An Arrhythmic Mutation E7K Facilitates TRPM4 Channel Activation via Enhanced PIP2 Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Gene Transfection
2.2. Solution
2.3. Electrophysiology
2.4. FRET Measurement
2.5. Statistical Evaluation
3. Results
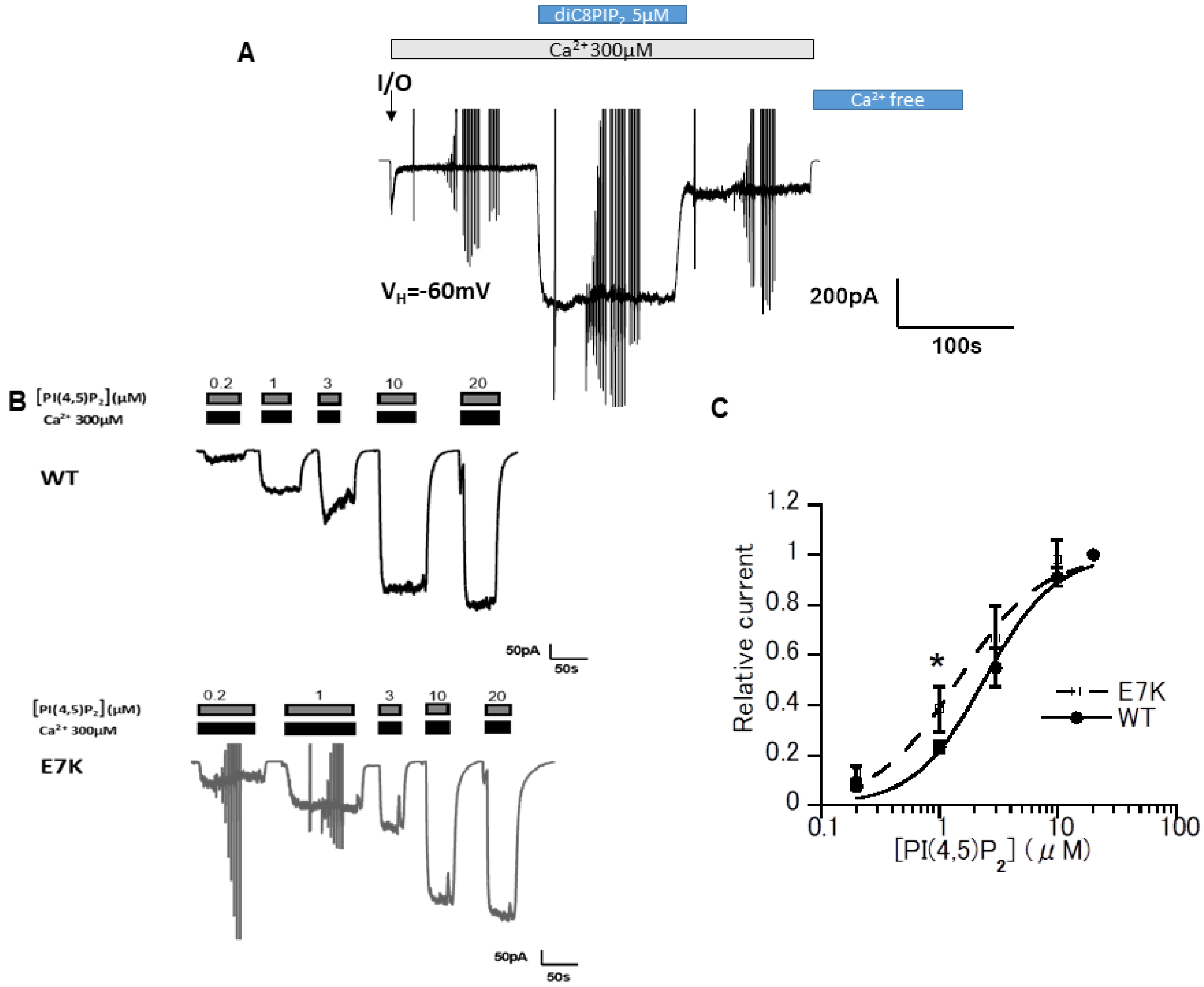
3.1. DiC8-PIP2 More Potently Reactivates E7K Than WT-TRPM4 Channels
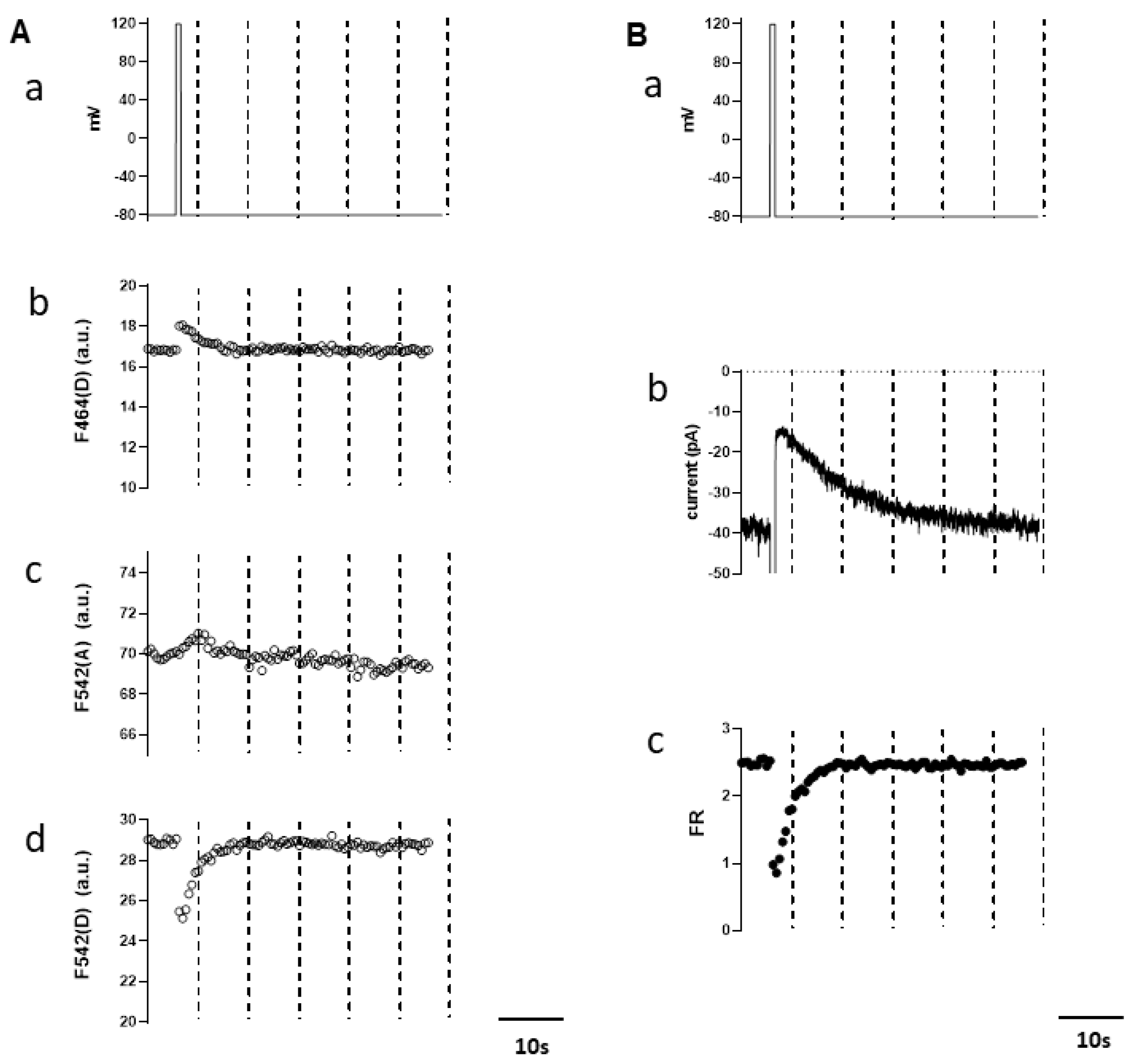
3.2. Simultaneous Measurements of Endogenous PI(4,5)P2 and TRPM4 Channel Activity
3.3. Differential PIP2 Sensitivities of WT and E7K-Mutant TRPM4 Channels
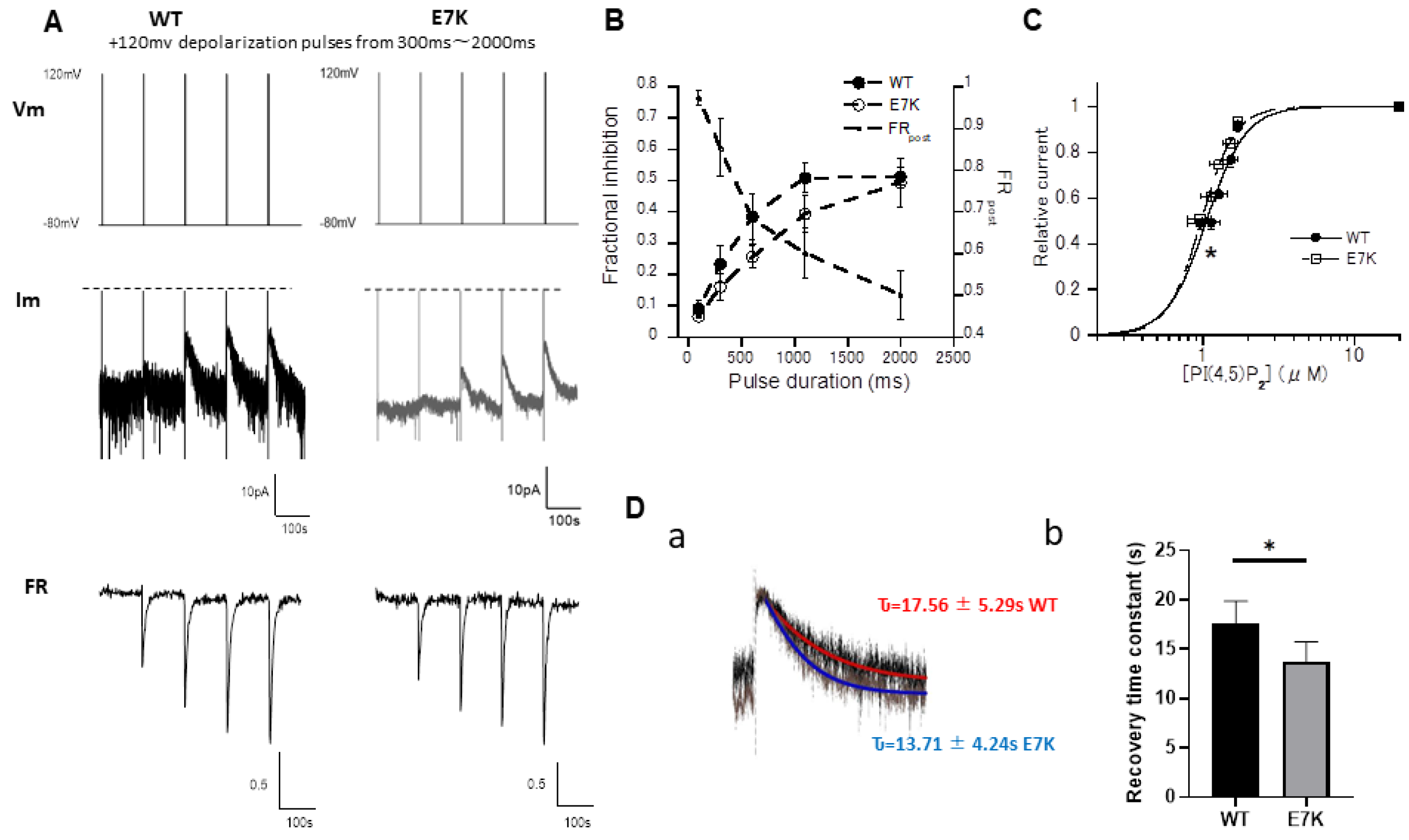
3.4. PIP2 Depletion Only Modestly Affects the Voltage-Dependent Activation of E7K Mutant Because of Its Higher PIP2 Affinity
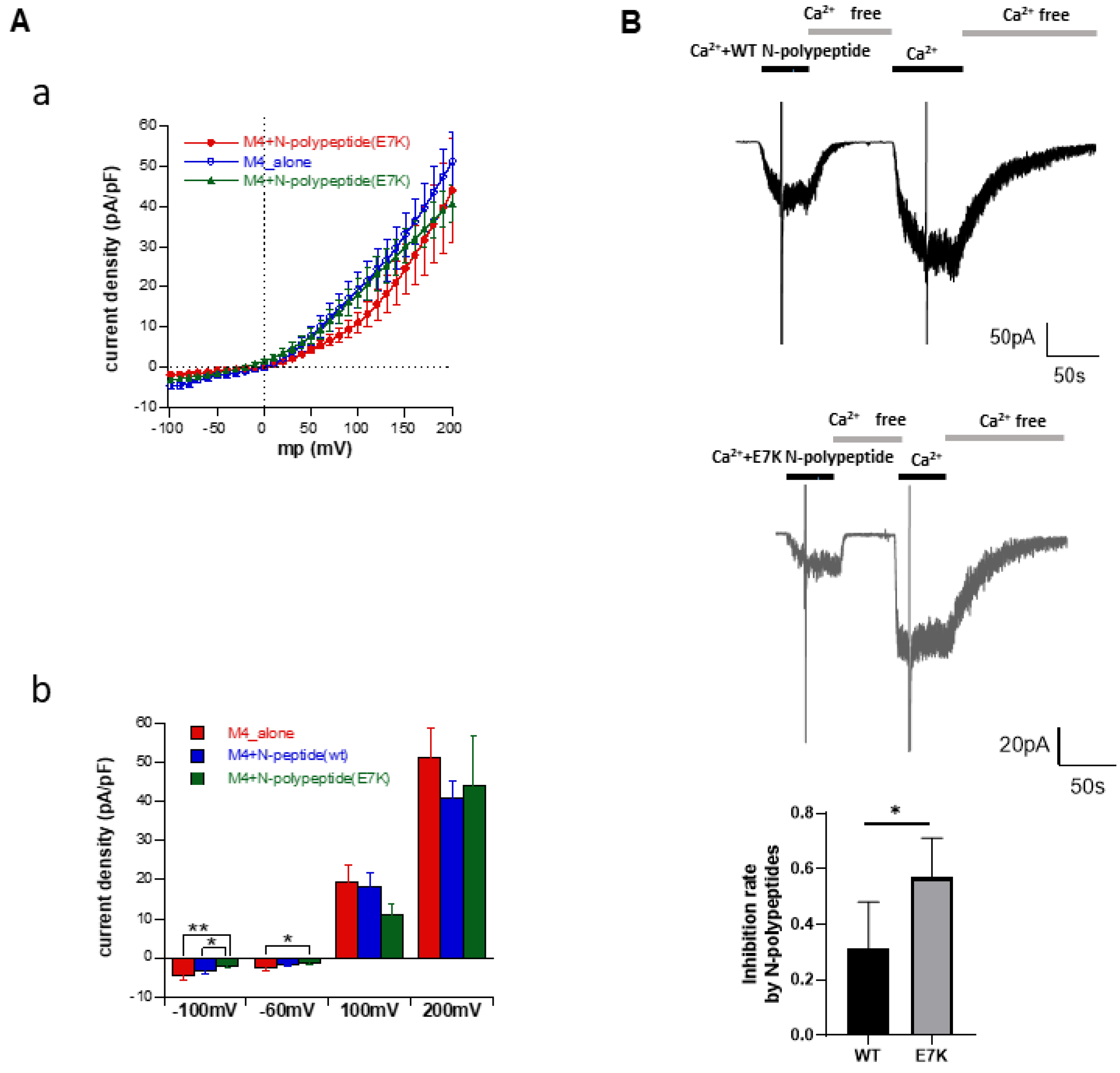
3.5. N-Terminal Polypeptides Inhibit TRPM4 Channel Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of sudden cardiac death: Global and regional perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef]
- Inoue, R.; Jensen, L.J.; Shi, J.; Morita, H.; Nishida, M.; Honda, A.; Ito, Y. Transient receptor potential channels in cardiovascular function and disease. Circ. Res. 2006, 99, 119–131. [Google Scholar] [CrossRef]
- Launay, P.; Fleig, A.; Perraud, A.-L.; Scharenberg, A.M.; Penner, R.; Kinet, J.-P. Trpm4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef]
- Simard, C.; Sallé, L.; Rouet, R.; Guinamard, R. Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br. J. Pharmacol. 2012, 165, 2354–2364. [Google Scholar] [CrossRef]
- Kruse, M.; Schulze-Bahr, E.; Corfield, V.; Beckmann, A.; Stallmeyer, B.; Kurtbay, G.; Ohmert, I.; Schulze-Bahr, E.; Brink, P.; Pongs, O. Impaired endocytosis of the ion channel trpm4 is associated with human progressive familial heart block type i. J. Clin. Investig. 2009, 119, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, S.; Duff, H.J. Genetic determinants of hereditary bradyarrhythmias: A contemporary review of a diverse group of disorders. Can. J. Cardiol. 2017, 33, 758–767. [Google Scholar] [CrossRef]
- Rosenhouse-Dantsker, A.; Mehta, D.; Levitan, I. Regulation of ion channels by membrane lipids. Compr. Physiol. 2011, 2, 31–68. [Google Scholar]
- Hilgemann, D.W.; Feng, S.; Nasuhoglu, C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, 2001, re19. [Google Scholar] [CrossRef]
- Barki-Harrington, L.; Luttrell, L.M.; Rockman, H.A. Dual inhibition of β-adrenergic and angiotensin ii receptors by a single antagonist: A functional role for receptor–receptor interaction in vivo. Circulation 2003, 108, 1611–1618. [Google Scholar] [CrossRef]
- Tappia, P.S.; Liu, S.-Y.; Shatadal, S.; Takeda, N.; Dhalla, N.S.; Panagia, V. Changes in sarcolemmal plc isoenzymes in postinfarct congestive heart failure: Partial correction by imidapril. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, H40–H49. [Google Scholar] [CrossRef]
- Nilius, B.; Mahieu, F.; Prenen, J.; Janssens, A.; Owsianik, G.; Vennekens, R.; Voets, T. The Ca2+-activated cation channel trpm4 is regulated by phosphatidylinositol 4, 5-biphosphate. EMBO J. 2006, 25, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Okawa, H.; Wang, Y.; Liman, E.R. Phosphatidylinositol 4, 5-bisphosphate rescues trpm4 channels from desensitization. J. Biol. Chem. 2005, 280, 39185–39192. [Google Scholar] [CrossRef]
- Murata, Y.; Iwasaki, H.; Sasaki, M.; Inaba, K.; Okamura, Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 2005, 435, 1239–1243. [Google Scholar] [CrossRef]
- van der Wal, J.; Habets, R.; Várnai, P.; Balla, T.; Jalink, K. Monitoring agonist-induced phospholipase c activation in live cells by fluorescence resonance energy transfer. J. Biol. Chem. 2001, 276, 15337–15344. [Google Scholar] [CrossRef] [PubMed]
- Itsuki, K.; Imai, Y.; Hase, H.; Okamura, Y.; Inoue, R.; Mori, M.X. PLC-mediated PI(4, 5) P2 hydrolysis regulates activation and inactivation of trpc6/7 channels. J. Gen. Physiol. 2014, 143, 183–201. [Google Scholar] [CrossRef]
- Inoue, R.; Okada, T.; Onoue, H.; Hara, Y.; Shimizu, S.; Naitoh, S.; Ito, Y.; Mori, Y. The transient receptor potential protein homologue trp6 is the essential component of vascular α1-adrenoceptor–activated Ca2+-permeable cation channel. Circ. Res. 2001, 88, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.G.; Liang, H.; Mori, M.X.; Yue, D.T. Fret two-hybrid mapping reveals function and location of L-type Ca2+ channel cam preassociation. Neuron 2003, 39, 97–107. [Google Scholar] [CrossRef]
- Hu, Y.; Duan, Y.; Takeuchi, A.; Hai-Kurahara, L.; Ichikawa, J.; Hiraishi, K.; Numata, T.; Ohara, H.; Iribe, G.; Nakaya, M. Uncovering the arrhythmogenic potential of trpm4 activation in atrial-derived HL-1 cells using novel recording and numerical approaches. Cardiovasc. Res. 2017, 113, 1243–1255. [Google Scholar] [CrossRef]
- Falkenburger, B.H.; Jensen, J.B.; Hille, B. Kinetics of PIP2 metabolism and kcnq2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 2010, 135, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Itsuki, K.; Okamura, Y.; Inoue, R.; Mori, M.X. A self-limiting regulation of vasoconstrictor-activated trpc3/c6/c7 channels coupled to PI(4, 5) P2-diacylglycerol signalling. J. Physiol. 2012, 590, 1101–1119. [Google Scholar] [CrossRef]
- Ishikawa, Y. The 95th annual meeting of the physiological society of Japan. J. Physiol. Sci. 2018, 68, 1–210. [Google Scholar] [CrossRef]
- Bousova, K.; Jirku, M.; Bumba, L.; Bednarova, L.; Sulc, M.; Franek, M.; Vyklicky, L.; Vondrasek, J.; Teisinger, J. PIP2 and PIP3 interact with n-terminus region of trpm4 channel. Biophys. Chem. 2015, 205, 24–32. [Google Scholar] [CrossRef]
- Holendova, B.; Grycova, L.; Jirku, M.; Teisinger, J. Ptdins (4, 5) P2 interacts with cam binding domains on trpm3 n-terminus. Channels 2012, 6, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Autzen, H.E.; Myasnikov, A.G.; Campbell, M.G.; Asarnow, D.; Julius, D.; Cheng, Y. Structure of the human trpm4 ion channel in a lipid nanodisc. Science 2018, 359, 228–232. [Google Scholar] [CrossRef]
- Zheng, W.; Cai, R.; Hofmann, L.; Nesin, V.; Hu, Q.; Long, W.; Fatehi, M.; Liu, X.; Hussein, S.; Kong, T. Direct binding between pre-s1 and trp-like domains in trpp channels mediates gating and functional regulation by PIP2. Cell Rep. 2018, 22, 1560–1573. [Google Scholar] [CrossRef]
- Hille, B. Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys. J. 1978, 22, 283–294. [Google Scholar] [CrossRef]
- Thompson, M.J.; Baenziger, J.E. Ion channels as lipid sensors: From structures to mechanisms. Nat. Chem. Biol. 2020, 16, 1331–1342. [Google Scholar] [CrossRef]
- Duan, J.; Li, Z.; Li, J.; Santa-Cruz, A.; Sanchez-Martinez, S.; Zhang, J.; Clapham, D.E. Structure of full-length human trpm4. Proc. Natl. Acad. Sci. USA 2018, 115, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Guinamard, R.; Demion, M.; Magaud, C.; Potreau, D.; Bois, P. Functional expression of the trpm4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension 2006, 48, 587–594. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Li, Q.; Kurahara, L.-H.; Shioi, N.; Hiraishi, K.; Fujita, T.; Zhu, X.; Inoue, R. An Arrhythmic Mutation E7K Facilitates TRPM4 Channel Activation via Enhanced PIP2 Interaction. Cells 2021, 10, 983. https://doi.org/10.3390/cells10050983
Hu Y, Li Q, Kurahara L-H, Shioi N, Hiraishi K, Fujita T, Zhu X, Inoue R. An Arrhythmic Mutation E7K Facilitates TRPM4 Channel Activation via Enhanced PIP2 Interaction. Cells. 2021; 10(5):983. https://doi.org/10.3390/cells10050983
Chicago/Turabian StyleHu, Yaopeng, Qin Li, Lin-Hai Kurahara, Narumi Shioi, Keizo Hiraishi, Takayuki Fujita, Xin Zhu, and Ryuji Inoue. 2021. "An Arrhythmic Mutation E7K Facilitates TRPM4 Channel Activation via Enhanced PIP2 Interaction" Cells 10, no. 5: 983. https://doi.org/10.3390/cells10050983
APA StyleHu, Y., Li, Q., Kurahara, L.-H., Shioi, N., Hiraishi, K., Fujita, T., Zhu, X., & Inoue, R. (2021). An Arrhythmic Mutation E7K Facilitates TRPM4 Channel Activation via Enhanced PIP2 Interaction. Cells, 10(5), 983. https://doi.org/10.3390/cells10050983





