Mind the Gap: LRRK2 Phenotypes in the Clinic vs. in Patient Cells
Abstract
1. Introduction
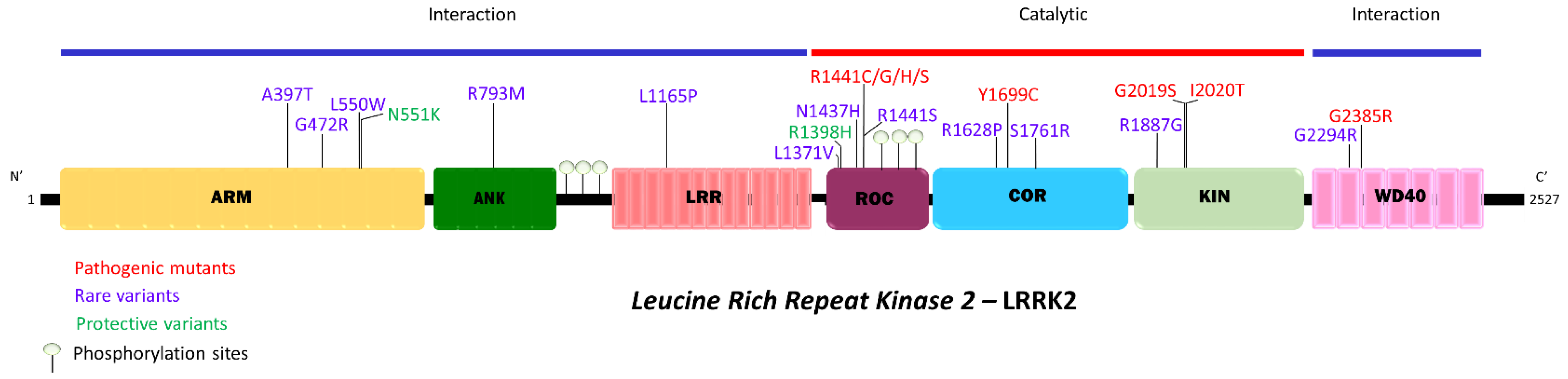
2. Understanding LRRK2 in PD through the Study of Disease-Linked or Associated Variants of LRRK2
2.1. ARM—ANK—LRR Domains
ARM—ANK—LRR Clinical Characteristics
2.2. ROC Domain
2.2.1. ROC—Clinical Characteristics
2.2.2. ROC—Patient-Derived Cell Phenotypes (See Table 1)
2.2.3. ROC—Cellular and Molecular Effects of the Mutation in Experimental Models
2.3. COR Domain
2.3.1. COR—Patient Characteristics
2.3.2. COR—Patient-Derived Cell Characteristics
2.3.3. COR—Cellular and Molecular Effects of the Mutation in Experimental Models
2.4. Kinase Domain
2.4.1. Kinase—Patient Characteristics
2.4.2. Kinase—Patient Cell Derived Characteristics
2.4.3. Kinase—Cellular and Molecular Effects of the Mutation in Experimental Models
2.5. WD-40 Domain
2.5.1. WD40—Patient Characteristics
2.5.2. WD40—Patient-Derived Cell Characteristics
2.5.3. WD40—Cellular and Molecular Effects of the Mutation in Experimental Models
| Cell Types | Nature of Patient-Derived Cells | Patient Cell Phenotypes | Reference | |
|---|---|---|---|---|
| N1437S | ||||
| 1. | Fibroblast cells | Skin biopsies obtained from four healthy, two N1437S PD patients, three G2019S patients and one R1441C patient. | No change was observed in cell adhesion between patient fibroblast and control cells in both basal and kinase inhibited state. | [57] |
| R1441C | ||||
| 2. | DA neurons | Fibroblast cells (from the Northwestern University Biorepository and NINDS human cell biorepository) were reprogrammed to iPSCs and differentiated to DA neurons. | The LRRK2 R1441C mutant depicted a reduction in lysosome specific GCase activity. The knockdown of Rab10 (bona fide substrate of LRRK2) resulted in reduced GCase activity. The R1441C mutant results in an increased level of phospho-Rab10 and over-expression of Rab10 increases the GCase activity. | [59] |
| 3. | Neural cells derived from iPSCs | The iPSC lines are derived from 2 (Twins—brother and sister) heterozygous LRRK2 R1441C (gene from father with PD) carriers. No clinical manifestation of PD in sister, however resting tremor observed in brother. | Lactate dehydrogenase (LDH) enzyme release was used in order to measure neuronal cell vulnerability, MTS assay— LDH/MTS assays, immunocytochemistry and cell counts depicted an increased vulnerability of iPSC derived neural cells from R1441C variant carriers on exposure to chemical stressors (concanamycin A, valinomycin), reduced oxygen consumption and dysfunctional mitochondrial mobility as compared to healthy controls. | [60] |
| 4. | The iPSCs from at risk PD related R1441C variant carriers were obtained from the Coriell BioBank. | Significant mtDNA damage observed in iPSC cell lines differentiated into neural cells using two separate protocols. | [58] | |
| 5. | Fibroblast cells | Skin biopsies obtained from four healthy subjects as well as, two N1437S, three G2019S and one R1441C PD patients. | No change was observed in cell adhesion between patient fibroblast and control cells in both basal and kinase inhibited state. | [57] |
| R1441G | ||||
| 6. | DA neurons | Fibroblast cells (from the Northwestern University Biorepository and NINDS human cell biorepository) were reprogrammed to iPSCs and differentiated to DA neurons. | A reduction in lysosome specific GCase activity was reported. | [59] |
| 7. | Fibroblast from PD patients with R1441G mutation in LRRK2 (n = 2) and matched healthy subjects. Controls include Human embryonic stem cell line (H9), so as to study effects of the lentiviral reprogramming on the cells. | This study was the first to report an iPSC line with the R1441G mutation. α-synuclein levels remained unchanged in the R1441G differentiated mature DA neurons as compared to controls. | [62] | |
| G2019S | ||||
| 8. | Neuro-progenitor cells and mature neural cells derived from iPSCs | Patient-derived fibroblasts are reprogrammed into iPSCs, three homozygous/heterozygous LRRK2 G2019S patient, three age matched healthy subjects w/o LRRK2 mutations | Significant mtDNA (mitochondrial DNA) damage observed in iPSC derived NPC and neural cells with G2019S mutation and not in the fibroblast cells or undifferentiated iPSCs. Indicating that mtDNA damage might be a neural specific phenotype. | [58] |
| 9. | Neural cells derived from iPSCs | The iPSC lines from 2 heterozygous R1441C variant carrier, 1 homozygous PD patient and healthy controls were used in this study | Lactate dehydrogenase (LDH) enzyme release was used in order to measure neuronal cell vulnerability, The G2019S mutant cells showed increased vulnerability to valinomycin, concanamycin and MPP+ (selected stressors directed to assess mitochondrial function or protein degradation) in neural cells in comparison to control fibroblast cells. | [60] |
| 10. | Neurons differentiated from iPSCs | Fibroblast from two patients harboring the LRRK2 G2019S mutations reprogrammed into iPSCs, further, differentiated into neurons. Nonisogenic healthy controls iPSCs were generated from healthy women. | The authors reported a prominent dysregulation by means of alterations in gene expression of CPNE8 (role in calcium mediated intracellular processes), MAP7 (involved in microtubule dynamics), UHRF2 (involved in cell-cycle regulation), ANXA1 (vital role in phospholipid binding), CADPS2 (involved in exocytosis of vesicles with neurotransmitters/neuropeptides), in differentiated neurons out of which 4 genes are known contributors of DA neurodegeneration. The mutation also results in an increase in extracellular signal regulated kinase 1/2 (ERK) phosphorylation | [97] |
| 11. | PD patient LRRK2 heterozygous G2019S iPSCs derived neurons and tested against control. | The study concludes that the LRRK2 plays a pivotal role in the Endoplasmic reticulum (ER) Ca2+ homeostasis, the upregulated kinase activity of the G2019S mutant. Alteration of calcium homeostasis along with ER stressors potentially result in the neurite collapse. | [142] | |
| 12. | Midbrain DA neurons derived from iPSCs | Fibroblast cells (from the Northwestern University Biorepository and NINDS human cell biorepository) were reprogrammed to iPSCs and differentiated to DA neurons. | Significant a reduction in the GCase activity of LRRK2 G2019S iPSC neuronal cells in comparison to the healthy controls, which was reversed on correction of the mutation using CRISPR-Cas9. | [59] |
| 13. | Fibroblast from 2 PD patients with G2019S mutation in LRRK2 and matched healthy subjects. Controls include Human embryonic stem cell like cells (H9) | α-synuclein levels were two-fold higher in the G2019S differentiated mature DA neurons than controls. Marked increase in autophagic mediator p62 (responsible for clearance of α-synuclein). The G2019S mutant results in an impaired NF-κB signaling and the gene transcription of NF-κB was altered in LRRK2 knockdown experiments. | [62] | |
| 14. | G2019S iPSCs generated from dermal fibroblast cells. Further differentiated into midbrain DA neurons. | No difference was observed between differentiated and undifferentiated iPSC cell from the G2019S and WT iPSCs and hESCs in terms of pluripotency markers, general morphology, differentiation potential, epigenetics and gene expression profiles. G2019S affected neurons displayed higher than normal expression of stress response genes altered α-synuclein proteins accumulations and increased vulnerability of neurons to neurotoxins (compared to unaffected controls). | [99] | |
| 15. | iPSCs- derived DA, glutaminergic and sensory neurons | Human iPSCs were obtained from two homozygous LRRK2 G2019S patient cells, one heterozygous G2019S carrier and three healthy controls from publicly available samples from NINDS Coriell Institute. | The study indicates that the G2019S results in an impaired mitochondrial respiration pattern in the iPSC derived DA and glutaminergic neurons, however, sensory neurons showed no such trends. The G2019S iPSC derived DA neurons displayed alternate mitochondrial distribution and trafficking patterns as well as cell specific bio-energetic modifications caused by reduced NAD+ and sirturin deacetylatse which is quite indicative of a disease specific phenotype. | [100] |
| 16. | Neuro-epithelial cells (NESCs) | The study looked at thirteen human iPSC-derived NESC lines obtained from three patients carrying the LRRK2-G2019S mutation with four healthy individuals (age/gender matched). Four isogenic NESC lines were generated, two of which had a mutation introduced and the other two in which the mutation was corrected. | Patient NESC have an increased number of fragmented mitochondria, reduced membrane potential and reduced mitophagic clearance via lysosomes in comparison to isogenic control lines. | [102] |
| 17. | Astrocytes | A Co-culture of iPSC derived astrocytes and ventral midbrain DA neurons (vmDAns) from familial G2019S PD patients | Chemical enhancement of Chaperone Mediated autophagy (CMA) protected PD astrocytes and vmDAns via clearance of α-synuclein accumulations. Non-cell autonomous contribution of astrocytes during PD pathogenesis. Possibility of exploring a therapeutic strategy of blocking pathogenic cross talk between neurons and glial cells. Dysfunctional CMA (chaperone mediated autophagy), impaired macroautophagy, progressive α-synuclein accumulation. | [107] |
| 18. | Fibroblast cells | 3 skin fibroblast groups - 1) Control (Patients who did not develop PD) 2) Idiopathic PD—IPD (Patients without G2019S PD) 3) G2019S PD patients The overall goal of the study is to examine variation in the levels of histone acetyltransferase (HAT) and histone deacetylase (HDAC), described as the key players of autophagy. | The fibroblasts from G2019S cells display an increased clearance of defective mitochondria where as those of IPD show a reduction of mitophagy and increased ROS. The acetylation proteins between the two groups vary, however, HDAC activity is reduced in IPD cells (HAT activity unchanged), the imbalance of autophagy enzymes leads to cell death. The inhibition of the HATs in IPD cell lines could be considered as cyto-protective in this case. | [103] |
| 19. | Skin biopsies collected from 5 individuals of confirmed G2019S PD diagnosis, results compared to 5 healthy age matched controls. | 1. Mitochondrial membrane potential (decrease by 45%) and ATP levels are reduced in LRRK2 mutant fibroblast cells 2. Increased elongation and interconnectivity of mitochondria in LRRK2 mutant patient cells. | [105] | |
| 20. | Skin biopsies donated by four G2019S PD patients and four healthy controls. | Lysosomes were enlarged/swollen, through microscopy they could be characterized by large translucent areas and clustered around the nucleus in G2019S patient-derived fibroblasts. These defects were reversed by inhibition of kinase activity using LRRK2In1, silencing of TPC2 (Two pore channels—acidic vesicles that comprise the endolysosomal system) and pharmacological inhibition of TPC regulators [Rab7, NAADP and PtdIns (3,5)P2] crucial to autophagy. | [109] | |
| 21. | Fibroblasts cultured from punch skin biopsies obtained from nine presymptomatic PD G2019S mutation carriers and age matched healthy controls | A reduction in the secretion of Progranulin (PGRN) was observed in supernatants of cultured human fibroblasts isolated from presymptomatic LRRK2 (G2019S) mutation carriers, and mitochondrial function remained unaffected. | [110] | |
| 22. | Fibroblast cells were obtained via skin biopsies from LRRK2 G2019S mutation carriers both with and without PD as well as healthy controls. | Cells from the G2019S mutant carriers w/o symptoms show an enhanced mitochondrial performance as well as upregulated autophagic flux in comparison to healthy controls and G2019S patients. Therefore, the hyperactivity of mitochondrial complex as well as autophagy could be the driving force for carriers to develop symptoms. | [106] | |
| 23. | Two separate (1 male, 1 female) LRRK2 G2019S PD patient fibroblast cell lines were obtained from the Coriell biorepository | Amplified mitophagy is observed in patient fibroblasts as compared to healthy controls, this is further supported by the evidence indicating a significant loss of mitochondrial membrane potential, reduction in mitochondrial mass and reduced citrate synthase activity accompanied by increased autophagic flux. These results indicate that G2019S mutant accelerates autophagy in cells. | [104] | |
| 24. | Skin biopsies were obtained from four healthy, two N1437S PD patients, three G2019S patients and one R1441C patient. | The G2019S mutant did not result in any change in cell adhesion patterns in fibroblast cells as compared to healthy control fibroblasts, neither in basal conditions nor in conditions of inhibition of LRRK2 kinase activity. | [57] | |
| 25. | Skin biopsies from three PD G2019S patients and four healthy individuals were obtained | An increase in kinase activity and autophagic flux in the mutant fibroblast cells was observed. However, MEK/ERK (MAPK signaling pathway) inhibition reduced the autophagic flux and sensitivity of the G2019S mutants in comparison to healthy controls. | [143] | |
| 26. | Skin biopsies obtained from G2019S mutation carriers (not directly related to each other) without clinical symptoms of PD. Control fibroblasts obtained from age/gender matched controls (n = 5) | Increased sensitivity of LRRK2 mutant fibroblasts to LatA (drug known to disrupt microfilament organization, cell shape [144]). They also observe a significant increase in F-Actin bundles with a decrease in filopodial length. This depicts a pattern of LRRK2 mutant dependent alteration of F-Actin dynamics. | [111] | |
| 27. | Lymphoblastoid cells (LCLs) | LCLs samples from six patients with heterozygous G2019S mutation, thirteen sporadic PD patients and thirteen unrelated gender/age matched controls. For the study additionally three gender/age matched LCL controls, six gender/age matched LCLs from heterozygous G2019S LRRK2 patients were obtained from the NINDS Coriell Cell Repository. | The authors concluded that the G2019S LCLs and a subset of LCLs derived from sporadic PD patients displayed a centrosomal cohesion deficit, reverted by LRRK2 kinase inhibition. | [114] |
| 28. | Endogenous LRRK2 activity was studied in EBV-transformed LCLs derived from a PD patient with homozygous G2019S mutation and one healthy control (no LRRK2 mutations) | LRRK2 levels were similar in both cell lines, however, a three-fold increase in kinase activity was observed in the G2019S mutant. The S910, S935 sites in both the mutant and healthy LCLs were equally phosphorylated. However, on treatment with kinase inhibitors (H-1152, Sunitib), the phosphorylation of S910, S935 was more potently inhibited in the G2019S mutant than the healthy control. | [112] | |
| 29. | Six iPSC line derived LCLs and healthy age matched controls were used for the study (Parkinson institute or NINDS Coriell biorepository). Further, skin biopsies from three G2019S PD patient and three healthy individuals were obtained to study fibroblast cells. | An increase in the mtDNA damage was observed in LCLs of G2019S mutation carriers (with PD) as compared to LCLs from age matched healthy controls. However, no change was observed in mtDNA damage in fibroblast cells obtained from G2019S mutation carriers in comparison to healthy subjects. | [113] | |
| G2019S Organoids | ||||
| 30. | Midbrain floor plate neural progenitor cells (mfNPCs) | mfNPC are differentiated into 2D midbrain DA neurons (mDANs) and 3D human midbrain-specific organoids (hMOs) from PD patients carrying the LRRK2-G2019S mutation. | The expression of DA neuron markers TH, AADC, VMAT2, and DAT are markedly decreased in LRRK2-G2019S organoids as compared to the controls at day 60. Mature neuronal genes such as NURR1, PITX3, EN1, TH, and MAPT are also reduced in the LRRK2-G2019S mutant organoids but not in 2D cultures (where their expression is very low regardless of genotype). The DA neurons in LRRK2-G2019S organoids exhibited a decrease in neurite length. | [115] |
| I2020T | ||||
| 31. | Neurons | I2020T PD patient fibroblast derived iPSCs were reprogrammed to neurons (Japanese Sagamihara kindred) | The I2020T neurons displayed reduced levels of phospho-AKT in comparison to control neurons, with an observable increase in apoptosis. An increase in glycogen synthase kinase-3β (GSK-3β)—a central intracellular regulator of vital functions—proliferation, migration, glucose metabolism) which is implicated in a number of diseases and high tau phosphorylation. Post-mortem histopathological studies reveal deposits of neurofibrillary tangles, increased tau phosphorylation of neurons. | [145] |
| G2294R | ||||
| 32. | Peripheral blood mononuclear cells (PBMC) | Monocytes were isolated from PBMCs of G2294R PD patient and gender matched controls. Further monocytes were differentiated to macrophages | LRRK2 protein levels are increased in LRRK2 G2294R monocytes and reduced in macrophages. Further Rab8 and Rab10 levels were also decreased in macrophages. | [133] |
| G2385R | ||||
| 33. | PBMC | PBMCs from G2385R patients were reprogrammed into iPSC cells and used to generate stable cell lines. | No characterization was reported. | [146] |
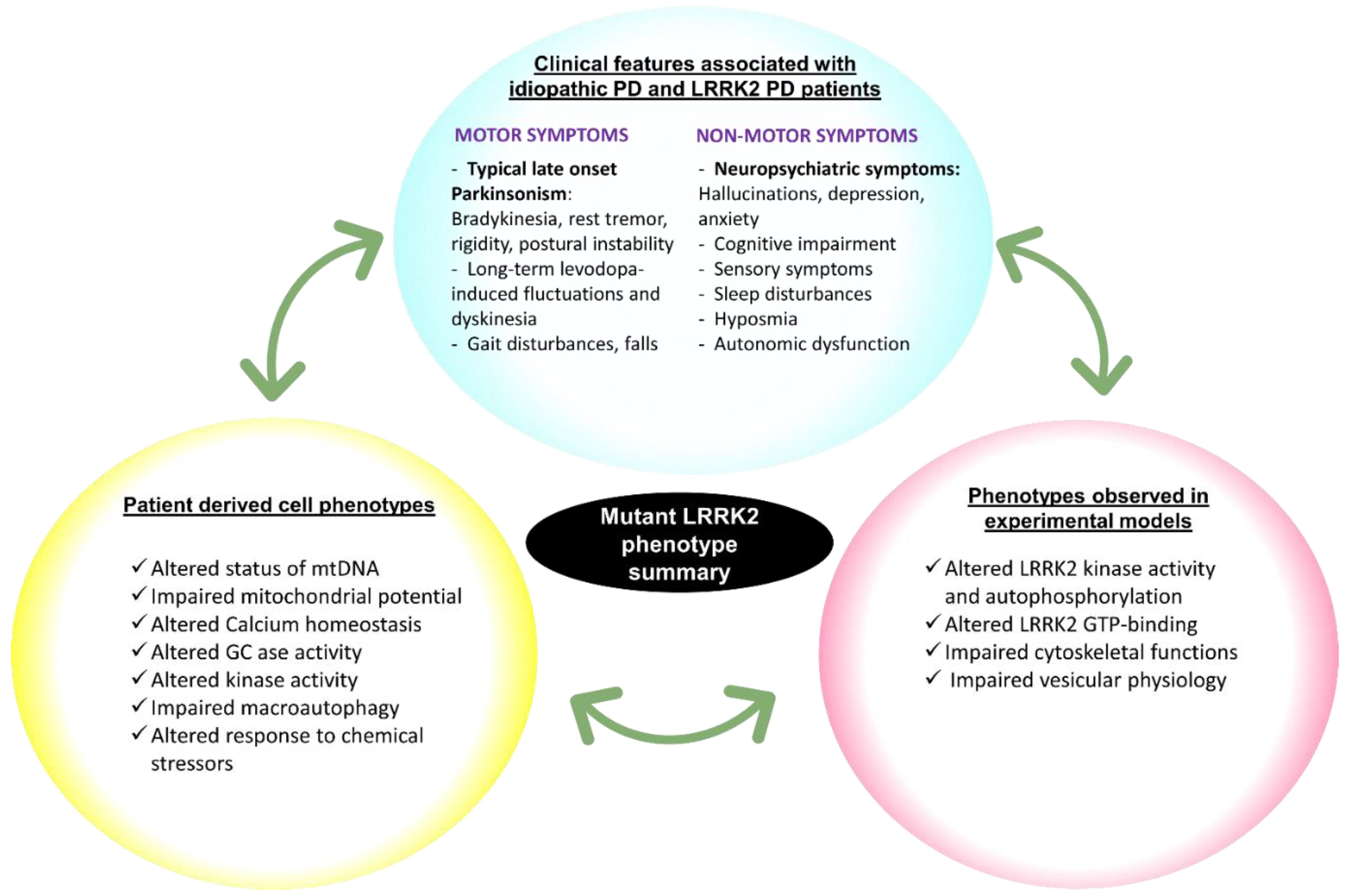
3. Conclusions and Perspectives
- -
- In order to establish better and more quantitative correlations between clinical patient phenotypes and cellular phenotypes of patient-derived cells, there is a need to implement biological marker readouts that are analogous in patients and patient cells.
- -
- Studies of clinical progression of disease are often performed on large cohorts of patients, while studies in patient-derived cell cultures are performed on average using a much lower numbers of patients. It is a challenge to determine what the optimal sample size is in studies with patient-derived cells, the task being to balance the need for high numbers to ensure the robustness of the findings with the limitations in throughput/capacity of research and clinical laboratories.
- -
- Studies are accumulating with cells derived from subjects with coding sequence variants of LRRK2. However little attention has been given thus far to analyzing cells from subjects who carry risk factor variants that do not affect the LRRK2 coding sequence. As these may constitute low risk but more common variants, it would be important to also pursue studies in cells with non-coding risk factor variants of LRRK2.
- -
- The usefulness of each patient-derived cell type in informing on the patient’s disease and/or response to therapy should be addressed. Similarly, situations can be identified where simple and quick protocols such as simple primary cultures relevant as opposed to other situations where more refined and sophisticated protocols are needed such as those involving cell de- and re-differentiation.
- -
- In addition to studies using homogeneous cell cultures of patient-derived cells, more sophisticated culture configurations, such as 3D cultures, co-cultures and organoid cultures should be evaluated for their tractability to provide correlative information about patients’ disease state, treatment options and prognosis.
- -
- In order to obtain PD specific information it is vital to have PD patients derived cells with non-LRRK2 mutations (mutations in other genes or idiopathic forms) as well as healthy controls to compare the baseline variation in the molecular mechanisms of reprogrammed cells among the three cohorts of cells derived. As PD is a progressive neurodegenerative disease it is vital to study the progressive changes in the molecular mechanisms of the patient-derived reprogrammed cells.
- -
- An additional opportunity is to carry out studies on LRRK2 variants that are inversely correlated to risk for PD (the so-called ‘protective’ variants). Such studies have the potential to reveal molecular pathways that could be exploited as a strategy to offer neuroprotection to PD patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray Dorsey, E.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- De Rijk, M.C.; Tzourio, C.; Breteler, M.M.B.; Dartigues, J.F.; Amaducci, L.; Lopez-Pousa, S.; Manubens-Bertran, J.M.; Alperovitch, A.; Rocca, W.A. Prevalence of parkinsonism and Parkinson’s disease in Europe: The EUROPARKINSON collaborative study. J. Neurol. Neurosurg. Psychiatry 1997, 62, 10–15. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Gomperts, S.N. Lewy body dementias: Dementia with Lewy bodies and Parkinson disease dementia. Contin. Lifelong Learn. Neurol. 2016, 22, 435–463. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, T.; Mattila, P.; Davies, P.; Wang, D.; Dickson, D.W. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J. Neuropathol. Exp. Neurol. 2003, 62, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V. Etiology of Parkinson’s disease. Neurology 2006, 66, S10–S23. [Google Scholar] [CrossRef]
- Ramirez, M.B.; Madero-Perez, J.; Rivero-Rios, P.; Martinez-Salvador, M.; Lara Ordonez, A.J.; Fernandez, B.; Fdez, E.; Hilfiker, S. LRRK2 and Parkinson’s Disease: From Lack of Structure to Gain of Function. Curr. Protein Pept. Sci. 2016, 18, 677–686. [Google Scholar] [CrossRef]
- Ross, O.A.; Soto-Ortolaza, A.I.; Heckman, M.G.; Aasly, J.O.; Abahuni, N.; Annesi, G.; Bacon, J.A.; Bardien, S.; Bozi, M.; Brice, A.; et al. Association of LRRK2 exonic variants with susceptibility to Parkinson’s disease: A case-control study. Lancet Neurol. 2011, 10, 898–908. [Google Scholar] [CrossRef]
- Cookson, M.R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 791–797. [Google Scholar] [CrossRef]
- Li, J.Q.; Tan, L.; Yu, J.T. The role of the LRRK2 gene in Parkinsonism. Mol. Neurodegener. 2014, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Versées, W.; Kortholt, A. Roco Proteins: GTPases with a Baroque Structure and Mechanism. Int. J. Mol. Sci. 2019, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Civiero, L.; Vancraenenbroeck, R.; Belluzzi, E.; Beilina, A.; Lobbestael, E.; Reyniers, L.; Gao, F.; Micetic, I.; de Maeyer, M.; Bubacco, L.; et al. Biochemical Characterization of Highly Purified Leucine-Rich Repeat Kinases 1 and 2 Demonstrates Formation of Homodimers. PLoS ONE 2012, 7, e43472. [Google Scholar] [CrossRef]
- Marchand, A.; Drouyer, M.; Sarchione, A.; Chartier-Harlin, M.C.; Taymans, J.M. LRRK2 Phosphorylation, More Than an Epiphenomenon. Front. Neurosci. 2020, 14, 527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mobley, J.A.; Delucas, L.J.; Kahn, R.A.; West, A.B. LRRK2 autophosphorylation enhances its GTPase activity. FASEB J. 2016, 30, 336–347. [Google Scholar] [CrossRef]
- Iannielli, A.; Ugolini, G.S.; Cordiglieri, C.; Bido, S.; Rubio, A.; Colasante, G.; Valtorta, M.; Cabassi, T.; Rasponi, M.; Broccoli, V. Understanding the GTPase activity of LRRK2: Regulation, function, and neurotoxicity. Cell Rep. 2019, 29, 4646–4656.e4. [Google Scholar] [CrossRef] [PubMed]
- Greggio, E. Role of LRRK2 kinase activity in the pathogenesis of Parkinson’s disease. Biochem. Soc. Trans. 2012, 40, 1058–1062. [Google Scholar] [CrossRef]
- Steger, M.; Tonelli, F.; Ito, G.; Davies, P.; Trost, M.; Vetter, M.; Wachter, S.; Lorentzen, E.; Duddy, G.; Wilson, S.; et al. Phosphoproteomics Reveals that Parkinson’s Disease Kinase LRRK2 Regulates a Subset of Rab GTPases. eLife 2016, 5, 1–28. [Google Scholar] [CrossRef]
- Xiong, Y.; Coombes, C.E.; Kilaru, A.; Li, X.; Gitler, A.D.; Bowers, W.J.; Dawson, V.L.; Dawson, T.M.; Moore, D.J. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Lewis, P.A.; Greggio, E.; Beilina, A.; Jain, S.; Baker, A.; Cookson, M.R. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem. Biophys. Res. Commun. 2007, 357, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Greggio, E.; Zambrano, I.; Kaganovich, A.; Beilina, A.; Taymans, J.M.; Daniëls, V.; Lewis, P.; Jain, S.; Ding, J.; Syed, A.; et al. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J. Biol. Chem. 2008, 283, 16906–16914. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.L.; Rovelli, G.; Springer, W.; Schall, C.; Gasser, T.; Kahle, P.J. Homo- and heterodimerization of ROCO kinases: LRRK2 kinase inhibition by the LRRK2 ROCO fragment. J. Neurochem. 2009, 111, 703–715. [Google Scholar] [CrossRef]
- Daniẽls, V.; Vancraenenbroeck, R.; Law, B.M.; Greggio, E.; Lobbestael, E.; Gao, F.; De Maeyer, M.; Cookson, M.R.; Harvey, K.; Baekelandt, V.; et al. Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant. J. Neurochem. 2011, 116, 304–315. [Google Scholar] [CrossRef]
- Taymans, J.-M.; Nkiliza, A.; Chartier-Harlin, M.-C. Deregulation of protein translation control, a potential game-changing hypothesis for Parkinson’s disease pathogenesis. Trends Mol. Med. 2015, 21, 466–472. [Google Scholar] [CrossRef]
- Cook, D.A.; Kannarkat, G.T.; Cintron, A.F.; Butkovich, L.M.; Fraser, K.B.; Chang, J.; Grigoryan, N.; Factor, S.A.; West, A.B.; Boss, J.M.; et al. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Park. Dis. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Rideout, H.J.; Stefanis, L. The Neurobiology of LRRK2 and its Role in the Pathogenesis of Parkinson’s Disease. Neurochem. Res. 2014, 39, 576–592. [Google Scholar] [CrossRef]
- Tomkins, J.E.; Dihanich, S.; Beilina, A.; Ferrari, R.; Ilacqua, N.; Cookson, M.R.; Lewis, P.A.; Manzoni, C. Comparative Protein Interaction Network Analysis Identifies Shared and Distinct Functions for the Human ROCO Proteins. Proteomics 2018, 18, 1700444. [Google Scholar] [CrossRef] [PubMed]
- Iannielli, A.; Ugolini, G.S.; Cordiglieri, C.; Bido, S.; Rubio, A.; Colasante, G.; Valtorta, M.; Cabassi, T.; Rasponi, M.; Broccoli, V. In silico and Wet Bench Interactomics Sheds Light on the Similitudes and Differences between Human ROCO Proteins. Cell Rep. 2019, 29, 4646–4656.e4. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.A.S.; Dave, K.D.; Frasier, M.A.; Sherer, T.B.; Greeley, M.; Beck, M.J.; Varsho, J.S.; Parker, G.A.; Moore, C.; Churchill, M.J.; et al. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS ONE 2013, 8, e80705. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yamaguchi, H.; Giaime, E.; Boyle, S.; Kopan, R.; Kelleher, R.J.; Shen, J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of α-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. USA 2010, 107, 9879–9884. [Google Scholar] [CrossRef]
- Iannielli, A.; Ugolini, G.S.; Cordiglieri, C.; Bido, S.; Rubio, A.; Colasante, G.; Valtorta, M.; Cabassi, T.; Rasponi, M.; Broccoli, V.; et al. LRRK2 at the Interface between Peripheral and Central Immune Function in Parkinson’s; Frontiers Media S.A.: Lausanne, Switzerland, 2020; Volume 14, p. 443. [Google Scholar]
- Andres-Mateos, E.; Mejias, R.; Sasaki, M.; Li, X.; Lin, B.M.; Biskup, S.; Zhang, L.; Banerjee, R.; Thomas, B.; Yang, L.; et al. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). J. Neurosci. 2009, 29, 15846–15850. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, J.; Krummey, S.; Lu, W.; Cai, H.; Lenardo, M.J. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat. Immunol. 2011, 12, 1063–1070. [Google Scholar] [CrossRef]
- Lebovitz, C.; Wretham, N.; Osooly, M.; Milne, K.; Dash, T.; Thornton, S.; Tessier-Cloutier, B.; Sathiyaseelan, P.; Bortnik, S.; Go, N.E.; et al. Loss of Parkinson’s susceptibility gene LRRK2 promotes carcinogen-induced lung tumorigenesis. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Daher, J.P.L.; Volpicelli-Daley, L.A.; Blackburn, J.P.; Moehle, M.S.; West, A.B. Abrogation of α-synuclein -mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl. Acad. Sci. USA 2014, 111, 9289–9294. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef]
- Pickrell, J.K.; Berisa, T.; Liu, J.Z.; Ségurel, L.; Tung, J.Y.; Hinds, D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016, 48, 709–717. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Saez-Atienzar, S.; Bonet-Ponce, L.; Billingsley, K.; Vitale, D.; Blauwendraat, C.; Gibbs, J.R.; Pihlstrøm, L.; Gan-Or, Z.; Noyce, A.J.; et al. The endocytic membrane trafficking pathway plays a major role in the risk of Parkinson’s disease. Mov. Disord. 2019, 34, 460–468. [Google Scholar] [CrossRef]
- Simón-Sánchez, J.; Schulte, C.; Bras, J.M.; Sharma, M.; Gibbs, J.R.; Berg, D.; Paisan-Ruiz, C.; Lichtner, P.; Scholz, S.W.; Hernandez, D.G.; et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009, 41, 1308–1312. [Google Scholar] [CrossRef]
- Parisiadou, L.; Xie, C.; Hyun, J.C.; Lin, X.; Gu, X.L.; Long, C.X.; Lobbestael, E.; Baekelandt, V.; Taymans, J.M.; Sun, L.; et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 2009, 29, 13971–13980. [Google Scholar] [CrossRef]
- Tan, E.K.; Peng, R.; Teo, Y.Y.; Tan, L.C.; Angeles, D.; Ho, P.; Chen, M.L.; Lin, C.H.; Mao, X.Y.; Chang, X.L.; et al. Multiple LRRK2 variants modulate risk of Parkinson disease: A Chinese multicenter study. Hum. Mutat. 2010, 31, 561–568. [Google Scholar] [CrossRef]
- Rudenko, I.N.; Cookson, M.R. Heterogeneity of Leucine-Rich Repeat Kinase 2 Mutations: Genetics, Mechanisms and Therapeutic Implications. Neurotherapeutics 2014, 11, 738–750. [Google Scholar] [CrossRef]
- Tolosa, E.; Vila, M.; Klein, C.; Rascol, O. LRRK2 in Parkinson disease: Challenges of clinical trials. Nat. Rev. Neurol. 2020, 16, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.R. LRRK2 Pathways Leading to Neurodegeneration. Curr. Neurol. Neurosci. Rep. 2015, 15, 42. [Google Scholar] [CrossRef]
- Kishore, A.; Ashok Kumar Sreelatha, A.; Sturm, M.; von-Zweydorf, F.; Pihlstrøm, L.; Raimondi, F.; Russell, R.; Lichtner, P.; Banerjee, M.; Krishnan, S.; et al. Understanding the role of genetic variability in LRRK2 in Indian population. Mov. Disord. 2019, 34, 496–505. [Google Scholar] [CrossRef]
- Iannielli, A.; Ugolini, G.S.; Cordiglieri, C.; Bido, S.; Rubio, A.; Colasante, G.; Valtorta, M.; Cabassi, T.; Rasponi, M.; Broccoli, V.; et al. Clinical and pathological characteristics of patients with leucine-rich repeat kinase-2 mutations. Mov. Disord. 2009, 24, 32–39. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Liu, Y.; Hong, H.; Wang, H.; Zheng, Y.; Zhou, H.; Chen, J.; Xian, W.; He, Y.; et al. LRRK2 R1398H polymorphism is associated with decreased risk of Parkinson’s disease in a Han Chinese population. Park. Relat. Disord. 2011, 17, 291–292. [Google Scholar] [CrossRef]
- Puschmann, A.; Englund, E.; Ross, O.A.; Vilariño-Güell, C.; Lincoln, S.J.; Kachergus, J.M.; Cobb, S.A.; Törnqvist, A.L.; Rehncrona, S.; Widner, H.; et al. First neuropathological description of a patient with Parkinson’s disease and LRRK2 p.N1437H mutation. Park. Relat. Disord. 2012, 18, 332–338. [Google Scholar] [CrossRef]
- Haugarvoll, K.; Rademakers, R.; Kachergus, J.M.; Nuytemans, K.; Ross, O.A.; Gibson, J.M.; Tan, E.K.; Gaig, C.; Tolosa, E.; Goldwurm, S.; et al. Lrrk2 R1441C parkinsonism is clinically similar to sporadic Parkinson disease. Neurology 2008, 70, 1456–1460. [Google Scholar] [CrossRef]
- Simón-Sánchez, J.; Martí-Massó, J.-F.; Sánchez-Mut, J.V.; Paisán-Ruiz, C.; Martínez-Gil, A.; Ruiz-Martínez, J.; Sáenz, A.; Singleton, A.B.; López de Munain, A.; Pérez-Tur, J. Parkinson’s disease due to the R1441G mutation in Dardarin: A founder effect in the basques. Mov. Disord. 2006, 21, 1954–1959. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Gorostidi, A.; Ibañez, B.; Alzualde, A.; Otaegui, D.; Moreno, F.; De Munain, A.L.; Bergareche, A.; Gómez-Esteban, J.C.; Massó, J.F.M. Penetrance in Parkinson’s disease related to the LRRK2 R1441G mutation in the Basque country (Spain). Mov. Disord. 2010, 25, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Funayama, M.; Kubo, S.I.; Mata, I.F.; Oji, Y.; Mori, A.; Zabetian, C.P.; Waldherr, S.M.; Yoshino, H.; Oyama, G.; et al. Identification of a Japanese family with LRRK2 p.R1441G-related Parkinson’s disease. Neurobiol. Aging 2014, 35, 2656.e17–2656.e23. [Google Scholar] [CrossRef]
- Fan, Y.; Nirujogi, R.S.; Garrido, A.; Martínez, J.R.; Bergareche-yarza, A.; Rezola, E.M.; Aragón, A.V.; Croitoru, I.; Pagola, A.G.; Markinez, P.; et al. R1441G but not G201S mutation enhances LRRK2 mediated Rab10 phosphorylation in human peripheral blood neutrophils. medRxiv 2021. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Guedes, L.C.; Rosa, M.M.; Coelho, M.; Van Doeselaar, M.; Schweiger, D.; Di Fonzo, A.; Oostra, B.A.; Sampaio, C.; Bonifati, V. High prevalence of LRRK2 mutations in familial and sporadic Parkinson’s disease in Portugal. Mov. Disord. 2007, 22, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.F.; Davis, M.Y.; Lopez, A.N.; Dorschner, M.O.; Martinez, E.; Yearout, D.; Cholerton, B.A.; Hu, S.C.; Edwards, K.L.; Bird, T.D.; et al. The discovery of LRRK2 p.R1441S, a novel mutation for Parkinson’s disease, adds to the complexity of a mutational hotspot. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 925–930. [Google Scholar] [CrossRef]
- Garcia-Miralles, M.; Coomaraswamy, J.; Häbig, K.; Herzig, M.C.; Funk, N.; Gillardon, F.; Maisel, M.; Jucker, M.; Gasser, T.; Galter, D.; et al. No Dopamine Cell Loss or Changes in Cytoskeleton Function in Transgenic Mice Expressing Physiological Levels of Wild Type or G2019S Mutant LRRK2 and in Human Fibroblasts. PLoS ONE 2015, 10, e0118947. [Google Scholar] [CrossRef]
- Sanders, L.; Laganière, J.; Cooper, O.S.M.-N. LRRK2 Mutations Cause Mitochondrial DNA Damage in iPSC-Derived Neural Cells from Parkinson’s Disease Patients: Reversal by Gene Correction; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ysselstein, D.; Nguyen, M.; Young, T.J.; Severino, A.; Schwake, M.; Merchant, K.; Krainc, D. LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson’s disease patients. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Cooper, O.; Seo, H.; Andrabi, S.; Guardia-Laguarta, C.; Graziotto, J.; Sundberg, M.; McLean, J.R.; Carrillo-Reid, L.; Xie, Z.; Osborn, T.; et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Bahnassawy, L.; Nicklas, S.; Palm, T.; Menzl, I.; Birzele, F.; Gillardon, F.; Schwamborn, J.C. The Parkinson’s disease-associated LRRK2 mutation R1441G inhibits neuronal differentiation of neural stem cells. Stem. Cells Dev. 2013, 22, 2487–2496. [Google Scholar] [CrossRef]
- López de Maturana, R.; Lang, V.; Zubiarrain, A.; Sousa, A.; Vázquez, N.; Gorostidi, A.; Águila, J.; López de Munain, A.; Rodríguez, M.; Sánchez-Pernaute, R. Mutations in LRRK2 impair NF-κB pathway in iPSC-derived neurons. J. Neuroinflammation 2016, 13, 295. [Google Scholar] [CrossRef]
- Nixon-Abell, J.; Berwick, D.C.; Grannó, S.; Spain, V.A.; Blackstone, C.; Harvey, K. Protective LRRK2 R1398H variant enhances GTPase and Wnt signaling activity. Front. Mol. Neurosci. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, C.; Park, Y.; Long, X.; Hoang, Q.Q.; Liao, J. The Parkinson’s disease–associated mutation N1437H impairs conformational dynamics in the G domain of LRRK2. FASEB J. 2019, 33, 4814–4823. [Google Scholar] [CrossRef]
- Ramírez, M.B.; Ordóñez, A.J.L.; Fdez, E.; Madero-Pérez, J.; Gonnelli, A.; Drouyer, M.; Chartier-Harlin, M.C.; Taymans, J.M.; Bubacco, L.; Greggio, E.; et al. GTP binding regulates cellular localization of Parkinson’s disease-associated LRRK2. Hum. Mol. Genet. 2017, 26, 2747–2767. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.J.; Dzamko, N.; Morrice, N.A.; Campbell, D.G.; Deak, M.; Ordureau, A.; Macartney, T.; Tong, Y.; Shen, J.; Prescott, A.; et al. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 2010, 430, 393–404. [Google Scholar] [CrossRef]
- Lobbestael, E.; Zhao, J.; Rudenko, I.N.; Beylina, A.; Gao, F.; Wetter, J.; Beullens, M.; Bollen, M.; Cookson, M.R.; Baekelandt, V.; et al. Identification of protein phosphatase 1 as a regulator of the LRRK2 phosphorylation cycle. Biochem. J. 2013, 456, 119–128. [Google Scholar] [CrossRef]
- Tong, Y.; Pisani, A.; Martella, G.; Karouani, M.; Yamaguchi, H.; Pothos, E.N.; Shen, J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 14622–14627. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Oo, T.F.; Wang, L.; Tang, Y.; Jackson-Lewis, V.; Zhou, C.; Geghman, K.; Bogdanov, M.; Przedborski, S.; et al. Mutant LRRK2R1441G BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat. Neurosci. 2009, 12, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wu, C.-X.; Burlak, C.; Zhang, S.; Sahm, H.; Wang, M.; Zhang, Z.-Y.; Vogel, K.W.; Federici, M.; Riddle, S.M.; et al. Parkinson disease-associated mutation R1441H in LRRK2 prolongs the “active state” of its GTPase domain. Proc. Natl. Acad. Sci. USA 2014, 111, 4055–4060. [Google Scholar] [CrossRef]
- Oosterveld, L.P.; Allen, J.C.; Ng, E.Y.L.; Seah, S.H.; Tay, K.Y.; Au, W.L.; Tan, E.K.; Tan, L.C.S. Greater motor progression in patients with Parkinson disease who carry LRRK2 risk variants. Neurology 2015, 85, 1039–1042. [Google Scholar] [CrossRef]
- Sosero, Y.L.; Yu, E.; Krohn, L.; Rudakou, U.; Mufti, K.; Ruskey, J.A.; Asayesh, F.; Laurent, S.B.; Spiegelman, D.; Fahn, S.; et al. LRRK2 p.M1646T is associated with glucocerebrosidase activity and with Parkinson’s disease. Neurobiol. Aging 2021. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.L.; Jain, S.; Lynch, J.M.; Pavese, N.; Abou-Sleiman, P.; Holton, J.L.; Healy, D.G.; Gilks, W.P.; Sweeney, M.G.; Ganguly, M.; et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: Clinical, pathological, olfactory and functional imaging and genetic data. Brain 2005, 128, 2786–2796. [Google Scholar] [CrossRef]
- Nicholl, D.J.; Vaughan, J.R.; Khan, N.L.; Ho, S.L.; Aldous, D.E.W.; Lincoln, S.; Farrer, M.; Gayton, J.D.; Davis, M.B.; Piccini, P.; et al. Two large British kindreds with familial Parkinson’s disease: A clinico-pathological and genetic study. Brain 2002, 125, 44–57. [Google Scholar] [CrossRef][Green Version]
- Lorenzo-Betancor, O.; Samaranch, L.; Ezquerra, M.; Tolosa, E.; Lorenzo, E.; Irigoyen, J.; Gaig, C.; Pastor, M.A.; Soto-Ortolaza, A.I.; Ross, O.A.; et al. LRRK2 haplotype-sharing analysis in Parkinson’s disease reveals a novel p.S1761R mutation. Mov. Disord. 2012, 27, 146–150. [Google Scholar] [CrossRef]
- Mata, I.F.; Alvarez, V.; Ribacoba, R.; Infante, J.; Sierra, M.; Gómez-Garre, P.; Mir, P.; Waldherr, S.; Yearout, D.; Zabetian, C.P. Novel Lrrk2-p.S1761R mutation is not a common cause of Parkinson’s disease in Spain. Mov. Disord. 2013, 28, 248. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ng, S.H.; Zeng, L.; Zhao, Y.; Tan, E.K. Generation of a human induced pluripotent stem cell (iPSC) line carrying the Parkinson’s disease linked LRRK2 variant S1647T. Stem Cell Res. 2017, 18, 54–56. [Google Scholar] [CrossRef]
- West, A.B.; Moore, D.J.; Choi, C.; Andrabi, S.A.; Li, X.; Dikeman, D.; Biskup, S.; Zhang, Z.; Lim, K.L.; Dawson, V.L.; et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 2007, 16, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dächsel, J.C.; Behrouz, B.; Yue, M.; Beevers, J.E.; Melrose, H.L.; Farrer, M.J. A comparative study of Lrrk2 function in primary neuronal cultures. Park. Relat. Disord. 2010, 16, 650–655. [Google Scholar] [CrossRef]
- Correia Guedes, L.; Ferreira, J.J.; Rosa, M.M.; Coelho, M.; Bonifati, V.; Sampaio, C. Worldwide frequency of G2019S LRRK2 mutation in Parkinson’s disease: A systematic review. Park. Relat. Disord. 2010, 16, 237–242. [Google Scholar] [CrossRef]
- Clark, L.N.; Wang, Y.; Karlins, E.; Saito, L.; Mejia-Santana, H.; Harris, J.; Louis, E.D.; Cote, L.J.; Andrews, H.; Fahn, S.; et al. Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology 2006, 67, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Kachergus, J.; Mata, I.F.; Hulihan, M.; Taylor, J.P.; Lincoln, S.; Aasly, J.; Gibson, J.M.; Ross, O.A.; Lynch, T.; Wiley, J.; et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: Evidence of a common founder across European populations. Am. J. Hum. Genet. 2005, 76, 672–680. [Google Scholar] [CrossRef]
- Mata, I.F.; Kachergus, J.M.; Taylor, J.P.; Lincoln, S.; Aasly, J.; Lynch, T.; Hulihan, M.M.; Cobb, S.A.; Wu, R.M.; Lu, C.S.; et al. Lrrk2 pathogenic substitutions in Parkinson’s disease. Neurogenetics 2005, 6, 171–177. [Google Scholar] [CrossRef]
- Healy, D.G.; Falchi, M.; O’Sullivan, S.S.; Bonifati, V.; Durr, A.; Bressman, S.; Brice, A.; Aasly, J.; Zabetian, C.P.; Goldwurm, S.; et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 2008, 7, 583–590. [Google Scholar] [CrossRef]
- Bonifati, V. Genetics of Parkinson’s disease. Minerva Med. 2005, 96, 175–186. [Google Scholar] [PubMed]
- Poulopoulos, M.; Levy, O.A.; Alcalay, R.N. The neuropathology of genetic Parkinson’s disease. Mov. Disord. 2012, 27, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Funayama, M.; Hasegawa, K.; Kowa, H.; Saito, M.; Tsuji, S.; Obata, F. A new locus for Parkinson’s Disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 2002, 51, 296–301. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E.; Hazrati, L.N.; Fujioka, S.; Wszolek, Z.K.; Dickson, D.W.; Ross, O.A.; Van Deerlin, V.M.; Trojanowski, J.Q.; Hurtig, H.I.; et al. Clinical correlations with lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015, 72, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ben Romdhan, S.; Farhat, N.; Nasri, A.; Lesage, S.; Hdiji, O.; Ben Djebara, M.; Landoulsi, Z.; Stevanin, G.; Brice, A.; Damak, M.; et al. LRRK2 G2019S Parkinson’s disease with more benign phenotype than idiopathic. Acta Neurol. Scand. 2018, 138, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Lanz, T.A.; Gorman, D.; Wolfe, M.; Joyce, A.; Cabrera, C.; Lawrence-Henderson, R.; Levers, N.; Joshi, N.; Ma, T.C.; et al. An Assessment of LRRK2 Serine 935 Phosphorylation in Human Peripheral Blood Mononuclear Cells in Idiopathic Parkinson’s Disease and G2019S LRRK2 Cohorts. J. Parkinsons. Dis. 2020, 10, 623–629. [Google Scholar] [CrossRef]
- Fraser, K.B.; Moehle, M.S.; Alcalay, R.N.; West, A.B. Urinary LRRK2 phosphorylation predicts parkinsonian phenotypes in G2019S LRRK2 carriers. Neurology 2016, 86, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Virreira Winter, S.; Karayel, O.; Strauss, M.T.; Padmanabhan, S.; Surface, M.; Merchant, K.; Alcalay, R.N.; Mann, M. Urinary proteome profiling for stratifying patients with familial Parkinson’s disease. EMBO Mol. Med. 2021, e13257. [Google Scholar] [CrossRef]
- Funayama, M.; Hasegawa, K.; Ohta, E.; Kawashima, N.; Komiyama, M.; Kowa, H.; Tsuji, S.; Obata, F. An LRRK2 mutation as a cause for the Parkinsonism in the original PARK8 family. Ann. Neurol. 2005, 57, 918–921. [Google Scholar] [CrossRef]
- Nukada, H.; Kowa, H.; Saitoh, T.; Tazaki, Y.; Miura, S. A big family of paralysis agitans (author’s transl). Clin. Neurol. 1978, 18, 627–634. [Google Scholar]
- Hasegawa, K.; Stoessl, A.J.; Yokoyama, T.; Kowa, H.; Wszolek, Z.K.; Yagishita, S. Familial parkinsonism: Study of original Sagamihara PARK8 (I2020T) kindred with variable clinicopathologic outcomes. Park. Relat. Disord. 2009, 15, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, S.; Hatano, T.; Kubo, S.I.; Imai, S.; Sato, S.; Uchihara, T.; Yagishita, S.; Hasegawa, K.; Kowa, H.; Sakai, F.; et al. LRRK2 I2020T mutation is associated with tau pathology. Park. Relat. Disord. 2012, 18, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, P.; Schmid, B.; Burbulla, L.F.; Schöndorf, D.C.; Wagner, L.; Glatza, M.; Höing, S.; Hargus, G.; Heck, S.A.; Dhingra, A.; et al. Genetic correction of a lrrk2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell Stem Cell 2013, 12, 354–367. [Google Scholar] [CrossRef]
- Korecka, J.A.; Thomas, R.; Christensen, D.P.; Hinrich, A.J.; Ferrari, E.J.; Levy, S.A.; Hastings, M.L.; Hallett, P.J.; Isacson, O. Mitochondrial clearance and maturation of autophagosomes are compromised in LRRK2 G2019S familial Parkinson’s disease patient fibroblasts. Hum. Mol. Genet. 2019, 28, 3232–3243. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Byers, B.; Cord, B.; Shcheglovitov, A.; Byrne, J.; Gujar, P.; Kee, K.; Schüle, B.; Dolmetsch, R.E.; Langston, W.; et al. LRRK2 mutant iPSC-derived da neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 2011, 8, 267–280. [Google Scholar] [CrossRef]
- Schwab, A.J.; Sison, S.L.; Meade, M.R.; Broniowska, K.A.; Corbett, J.A.; Ebert, A.D. Decreased Sirtuin Deacetylase Activity in LRRK2 G2019S iPSC-Derived Dopaminergic Neurons. Stem Cell Rep. 2017, 9, 1839–1852. [Google Scholar] [CrossRef]
- Iannielli, A.; Ugolini, G.S.; Cordiglieri, C.; Bido, S.; Rubio, A.; Colasante, G.; Valtorta, M.; Cabassi, T.; Rasponi, M.; Broccoli, V.; et al. LRRK2 and mitochondria: Recent advances and current views. Brain Res. 2019, 1702, 96–104. [Google Scholar] [CrossRef]
- Walter, J.; Bolognin, S.; Antony, P.M.A.; Nickels, S.L.; Poovathingal, S.K.; Salamanca, L.; Magni, S.; Perfeito, R.; Hoel, F.; Qing, X.; et al. Neural Stem Cells of Parkinson’s Disease Patients Exhibit Aberrant Mitochondrial Morphology and Functionality. Stem Cell Rep. 2019, 12, 878–889. [Google Scholar] [CrossRef]
- Yakhine-Diop, S.M.S.; Niso-Santano, M.; Rodríguez-Arribas, M.; Gómez-Sánchez, R.; Martínez-Chacón, G.; Uribe-Carretero, E.; Navarro-García, J.A.; Ruiz-Hurtado, G.; Aiastui, A.; Cooper, J.M.; et al. Impaired Mitophagy and Protein Acetylation Levels in Fibroblasts from Parkinson’s Disease Patients. Mol. Neurobiol. 2019, 56, 2466–2481. [Google Scholar] [CrossRef]
- Su, Y.C.; Guo, X.; Qi, X. Threonine 56 phosphorylation of Bcl-2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy. Biochim. Biophys. Acta—Mol. Basis Dis. 2015, 1852, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mortiboys, H.; Johansen, K.K.; Aasly, J.O.; Bandmann, O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology 2010, 75, 2017–2020. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Flores, D.L.; González-Casacuberta, I.; Ezquerra, M.; Bañó, M.; Carmona-Pontaque, F.; Catalán-García, M.; Guitart-Mampel, M.; Rivero, J.J.; Tobias, E.; Milisenda, J.C.; et al. Exhaustion of mitochondrial and autophagic reserve may contribute to the development of LRRK2 G2019S -Parkinson’s disease. J. Transl. Med. 2018, 16, 160. [Google Scholar] [CrossRef] [PubMed]
- di Domenico, A.; Carola, G.; Calatayud, C.; Pons-Espinal, M.; Muñoz, J.P.; Richaud-Patin, Y.; Fernandez-Carasa, I.; Gut, M.; Faella, A.; Parameswaran, J.; et al. Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson’s Disease. Stem. Cell Rep. 2019, 12, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.H.J.H.; Choi, H.; Chang, Y.; Park, H.H.J.H.; Shin, J.; Kim, J.J.J.J.; Lengner, C.J.; Lee, Y.K.; Kim, J.J.J.J. Modeling G2019S-LRRK2 Sporadic Parkinson’s Disease in 3D Midbrain Organoids. Stem. Cell Rep. 2019, 12, 518–531. [Google Scholar] [CrossRef]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Cristina Brailoiu, G.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Caesar, M.; Felk, S.; Zach, S.; Brønstad, G.; Aasly, J.O.; Gasser, T.; Gillardon, F. Changes in matrix metalloprotease activity and progranulin levels may contribute to the pathophysiological function of mutant leucine-rich repeat kinase 2. Glia 2014, 62, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Caesar, M.; Felk, S.; Aasly, J.O.; Gillardon, F. Changes in actin dynamics and F-actin structure both in synaptoneurosomes of LRRK2(R1441G) mutant mice and in primary human fibroblasts of LRRK2(G2019S) mutation carriers. Neuroscience 2015, 284, 311–324. [Google Scholar] [CrossRef]
- Dzamko, N.; Deak, M.; Hentati, F.; Reith, A.D.; Prescott, A.R.; Alessi, D.R.; Nichols, R.J. Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser 910/Ser935, disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem. J. 2010, 430, 405–413. [Google Scholar] [CrossRef]
- Howlett, E.H.; Jensen, N.; Belmonte, F.; Zafar, F.; Hu, X.; Kluss, J.; Schüle, B.; Kaufman, B.A.; Greenamyre, J.T.; Sanders, L.H. LRRK2 G2019S-induced mitochondrial DNA damage is LRRK2 kinase dependent and inhibition restores mtDNA integrity in Parkinson’s disease. Hum. Mol. Genet. 2017, 26, 4340–4351. [Google Scholar] [CrossRef]
- Fernández, B.; Lara Ordóñez, A.J.; Fdez, E.; Mutez, E.; Comptdaer, T.; Leghay, C.; Kreisler, A.; Simonin, C.; Vandewynckel, L.; Defebvre, L.; et al. Centrosomal cohesion deficits as cellular biomarker in lymphoblastoid cell lines from LRRK2 Parkinson’s disease patients. Biochem. J. 2019, 476, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s disease in midbrain-like organoids. NPJ Park. Dis. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Iannielli, A.; Ugolini, G.S.; Cordiglieri, C.; Bido, S.; Rubio, A.; Colasante, G.; Valtorta, M.; Cabassi, T.; Rasponi, M.; Broccoli, V. Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Cell Rep. 2019, 29, 4646–4656.e4. [Google Scholar] [CrossRef]
- Karayel, Ö.; Tonelli, F.; Winter, S.V.; Geyer, P.E.; Fan, Y.; Sammler, E.M.; Alessi, D.R.; Steger, M.; Mann, M. Accurate MS-based Rab10 Phosphorylation Stoichiometry Determination as Readout for LRRK2 Activity in Parkinson’s Disease. Mol. Cell. Proteom. 2020, 19, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Liu, M. Current understanding of LRRK2 in Parkinsons disease: Biochemical and structural features and inhibitor design. Future Med. Chem. 2012, 4, 1701–1713. [Google Scholar] [CrossRef]
- Vancraenenbroeck, R.; Lobbestael, E.; De Maeyer, M.; Baekelandt, V.; Taymans, J.-M. Kinases as Targets for Parkinson’s Disease: From Genetics to Therapy. CNS Neurol. Disord.—Drug Targets 2012, 10, 724–740. [Google Scholar] [CrossRef]
- Greggio, E.; Jain, S.; Kingsbury, A.; Bandopadhyay, R.; Lewis, P.; Kaganovich, A.; van der Brug, M.P.; Beilina, A.; Blackinton, J.; Thomas, K.J.; et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 2006, 23, 329–341. [Google Scholar] [CrossRef] [PubMed]
- West, A.B.; Moore, D.J.; Biskup, S.; Bugayenko, A.; Smith, W.W.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. USA 2005, 102, 16842–16847. [Google Scholar] [CrossRef]
- Ramonet, D.; Daher, J.P.L.; Lin, B.M.; Stafa, K.; Kim, J.; Banerjee, R.; Westerlund, M.; Pletnikova, O.; Glauser, L.; Yang, L.; et al. Dopaminergic Neuronal loss, Reduced Neurite Complexity and Autophagic Abnormalities in Transgenic Mice Expressing G2019S Mutant LRRK2. PLoS ONE 2011, 6, e18568. [Google Scholar] [CrossRef]
- Lee, B.D.; Shin, J.H.; Vankampen, J.; Petrucelli, L.; West, A.B.; Ko, H.S.; Lee, Y.I.; Maguire-Zeiss, K.A.; Bowers, W.J.; Federoff, H.J.; et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat. Med. 2010, 16, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.; Dowman, J.; Hammond, R.; Leete, T.; Inoue, K.; Abeliovich, A. The Familial Parkinsonism Gene LRRK2 Regulates Neurite Process Morphology. Neuron 2006, 52, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, M.; Nichols, R.J.; Deak, M.; Campbell, D.G.; Gillardon, F.; Knebel, A.; Alessi, D.R. LRRK2 phosphorylates moesin at threonine-558: Characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 2007, 405, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Abdelmotilib, H.; Liu, Z.; Stoyka, L.; Daher, J.P.L.; Milnerwood, A.J.; Unni, V.K.; Hirst, W.D.; Yue, Z.; Zhao, H.T.; et al. G2019s-LRRK2 expression augments α-synuclein sequestration into inclusions in neurons. J. Neurosci. 2016, 36, 7415–7427. [Google Scholar] [CrossRef]
- Hu, D.; Niu, J.Y.; Xiong, J.; Nie, S.K.; Zeng, F.; Zhang, Z. hui LRRK2 G2019S Mutation Inhibits Degradation of α-Synuclein in an In Vitro Model of Parkinson’s Disease. Curr. Med. Sci. 2018, 38, 1012–1017. [Google Scholar] [CrossRef]
- Hinkle, K.M.; Yue, M.; Behrouz, B.; Dächsel, J.C.; Lincoln, S.J.; Bowles, E.E.; Beevers, J.E.; Dugger, B.; Winner, B.; Prots, I.; et al. LRRK2 knockout mice have an intact dopaminergic system but display alterations in exploratory and motor co-ordination behaviors. Mol. Neurodegener. 2012, 7, 25. [Google Scholar] [CrossRef]
- Yue, M.; Hinkle, K.M.; Davies, P.; Trushina, E.; Fiesel, F.C.; Christenson, T.A.; Schroeder, A.S.; Zhang, L.; Bowles, E.; Behrouz, B.; et al. Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol. Dis. 2015, 78, 172–195. [Google Scholar] [CrossRef]
- Xiong, Y.; Neifert, S.; Karuppagounder, S.S.; Stankowski, J.N.; Lee, B.D.; Grima, J.C.; Chen, G.; Ko, H.S.; Lee, Y.; Swing, D.; et al. Overexpression of Parkinson’s disease-associated mutation LRRK2 G2019S in mouse forebrain induces behavioral deficits and α-synuclein pathology. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Longo, F.; Russo, I.; Shimshek, D.R.; Greggio, E.; Morari, M. Genetic and pharmacological evidence that G2019S LRRK2 confers a hyperkinetic phenotype, resistant to motor decline associated with aging. Neurobiol. Dis. 2014, 71, 62–73. [Google Scholar] [CrossRef]
- Gloeckner, C.J.; Kinkl, N.; Schumacher, A.; Braun, R.J.; O’Neill, E.; Meitinger, T.; Kolch, W.; Prokisch, H.; Ueffing, M. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum. Mol. Genet. 2006, 15, 223–232. [Google Scholar] [CrossRef]
- Ogata, J.; Hirao, K.; Nishioka, K.; Hayashida, A.; Li, Y.; Yoshino, H.; Shimizu, S.; Hattori, N.; Imai, Y. A Novel LRRK2 Variant p.G2294R in the WD40 Domain Identified in Familial Parkinson’s Disease Affects LRRK2 Protein Levels. Int. J. Mol. Sci. 2021, 22, 3708. [Google Scholar] [CrossRef]
- Di Fonzo, A.; Wu-Chou, Y.H.; Lu, C.S.; Van Doeselaar, M.; Simons, E.J.; Rohé, C.F.; Chang, H.C.; Chen, R.S.; Weng, Y.H.; Vanacore, N.; et al. A common missense variant in the LRRK2 gene, Gly2385Arg, associated with Parkinson’s disease risk in Taiwan. Neurogenetics 2006, 7, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Funayama, M.; Li, Y.; Tomiyama, H.; Yoshino, H.; Imamichi, Y.; Yamamoto, M.; Murata, M.; Toda, T.; Mizuno, Y.; Hattori, N. Leucine-Rich Repeat kinase 2 G2385R variant is a risk factor for Parkinson disease in Asian population. Neuroreport 2007, 18, 273–275. [Google Scholar] [CrossRef]
- Tan, E.K.; Peng, R.; Wu, Y.R.; Wu, R.M.; Wu-Chou, Y.H.; Tan, L.C.; An, X.K.; Chen, C.M.; Fook-Chong, S.; Lu, C.S. LRRK2 G2385R modulates age at onset in Parkinson’s disease: A multi-center pooled analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150, 1022–1023. [Google Scholar] [CrossRef]
- Fung, H.C.; Chen, C.M.; Hardy, J.; Singleton, A.B.; Wu, Y.R. A common genetic factor for Parkinson disease in ethnic Chinese population in Taiwan. BMC Neurol. 2006, 6, 47. [Google Scholar] [CrossRef]
- Shu, L.; Zhang, Y.; Pan, H.; Xu, Q.; Guo, J.; Tang, B.; Sun, Q. Clinical heterogeneity among lrrk2variants in Parkinson’s disease: A Meta-analysis. Front. Aging Neurosci. 2018, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, I.N.; Kaganovich, A.; Hauser, D.N.; Beylina, A.; Chia, R.; Ding, J.; Maric, D.; Jaffe, H.; Cookson, M.R. The G2385R variant of leucine-rich repeat kinase 2 associated with Parkinson’s disease is a partial loss-of-function mutation. Biochem. J. 2012, 446, 99–111. [Google Scholar] [CrossRef]
- Carrion, M.D.P.; Marsicano, S.; Daniele, F.; Marte, A.; Pischedda, F.; Cairano, E.D.; Piovesana, E.; Von Zweydorf, F.; Kremmer, E.; Gloeckner, C.J.; et al. The LRRK2 G2385R variant is a partial loss-of-function mutation that affects synaptic vesicle trafficking through altered protein interactions. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Rudenko, I.N.; Kaganovich, A.; Langston, R.G.; Beilina, A.; Ndukwe, K.; Kumaran, R.; Dillman, A.A.; Chia, R.; Cookson, M.R. The G2385R risk factor for Parkinson’s disease enhances CHIP-dependent intracellular degradation of LRRK2. Biochem. J. 2017, 474, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Korecka, J.A.; Talbot, S.; Osborn, T.M.; de Leeuw, S.M.; Levy, S.A.; Ferrari, E.J.; Moskites, A.; Atkinson, E.; Jodelka, F.M.; Hinrich, A.J.; et al. Neurite Collapse and Altered ER Ca 2+ Control in Human Parkinson Disease Patient iPSC-Derived Neurons with LRRK2 G2019S Mutation. Stem Cell Rep. 2019, 12, 29–41. [Google Scholar] [CrossRef]
- Bravo-San Pedro, J.M.; Niso-Santano, M.; Gómez-Sánchez, R.; Pizarro-Estrella, E.; Aiastui-Pujana, A.; Gorostidi, A.; Climent, V.; López De Maturana, R.; Sanchez-Pernaute, R.; López De Munain, A.; et al. The LRRK2 G2019S mutant exacerbates basal autophagy through activation of the MEK/ERK pathway. Cell. Mol. Life Sci. 2013, 70, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanger, J.M.; Sanger, J.W. Differential effects of Latrunculin-A on myofibrils in cultures of skeletal muscle cells: Insights into mechanisms of myofibrillogenesis. Cell Motil. Cytoskeleton 2005, 62, 35–47. [Google Scholar] [CrossRef]
- Ohta, E.; Nihira, T.; Uchino, A.; Imaizumi, Y.; Okada, Y.; Akamatsu, W.; Takahashi, K.; Hayakawa, H.; Nagai, M.; Ohyama, M.; et al. I2020T mutant LRRK2 iPSC-derived neurons in the Sagamihara family exhibit increased Tau phosphorylation through the AKT/GSK-3ß signaling pathway. Hum. Mol. Genet. 2015, 24, 4879–4900. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Huang, C.Y.; Ho, M.C.; Hsu, Y.H.; Syu, S.H.; Lu, H.E.; Lin, H.I.; Lin, C.H.; Hsieh, P.C.H. Generation of 2 induced pluripotent stem cell lines derived from patients with Parkinson’s disease carrying LRRK2 G2385R variant. Stem. Cell Res. 2018, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blauwendraat, C.; Reed, X.; Kia, D.A.; Gan-Or, Z.; Lesage, S.; Pihlstrøm, L.; Guerreiro, R.; Gibbs, J.R.; Sabir, M.; Ahmed, S.; et al. Frequency of loss of function variants in LRRK2 in Parkinson disease. JAMA Neurol. 2018, 75, 1416–1422. [Google Scholar] [CrossRef]
- Whiffin, N.; Armean, I.M.; Kleinman, A.; Marshall, J.L.; Minikel, E.V.; Goodrich, J.K.; Quaife, N.M.; Cole, J.B.; Wang, Q.; Karczewski, K.J.; et al. The effect of LRRK2 loss-of-function variants in humans. Nat. Med. 2020, 26, 869–877. [Google Scholar] [CrossRef]
- Haenseler, W.; Sansom, S.N.; Buchrieser, J.; Newey, S.E.; Moore, C.S.; Nicholls, F.J.; Chintawar, S.; Schnell, C.; Antel, J.P.; Allen, N.D.; et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Rep. 2017, 8, 1727–1742. [Google Scholar] [CrossRef]
- Iannielli, A.; Ugolini, G.S.; Cordiglieri, C.; Bido, S.; Rubio, A.; Colasante, G.; Valtorta, M.; Cabassi, T.; Rasponi, M.; Broccoli, V. Reconstitution of the Human Nigro-striatal Pathway on-a-Chip Reveals OPA1-Dependent Mitochondrial Defects and Loss of Dopaminergic Synapses. Cell Rep. 2019, 29, 4646–4656.e4. [Google Scholar] [CrossRef] [PubMed]
- Taymans, J.-M.M.; Van den Haute, C.; Baekelandt, V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J. Neurochem. 2006, 98, 951–961. [Google Scholar] [CrossRef] [PubMed]
- West, A.B.; Cowell, R.M.; Daher, J.P.L.; Moehle, M.S.; Hinkle, K.M.; Melrose, H.L.; Standaert, D.G.; Volpicelli-Daley, L.A. Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J. Comp. Neurol. 2014, 522, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Mandemakers, W.; Snellinx, A.; O’Neill, M.J.; de Strooper, B. LRRK2 expression is enriched in the striosomal compartment of mouse striatum. Neurobiol. Dis. 2012, 48, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Melrose, H.L.; Yue, M.; Pare, J.F.; Farrer, M.J.; Smith, Y. Lrrk2 localization in the primate basal ganglia and thalamus: A light and electron microscopic analysis in monkeys. Exp. Neurol. 2010, 224, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; James, W.S.; Cowley, S.A. LRRK2 in peripheral and central nervous system innate immunity: Its link to Parkinson’s disease. Biochem. Soc. Trans. 2017, 45, 131–139. [Google Scholar] [CrossRef]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; West, A.B. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Z.; Ye, T.; Mabrouk, O.S.; Maltbie, T.; Aasly, J.; West, A.B. Elevated LRRK2 autophosphorylation in brain-derived and peripheral exosomes in LRRK2 mutation carriers. Acta Neuropathol. Commun. 2017, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Howden, A.J.M.M.; Sarhan, A.R.; Lis, P.; Ito, G.; Martinez, T.N.; Brockmann, K.; Gasser, T.; Alessi, D.R.; Sammler, E.M.; et al. Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem. J. 2018, 475, 23–44. [Google Scholar] [CrossRef]
- Thirstrup, K.; Dächsel, J.C.; Oppermann, F.S.; Williamson, D.S.; Smith, G.P.; Fog, K.; Christensen, K.V. Selective LRRK2 kinase inhibition reduces phosphorylation of endogenous Rab10 and Rab12 in human peripheral mononuclear blood cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Atashrazm, F.; Hammond, D.; Perera, G.; Bolliger, M.F.; Matar, E.; Halliday, G.M.; Schüle, B.; Lewis, S.J.G.; Nichols, R.J.; Dzamko, N. LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson’s disease patients. Mov. Disord. 2019, 34, 406–415. [Google Scholar] [CrossRef]
- Rideout, H.J.; Chartier-Harlin, M.-C.C.; Fell, M.J.; Hirst, W.D.; Huntwork-Rodriguez, S.; Leyns, C.E.G.G.; Mabrouk, O.S.; Taymans, J.-M.M. The Current State-of-the Art of LRRK2-Based Biomarker Assay Development in Parkinson’s Disease. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.K.Y.; Lin, S.; Mok, V.C.T. Neuroimaging in Vascular Parkinsonism. Curr. Neurol. Neurosci. Rep. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Fuji, R.N.; Flagella, M.; Baca, M.; Baptista, M.A.S.; Brodbeck, J.; Chan, B.K.; Fiske, B.K.; Honigberg, L.; Jubb, A.M.; Katavolos, P.; et al. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci. Transl. Med. 2015, 7, 273ra15. [Google Scholar] [CrossRef] [PubMed]
- Derkinderen, P.; Rouaud, T.; Lebouvier, T.; Bruley Des Varannes, S.; Neunlist, M.; De Giorgio, R. Parkinson disease: The enteric nervous system spills its guts. Neurology 2011, 77, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.Y.; Fernandez-Hernandez, H.; Hu, J.; Schaffner, A.; Pankratz, N.; Hsu, N.Y.; Chuang, L.S.; Carmi, S.; Villaverde, N.; Li, X.; et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Cabezudo, D.; Baekelandt, V.; Lobbestael, E. Multiple-Hit Hypothesis in Parkinson’s Disease: LRRK2 and Inflammation. Front. Neurosci. 2020, 14, 939–953. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goveas, L.; Mutez, E.; Chartier-Harlin, M.-C.; Taymans, J.-M. Mind the Gap: LRRK2 Phenotypes in the Clinic vs. in Patient Cells. Cells 2021, 10, 981. https://doi.org/10.3390/cells10050981
Goveas L, Mutez E, Chartier-Harlin M-C, Taymans J-M. Mind the Gap: LRRK2 Phenotypes in the Clinic vs. in Patient Cells. Cells. 2021; 10(5):981. https://doi.org/10.3390/cells10050981
Chicago/Turabian StyleGoveas, Liesel, Eugénie Mutez, Marie-Christine Chartier-Harlin, and Jean-Marc Taymans. 2021. "Mind the Gap: LRRK2 Phenotypes in the Clinic vs. in Patient Cells" Cells 10, no. 5: 981. https://doi.org/10.3390/cells10050981
APA StyleGoveas, L., Mutez, E., Chartier-Harlin, M.-C., & Taymans, J.-M. (2021). Mind the Gap: LRRK2 Phenotypes in the Clinic vs. in Patient Cells. Cells, 10(5), 981. https://doi.org/10.3390/cells10050981







