Hypertension and Aging Affect Liver Sulfur Metabolism in Rats
Abstract
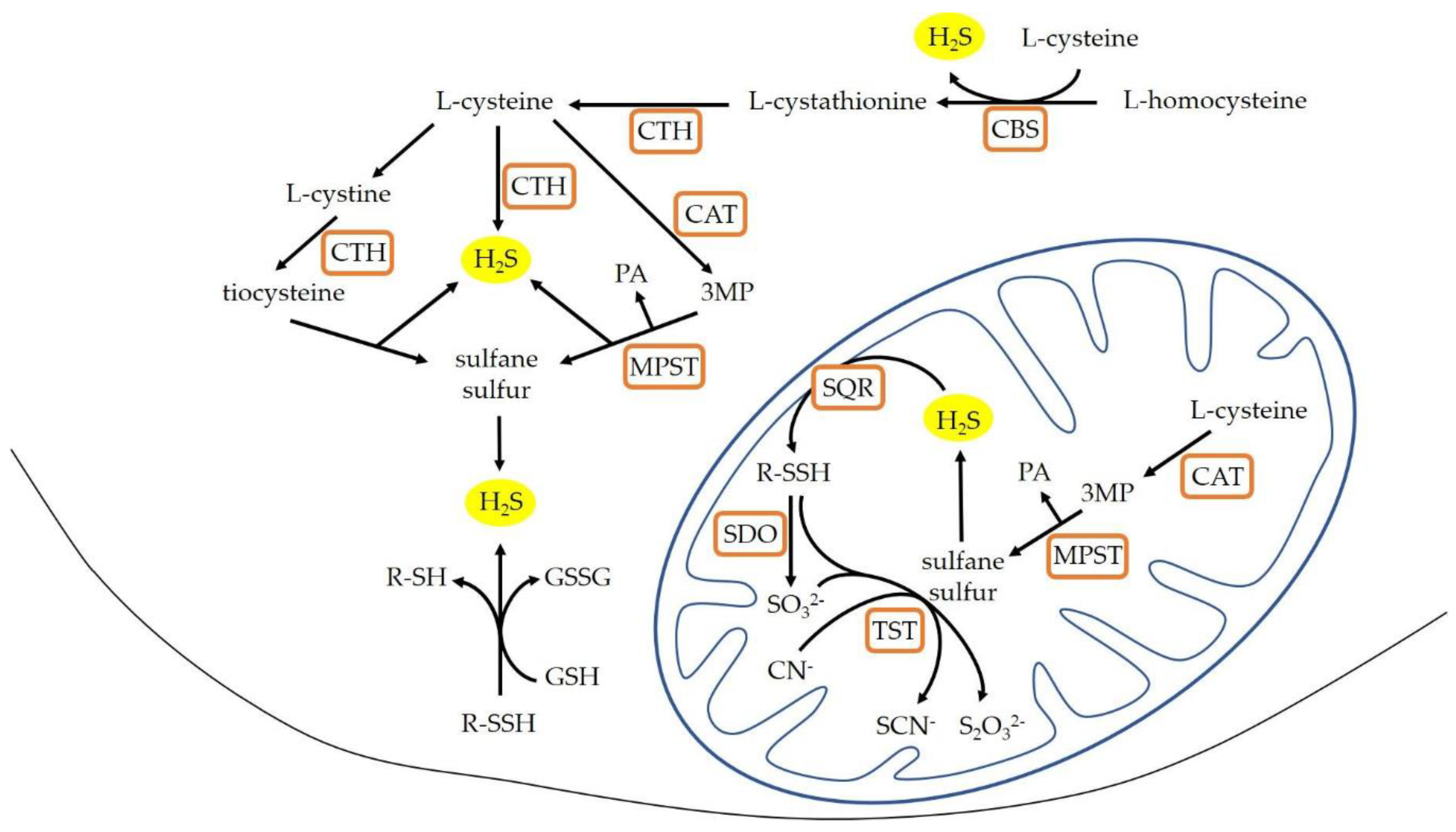
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
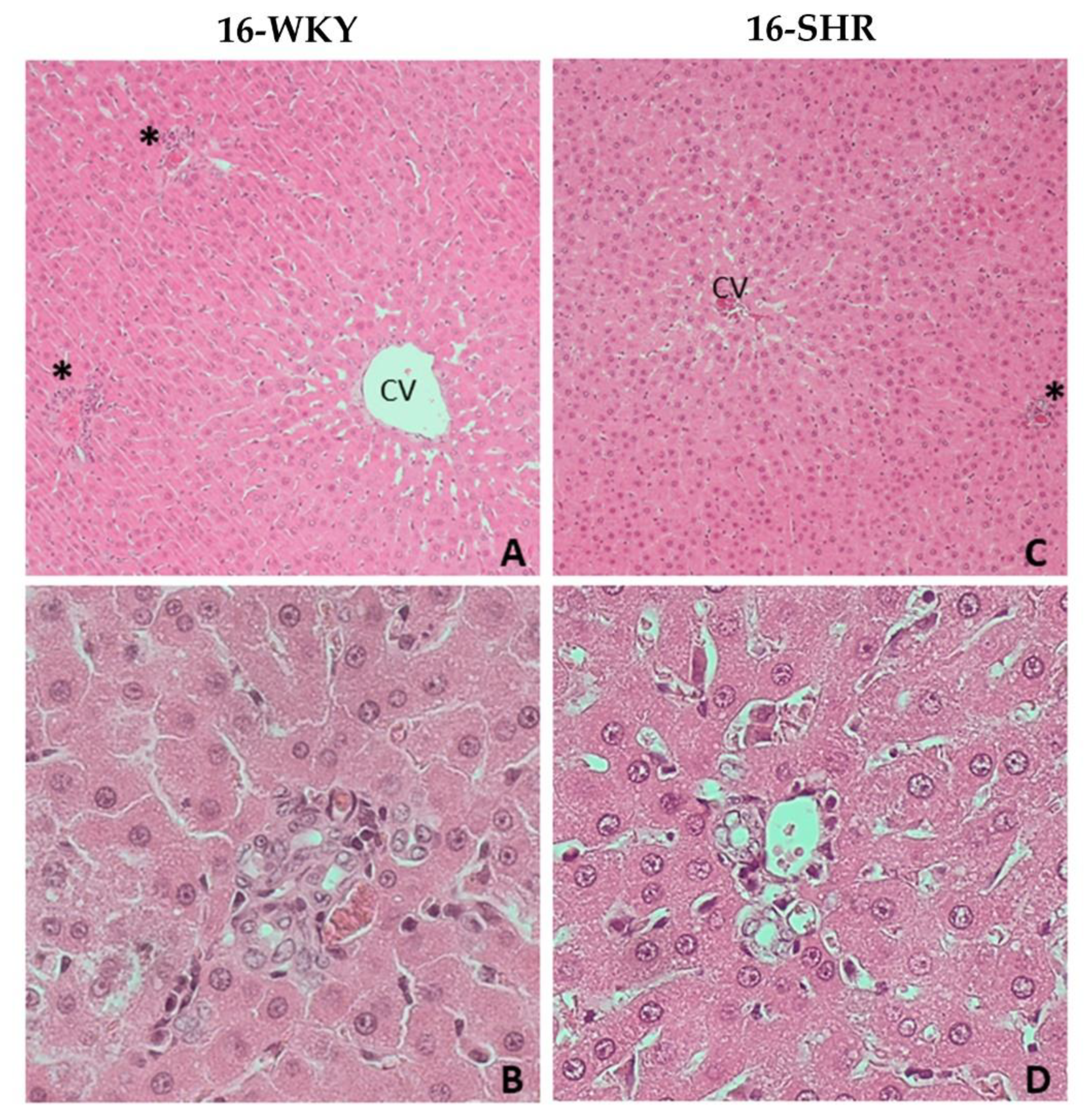
2.3. Histopathological Examination
2.4. Tissue Homogenates
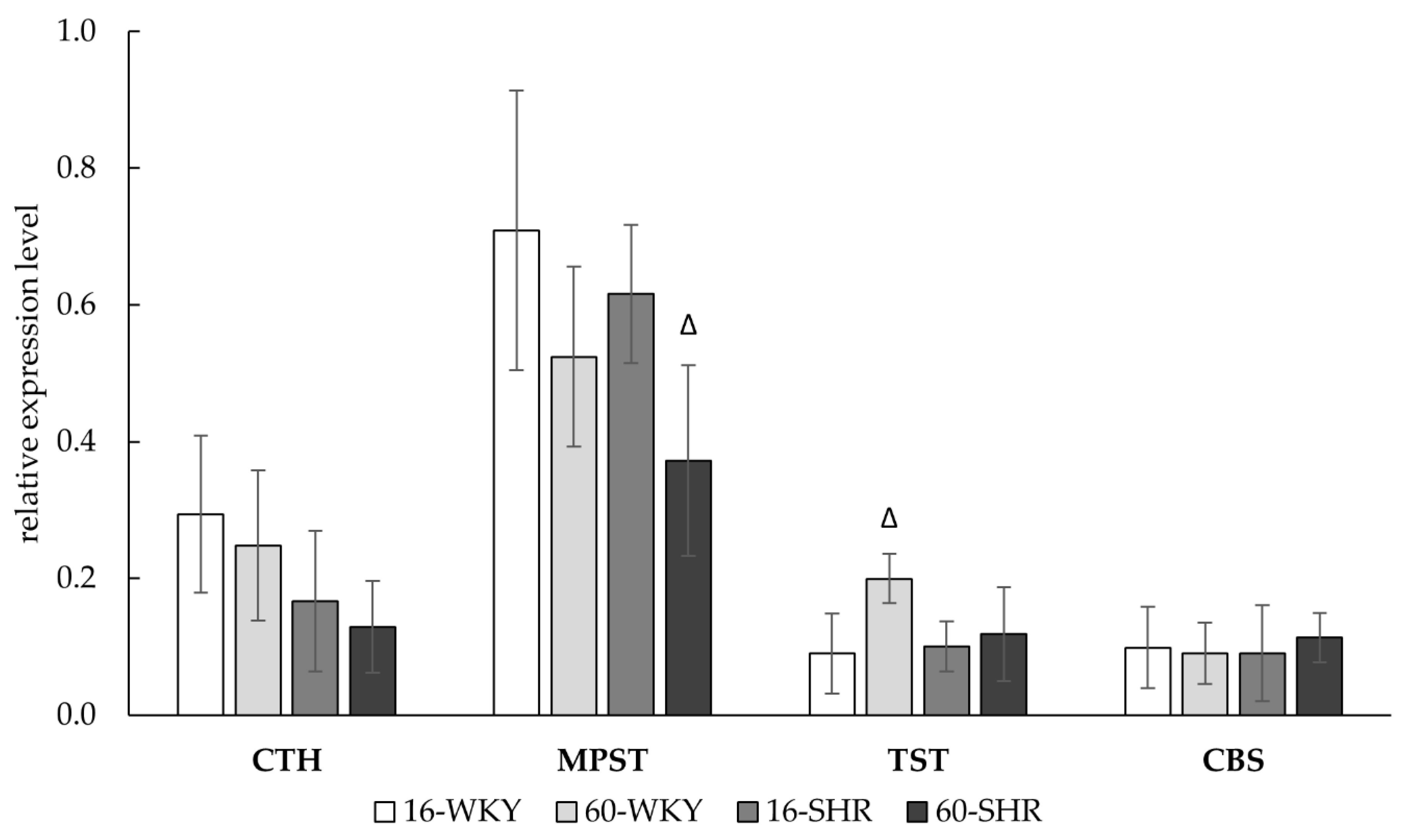
2.5. Expression of CTH, MPST, TST, and CBS Gene
2.5.1. Isolation of RNA
2.5.2. Reverse Transcription of RNA
2.5.3. Polymerase Chain Reaction (PCR)
2.6. Enzyme Assay
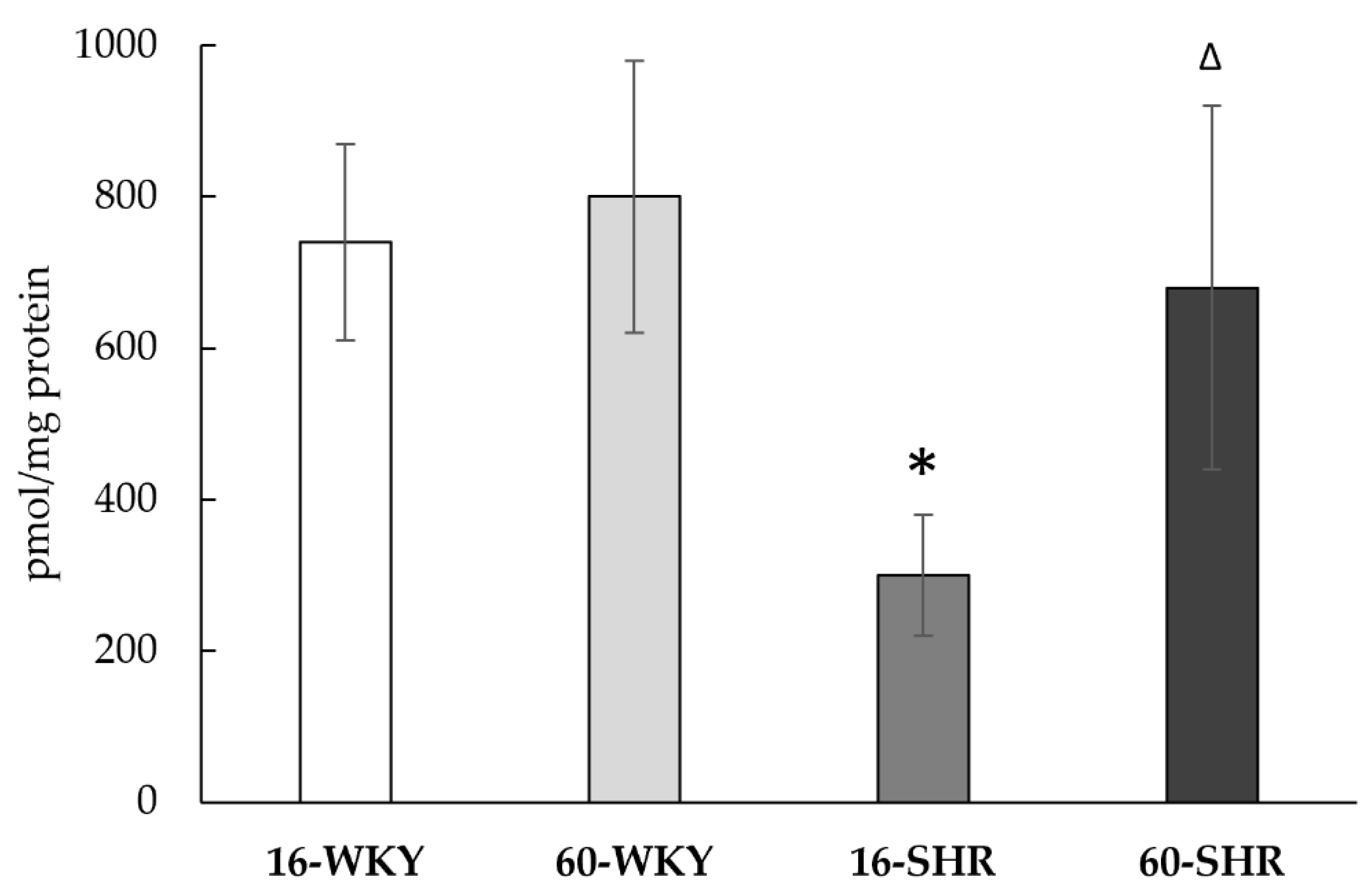
2.7. Sulfane Sulfur
2.8. Protein Level
2.9. Low Molecular Sulfur-Containing Compounds Determination Using RP-HPLC
2.10. Detection of H2S Production in Tissue Homogenates
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. 2008–2013 Action Plan for the Global Strategy for the Prevention and Control of Noncommunicable Diseases; WHO Document Production Services: Geneva, Switzerland, 2009; p. 5. ISBN 9789241597418. [Google Scholar]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide and polysulfides as signaling molecules. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2015, 91, 131–159. [Google Scholar] [CrossRef] [PubMed]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the Human Mitochondrial Hydrogen Sulfide Oxidation Pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Hunt, N.J.; Kang, S.W.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Mani, S.; Cao, W.; Wu, L.; Wang, R. Hydrogen sulfide and the liver. Nitric Oxide 2014, 41, 62–71. [Google Scholar] [CrossRef]
- Wu, D.-D.; Wang, D.-Y.; Li, H.-M.; Guo, J.-C.; Duan, S.-F.; Ji, X.-Y. Hydrogen Sulfide as a Novel Regulatory Factor in Liver Health and Disease. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Antonelli, E.; Mencarelli, A.; Orlandi, S.; Renga, B.; Rizzo, G.; Distrutti, E.; Shah, V.; Morelli, A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 2005, 42, 539–548. [Google Scholar] [CrossRef]
- Kang, K.; Zhao, M.; Jiang, H.; Tan, G.; Pan, S.; Sun, X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transplant. 2009, 15, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, M.; Tian, W.; Wang, S.; Cui, L.; Li, H.; Wang, H.; Ji, A.; Li, Y. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef]
- Sheedfar, F.; Di Biase, S.; Koonen, D.; Vinciguerra, M. Liver diseases and aging: Friends or foes? Aging Cell 2013, 12, 950–954. [Google Scholar] [CrossRef]
- Huc, T.; Drapala, A.; Gawrys, M.; Konop, M.; Bielinska, K.; Zaorska, E.; Samborowska, E.; Wyczalkowska-Tomasik, A.; Pączek, L.; Dadlez, M.; et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Circ. Physiol. 2018, 315, H1805–H1820. [Google Scholar] [CrossRef] [PubMed]
- Bronowicka-Adamska, P.; Wróbel, M.; Magierowski, M.; Magierowska, K.; Kwiecień, S.; Brzozowski, T. Hydrogen Sulphide Production in Healthy and Ulcerated Gastric Mucosa of Rats. Molecules 2017, 22, 530. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Hutsch, T.; Gawryś-Kopczyńska, M.; Maksymiuk, K.; Wróbel, M. Hydrogen sulfide formation in experimental model of acute pancreatitis. Acta Biochim. Pol. 2019, 66, 611–618. [Google Scholar] [CrossRef]
- Mard, S.A.; Veisi, A.; Ahangarpour, A.; Gharib-Naseri, M.K. Mucosal acidification increases hydrogen sulfide release through up-regulating gene and protein expressions of cystathionine gamma-lyase in the rat gastric mucosa. Iran. J. Basic Med. Sci. 2016, 19, 172–177. [Google Scholar]
- Matsuo, Y.; Greenberg, D.M. A Crystalline Enzyme that Cleaves Homoserine and Cystathionine. J. Biol. Chem. 1958, 230, 545–560. [Google Scholar] [CrossRef]
- Czubak, J.; Wróbel, M.; Jurkowska, H. Cystathionine γ-lyase (EC: 4.4.1.1) an enzymatic assay of α-ketobutyrate using lactate dehydrogenase. Acta Biol. Crac. Ser. Zool. 2002, 44, 113–117. [Google Scholar]
- Valentine, W.N.; Frankenfeld, J.K. 3-mercaptopyruvate sulfurtransferase (EC 2.8.1.2): A simple assay adapted to human blood cells. Clin. Chim. Acta 1974, 51, 205–210. [Google Scholar] [CrossRef]
- Wróbel, M.; Jurkowska, H.; Śliwa, L.; Srebro, Z. Sulfurtransferases and Cyanide Detoxification in Mouse Liver, Kidney, and Brain. Toxicol. Mech. Methods 2004, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Sörbo, B. Rhodanese. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 2, pp. 334–337. [Google Scholar]
- Bronowicka-Adamska, P.; Zagajewski, J.; Wróbel, M. An application of RP-HPLC for determination of the activity of cystathionine β-synthase and γ-cystathionase in tissue homogenates. Nitric Oxide 2015, 46, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.L. Sulfane sulfur. Methods Enzymol. 1987, 143, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dominick, P.K.; Cassidy, P.B.; Roberts, J.C. A new and versatile method for determination of thiolamines of biological importance. J. Chromatogr. B Biomed. Sci. Appl. 2001, 761, 1–12. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Zagajewski, J.; Czubak, J.; Wróbel, M. RP-HPLC method for quantitative determination of cystathionine, cysteine and glutathione: An application for the study of the metabolism of cysteine in human brain. J. Chromatogr. B 2011, 879, 2005–2009. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef]
- Collin, M.; Anuar, F.B.M.; Murch, O.; Bhatia, M.; Moore, P.K.; Thiemermann, C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J. Pharmacol. 2005, 146, 498–505. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Pan, S.; Li, J.; Dong, X.; Kang, K.; Zhao, M.; Jiang, X.; Kanwar, J.R.; Qiao, H.; Jiang, H.; et al. Hydrogen Sulfide Attenuates Carbon Tetrachloride-Induced Hepatotoxicity, Liver Cirrhosis and Portal Hypertension in Rats. PLoS ONE 2011, 6, e25943. [Google Scholar] [CrossRef]
- Predmore, B.L.; Alendy, M.J.; Ahmed, K.I.; Leeuwenburgh, C.; Julian, D. The hydrogen sulfide signaling system: Changes during aging and the benefits of caloric restriction. AGE 2010, 32, 467–481. [Google Scholar] [CrossRef]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The Quantitative Significance of the Transsulfuration Enzymes for H2S Production in Murine Tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Mars, W.M.; Bowen, W.C.; Barua, L.; Yang, Y.; Guo, L.; St.-Arnaud, R.; Dedhar, S.; Wu, C.; Michalopoulos, G.K. Loss of integrin linked kinase from mouse hepatocytesin vitro andin vivo results in apoptosis and hepatitis. Hepatology 2007, 45, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Goodman, Z.D.; Ishak, K.G. Hepatobiliary System and Pancreas. In Surgical Pathology and Cytopathology, 4th ed.; Silverberg, S.G., Ed.; Elsevier: Oxford, UK, 2006; Volume 2, pp. 1465–1526. [Google Scholar]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Kietzmann, T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017, 11, 622–630. [Google Scholar] [CrossRef]
- Percy, D.H.; Barthold, S.W. Pathology of Laboratory Rodents and Rabbits, 3rd ed.; Blackwell Publishing: Ames, IA, USA, 2007; p. 356. ISBN 9780813821016. [Google Scholar]
- Nakajima, T. Roles of Sulfur Metabolism and Rhodanese in Detoxification and Anti-Oxidative Stress Functions in the Liver: Responses to Radiation Exposure. Med. Sci. Monit. 2015, 21, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Burkhardt, B.; Fischer, L.; Beirow, M.; Bork, N.; Wönne, E.C.; Wagner, C.; Husen, B.; Zeilinger, K.; Liu, L.; et al. Age-dependent changes of the antioxidant system in rat livers are accompanied by altered MAPK activation and a decline in motor signaling. EXCLI J. 2015, 14, 1273–1290. [Google Scholar] [PubMed]
- Toohey, J. Possible involvement of sulfane sulfur in homocysteine-induced atherosclerosis. Med. Hypotheses 2001, 56, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Dysregulation of glutathione synthesis in liver disease. Liver Res. 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Yu, X.; Long, Y.C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Handler, J.A.; Genell, C.A.; Goldstein, R.S. Hepatobiliary function in senescent male Sprague-Dawley rats. Hepatology 1994, 19, 1496–1503. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Markel, T.A.; Drucker, N.; Boone, D.; Whiteman, M.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; et al. Extended hypoxia-mediated H2S production provides for long-term oxygen sensing. Acta Physiol. 2020, 228, e13368. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef]
- Guo, W.; Kan, J.-T.; Cheng, Z.-Y.; Chen, J.-F.; Shen, Y.-Q.; Xu, J.; Wu, D.; Zhu, Y.-Z. Hydrogen Sulfide as an Endogenous Modulator in Mitochondria and Mitochondria Dysfunction. Oxidative Med. Cell. Longev. 2012, 2012, 1–9. [Google Scholar] [CrossRef][Green Version]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef] [PubMed]

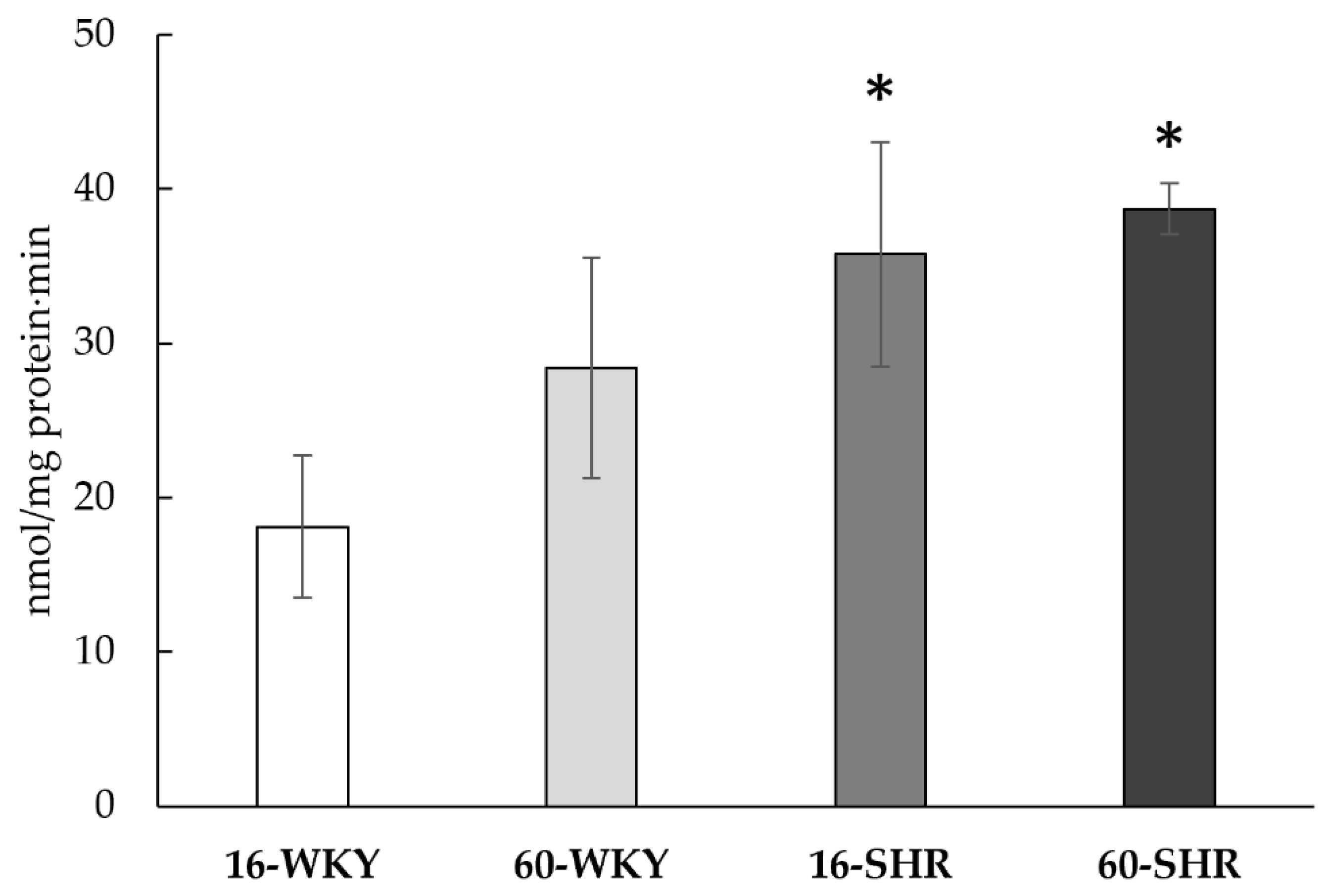

| Experimental Group | CTH | MPST | TST | CBS | Sulfane Sulfur |
|---|---|---|---|---|---|
| nmol/mg protein·min | pmol/mg protein·min | nmol/mg protein | |||
| 16-WKY | 14.7 ± 2.8 | 6713 ± 551 | 5244 ± 775 | 1.8 ± 0.8 | 249 ± 46 |
| 60-WKY | 16.9 ± 3.3 | 7827 ± 517 Δ | 8448 ± 539 Δ | 2.7 ± 0.7 | 288 ± 23 Δ |
| 16-SHR | 16.7 ± 2.7 | 6684 ± 530 | 9545 ± 1383 * | ND | 223 ± 19 * |
| 60-SHR | 21.4 ± 2.9 *Δ | 6164 ± 592 *Δ | 8120 ± 2822 | 2.3 ± 0.4 | 358 ± 87 *Δ |
| Experimental Group | GSH | GSSG | Total Glutathione (2GSSG+GSH) | CSH | CSSC | Total Cysteine (2CSSC+CSH) |
|---|---|---|---|---|---|---|
| nmol/mg protein | ||||||
| 16-WKY | 7.6 ±1.8 | 11.1 ± 3.0 | 28.4 ± 5.5 | 0.4 ± 0.3 | 4.3 ± 0.7 | 9.0 ± 1.4 |
| 60-WKY | 20.2 ± 3.6 Δ | 10.3 ± 2.4 | 41.4 ± 6.8 Δ | 1.0 ± 0.6 | 4.1 ± 1.0 | 9.2 ± 2.8 |
| 16-SHR | 32.3 ± 5.7 * | 2.9 ± 0.7 * | 38.3 ± 7.2 * | 3.0 ± 0.7 * | 3.9 ± 1.0 | 10.8 ± 2.5 |
| 60-SHR | 15.6 ± 2.9 *Δ | 4.2 ± 0.9 *Δ | 21.9 ± 3.5 *Δ | 7.9 ± 2.8 *Δ | 7.3 ± 1.6 *Δ | 22.2 ± 6.0 *Δ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlęzak, D.; Bronowicka-Adamska, P.; Hutsch, T.; Ufnal, M.; Wróbel, M. Hypertension and Aging Affect Liver Sulfur Metabolism in Rats. Cells 2021, 10, 1238. https://doi.org/10.3390/cells10051238
Szlęzak D, Bronowicka-Adamska P, Hutsch T, Ufnal M, Wróbel M. Hypertension and Aging Affect Liver Sulfur Metabolism in Rats. Cells. 2021; 10(5):1238. https://doi.org/10.3390/cells10051238
Chicago/Turabian StyleSzlęzak, Dominika, Patrycja Bronowicka-Adamska, Tomasz Hutsch, Marcin Ufnal, and Maria Wróbel. 2021. "Hypertension and Aging Affect Liver Sulfur Metabolism in Rats" Cells 10, no. 5: 1238. https://doi.org/10.3390/cells10051238
APA StyleSzlęzak, D., Bronowicka-Adamska, P., Hutsch, T., Ufnal, M., & Wróbel, M. (2021). Hypertension and Aging Affect Liver Sulfur Metabolism in Rats. Cells, 10(5), 1238. https://doi.org/10.3390/cells10051238







