerve injury in rats. Adv. Med Sci. 2010, 55, 32–42. [Google Scholar] [CrossRef]
- Widenfalk, J.; Lundströmer, K.; Jubran, M.; Brené, S.; Olson, L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci. 2001, 21, 3457–3475. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Smith, G.M.; Wen, X.; Pressman, Y.; Wood, P.M.; Xu, X.M. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia 2009, 57, 1178–1191. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Tomchuck, S.L.; Zwezdaryk, K.J.; Coffelt, S.B.; Waterman, R.S.; Danka, E.S.; Scandurro, A.B. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 2008, 26, 99–107. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Delarosa, O.; Dalemans, W.; Lombardo, E. Toll-like receptors as modulators of mesenchymal stem cells. Front. Immunol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; François, M.; Boivin, M.N.; Bouchentouf, M.; Spaner, D.E.; Galipeau, J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J. Immunol. 2009, 182, 7963–7973. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, A.; Stein, A.; Bank, M.; Sison, C.P.; Gibbs, K.; Davies, P.; Bloom, O. High-Mobility Group Box 1 (HMGB1) Is Elevated Systemically in Persons with Acute or Chronic Traumatic Spinal Cord Injury. J. Neurotrauma 2017, 34, 746–754. [Google Scholar] [CrossRef]
- Blight, A.R. Macrophages and inflammatory damage in spinal cord injury. J. Neurotrauma 1992, 9, S83–S91. [Google Scholar]
- Kopper, T.J.; McFarlane, K.E.; Bailey, W.M.; Orr, M.B.; Zhang, B.; Gensel, J.C. Delayed Azithromycin Treatment Improves Recovery After Mouse Spinal Cord Injury. Front. Cell Neurosci. 2019, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Bin, S.; Zhou, N.F.; Pan, J.; Pan, F.M.; Wu, X.F.; Zhou, Z.H. Nano-carrier mediated co-delivery of methyl prednisolone and minocycline for improved post-traumatic spinal cord injury conditions in rats. Drug Dev. Ind. Pharm. 2017, 43, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.S.; Galleu, A.; von Bonin, M.; Bornhäuser, M.; Dazzi, F. Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: Implications for the monitoring of mesenchymal stromal cell activity. Haematologica 2019, 104, e438. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mohsin, S.; Akhtar, S.; Ali, F.; Khan, S.N.; Riazuddin, S. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J. Cell. Mol. Med. 2012, 16, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Lutton, C.; Young, Y.W.; Williams, R.; Meedeniya, A.C.; Mackay-Sim, A.; Goss, B. Combined VEGF and PDGF treatment reduces secondary degeneration after spinal cord injury. J. Neurotrauma 2012, 29, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Oudega, M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012, 349, 269–288. [Google Scholar] [CrossRef]
- Ballabh, P.; Hu, F.; Kumarasiri, M.; Braun, A.; Nedergaard, M. Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatr. Res. 2005, 58, 791–798. [Google Scholar] [CrossRef]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef]
- De Becker, A.; Riet, I.V. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells 2016, 8, 73–87. [Google Scholar] [CrossRef]
- Zachar, L.; Bačenková, D.; Rosocha, J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J. Inflamm. Res. 2016, 9, 231. [Google Scholar] [CrossRef]
- Caffi, V.; Espinosa, G.; Gajardo, G.; Morales, N.; Durán, M.C.; Uberti, B.; Morán, G.; Plaza, A.; Henríquez, C. Pre-conditioning of Equine Bone Marrow-Derived Mesenchymal Stromal Cells Increases Their Immunomodulatory Capacity. Front. Vet. Sci. 2020, 7, 318. [Google Scholar] [CrossRef]
- Rafat, A.; Mohammadi Roushandeh, A.; Alizadeh, A.; Hashemi-Firouzi, N.; Golipoor, Z. Comparison of The Melatonin Preconditioning Efficacy between Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. Cell J. 2019, 20, 450–458. [Google Scholar] [CrossRef]
- Pourjafar, M.; Saidijam, M.; Mansouri, K.; Ghasemibasir, H.; Karimi Dermani, F.; Najafi, R. All-trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo. Cell Prolif. 2017, 50, e12315. [Google Scholar] [CrossRef]
- Tsai, L.K.; Wang, Z.; Munasinghe, J.; Leng, Y.; Leeds, P.; Chuang, D.M. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke 2011, 42, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Wang, Y.; Jin, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Shen, B.; Yin, S.; Liu, W.; Cui, L.; et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008, 18, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Freytes, D.O.; Kang, J.W.; Marcos-Campos, I.; Vunjak-Novakovic, G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 220–229. [Google Scholar] [CrossRef]
- Maldonado-Lasuncion, I.; Verhaagen, J.; Oudega, M. Mesenchymal Stem Cell-Macrophage Choreography Supporting Spinal Cord Repair. Neurotherapeutics 2018, 15, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, M.M.; Tu, M.K.; Keirstead, H.S. The extent of myelin pathology differs following contusion and transection spinal cord injury. J. Neurotrauma 2007, 24, 1631–1646. [Google Scholar] [CrossRef]
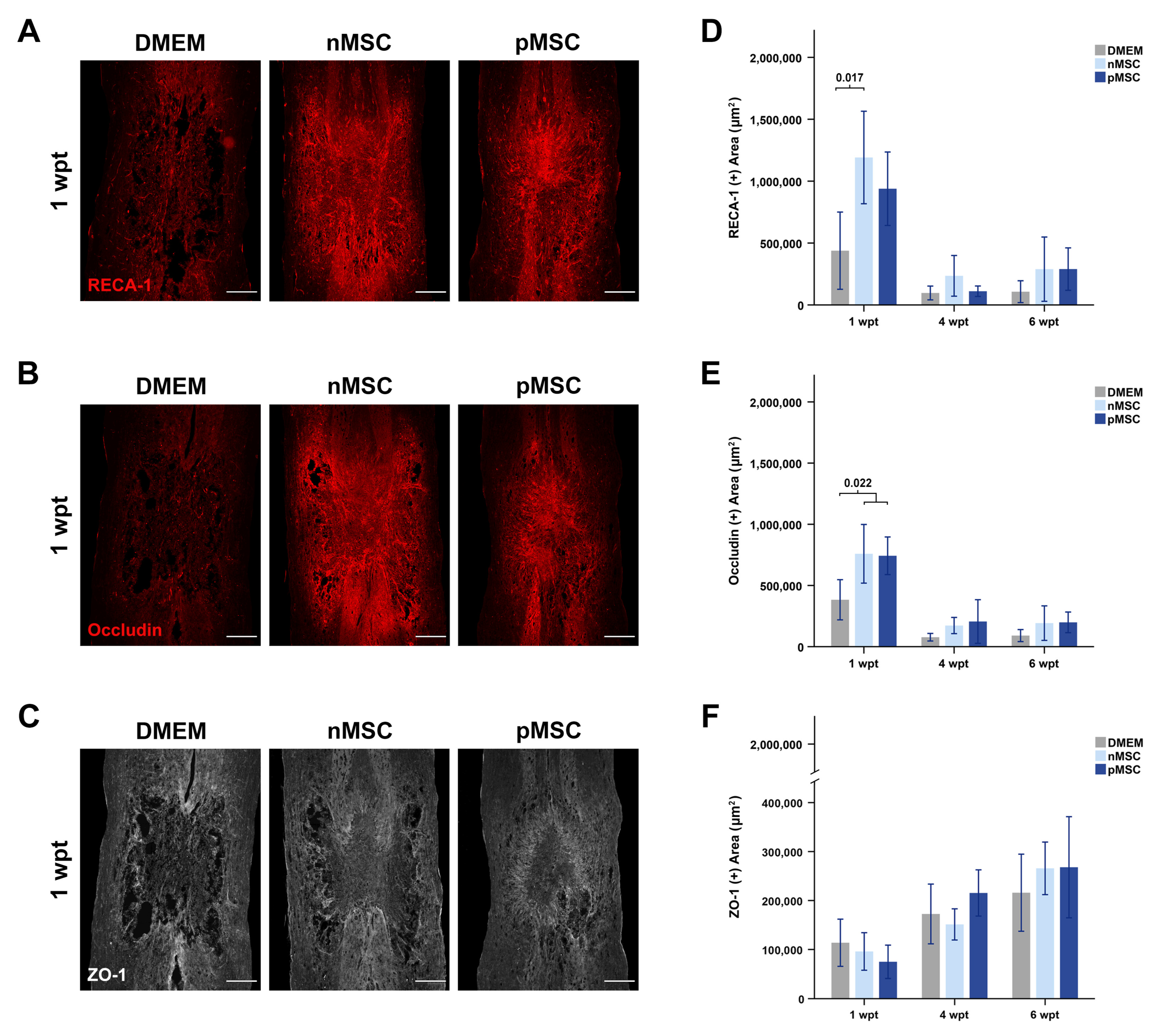
| Repair Effect | Outcome | Group | 1 wpt | 4 wpt | 6 wpt | Correlation † |
|---|---|---|---|---|---|---|
| Neuroprotection | Tissue sparing a (%) | pMSC | 33.1 ± 3.4 | 20.2 ± 4.3 | 19.3 ± 3.5 | 0.592 ** |
| nMSC | 33.0 ± 4.2 | 30.9 ± 6.4 | 11.3 ± 1.5 | 0.051 | ||
| DMEM | 25.5 ± 6.5 | 17.0 ± 3.5 | 25.8 ± 6.2 | N/A | ||
| Neuronal survival b (counts) | pMSC | 489 ± 113 | 603 ± 301 | 288 ± 84 | 0.398 | |
| nMSC | 834 ± 207 | 637 ± 217 | 717 ± 247 | 0.266 | ||
| DMEM | 580 ± 243 | 524 ± 188 | 696 ± 271 | N/A | ||
| Axonal presence c (µm2) | pMSC | 169,261 ± 7684 | 166,872 ± 13,592 | 173,308 ± 13,812 | −0.255 | |
| nMSC | 187,484 ± 17,010 | 167,010 ± 19,376 | 159,952 ± 18,065 | 0.355 | ||
| DMEM | 207,198 ± 30,776 | 183,902 ± 13,679 | 202,085 ± 12,265 | N/A | ||
| Immunomodulation | Pro-inflammatory d (%) | pMSC | 82.3 ± 2.8 | 62.2 ± 3.6 | 62.8 ± 3.4 | 0.340 |
| nMSC | 68.3 ± 6.4 | 61.2 ± 1.3 | 54.2 ± 3.0 | 0.425 | ||
| DMEM | 82.3 ± 7.9 | 56.2 ± 3.1 | 69.3 ± 5.5 | N/A | ||
| Early anti-inflammatory e (%) | pMSC | 91.3 ± 6.1 | 69.2 ± 5.5 * | 63.9 ± 5.1 | 0.298 | |
| nMSC | 75.8 ± 12.1 | 59.5 ± 4.1 | 57.5 ± 2.5 | 0.573 * | ||
| DMEM | 92.9 ± 6.0 | 52.5 ± 2.9 | 65.3 ± 2.2 | N/A | ||
| Late anti-inflammatory f (%) | pMSC | 14.51 ± 2.5 | 37.6 ± 6.2 | 34.99 ± 2.5 | −0.237 | |
| nMSC | 13.46 ± 2.7 | 30.06 ± 5.1 | 40.09 ± 5.5 | −0.550 * | ||
| DMEM | 18.08 ± 4.2 | 34.2 ± 3.2 | 29.6 ± 5.2 | N/A | ||
| Revascularization | Blood vessels g (µm2) | pMSC | 938,637 ± 148,407 | 110,756 ± 21,157 | 289,606 ± 85,683 | 0.614 ** |
| nMSC | 1,191,049 ± 186,800 * | 235,060 ± 82,194 | 288,927 ± 129,815 | 0.645 ** | ||
| DMEM | 438,311 ± 155,990 | 96,567 ± 27,864 | 107,048 ± 44,112 | N/A | ||
| Occludin h (µm2) | pMSC | 742,582 ± 76,849 * | 205,501 ± 89,115 | 198,592 ± 42,185 | 0.606 ** | |
| nMSC | 758,800 ± 119,874 * | 172,563 ± 33,095 | 192,260 ± 70,535 | 0.568 * | ||
| DMEM | 383,145 ± 82,063 | 771,11 ± 15,488 | 90,500 ± 24,577 | N/A | ||
| ZO-1 i (µm2) | pMSC | 74,904 ± 16,987 | 215,440 ± 23,596 | 267,937 ± 516,33 | −0.595 ** | |
| nMSC | 95,962 ± 19,165 | 151,201 ± 15,934 | 265,711 ± 26,818 | −0.304 | ||
| DMEM | 113,779 ± 24,084 | 172,522 ± 30,525 | 215,962 ± 39,358 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Lasunción, I.; Haggerty, A.E.; Okuda, A.; Mihara, T.; de la Oliva, N.; Verhaagen, J.; Oudega, M. The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair. Cells 2021, 10, 1316. https://doi.org/10.3390/cells10061316
Maldonado-Lasunción I, Haggerty AE, Okuda A, Mihara T, de la Oliva N, Verhaagen J, Oudega M. The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair. Cells. 2021; 10(6):1316. https://doi.org/10.3390/cells10061316
Chicago/Turabian StyleMaldonado-Lasunción, Inés, Agnes E. Haggerty, Akinori Okuda, Tokumitsu Mihara, Natalia de la Oliva, Joost Verhaagen, and Martin Oudega. 2021. "The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair" Cells 10, no. 6: 1316. https://doi.org/10.3390/cells10061316
APA StyleMaldonado-Lasunción, I., Haggerty, A. E., Okuda, A., Mihara, T., de la Oliva, N., Verhaagen, J., & Oudega, M. (2021). The Effect of Inflammatory Priming on the Therapeutic Potential of Mesenchymal Stromal Cells for Spinal Cord Repair. Cells, 10(6), 1316. https://doi.org/10.3390/cells10061316







