Metastatic Esophageal Carcinoma Cells Exhibit Reduced Adhesion Strength and Enhanced Thermogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culturing Conditions
2.2. Isothermal Microcalorimetry
2.3. Shear Stress Adhesion Assays
3. Results
3.1. Origin of Cell Lines
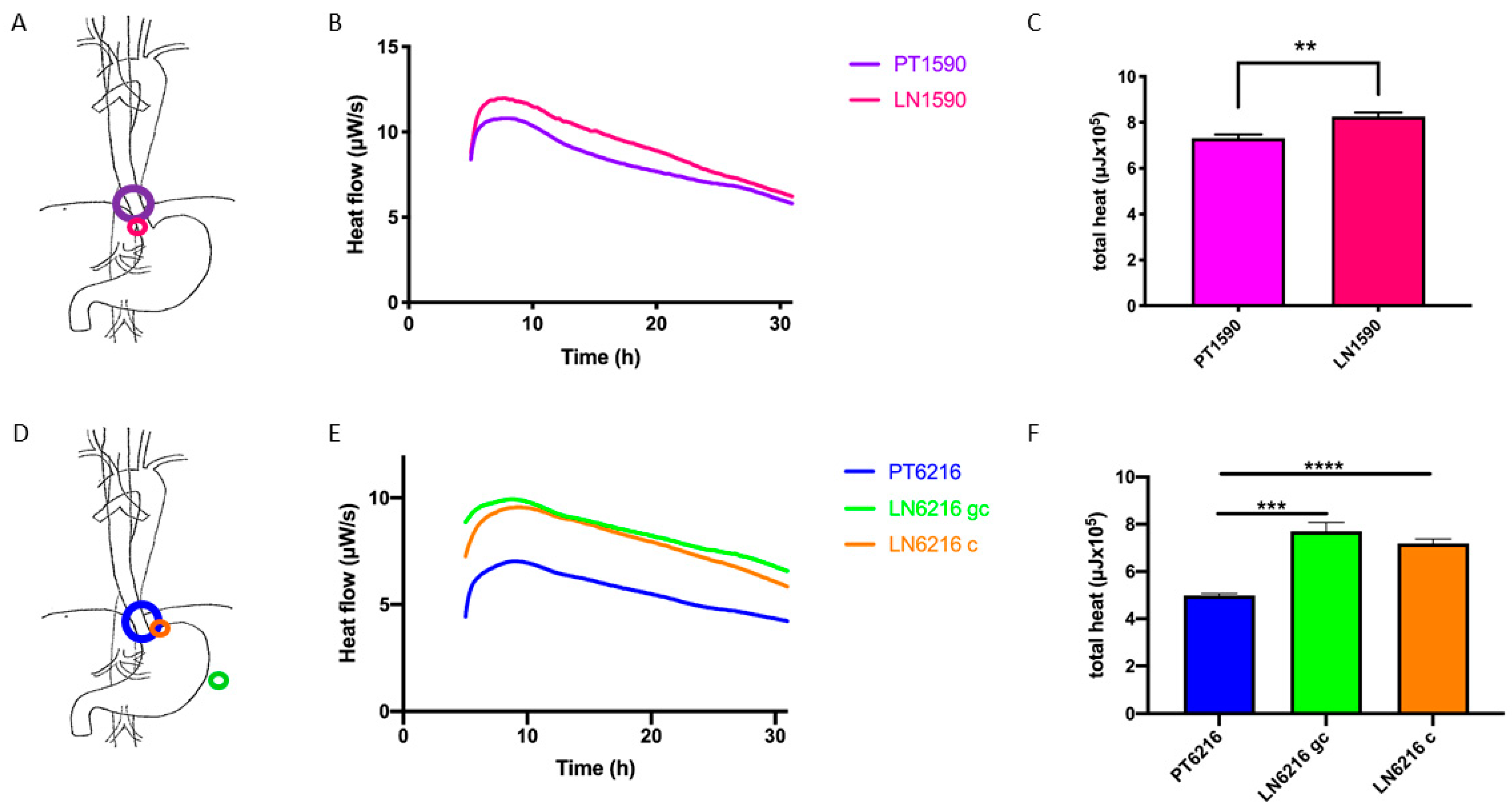
3.2. Microcalorimteric Assessment of Tumor Cells
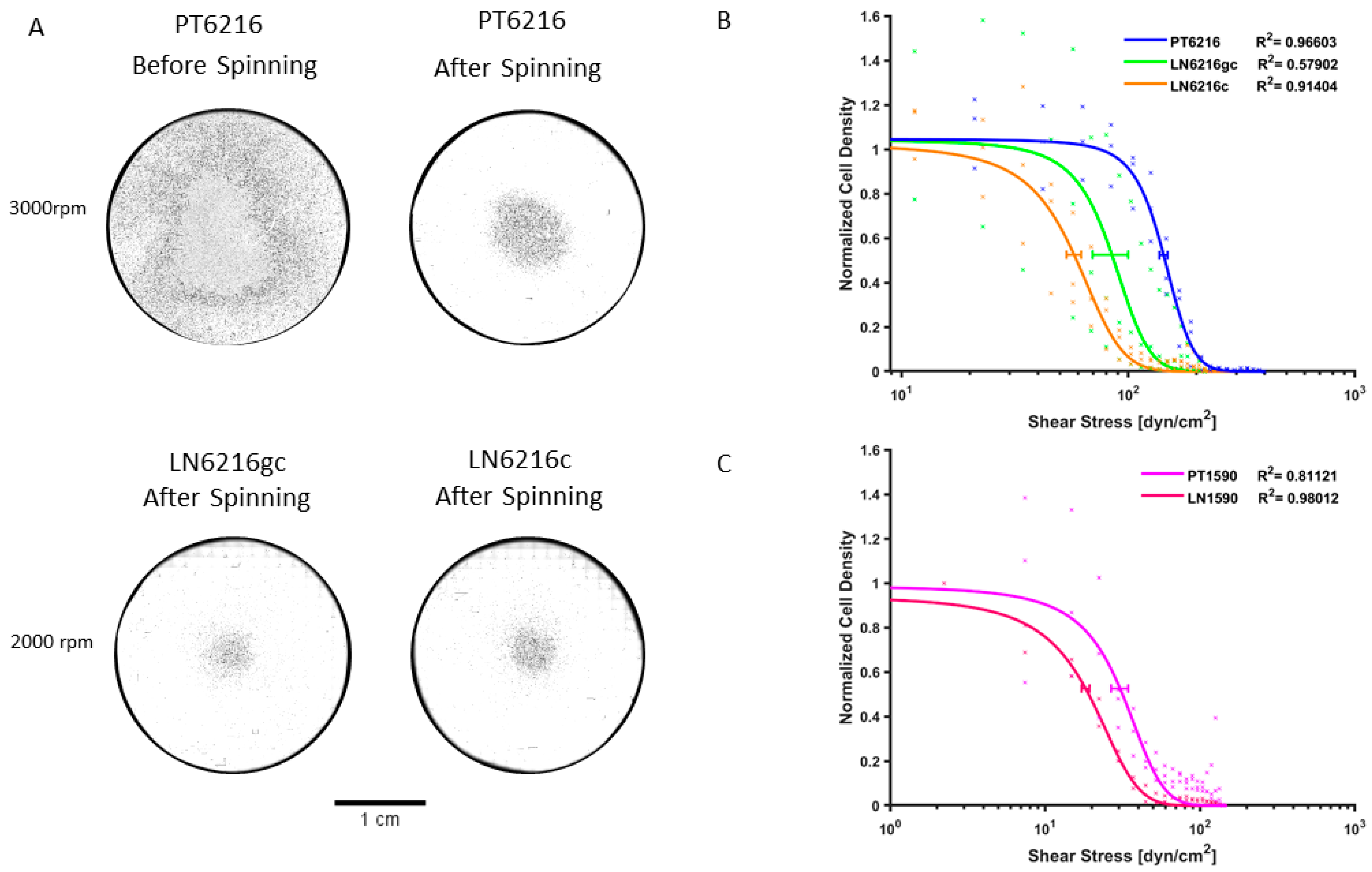
3.3. Shear Stress Adhesion Assay
4. Discussion
4.1. Biophysical Assays to Determine Metastatic Potential
4.2. Origin and Localization of Metastases
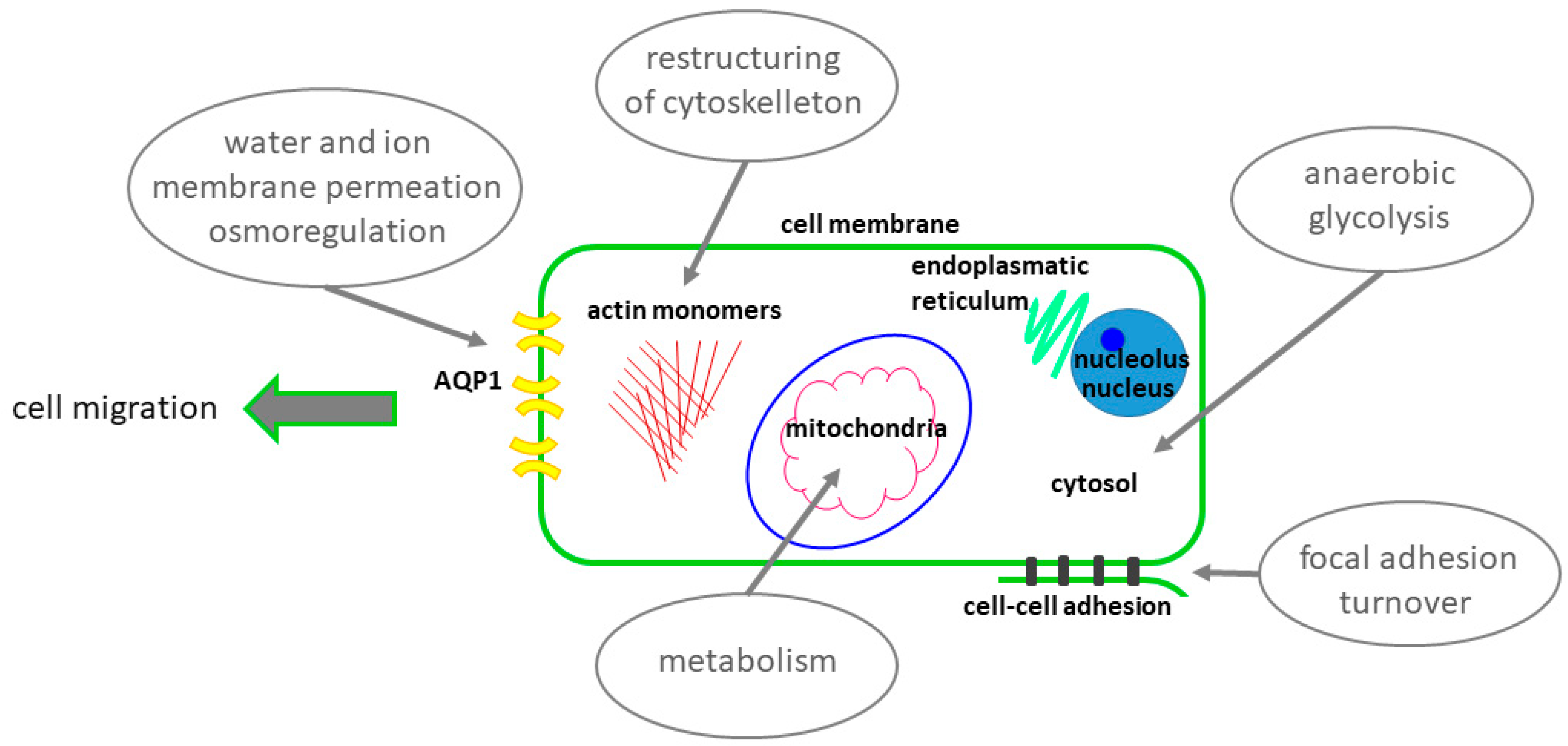
4.3. Possible Heat Generators and Sources of Thermogenesis in the Migrating Cell
4.4. Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thrift, A.P. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016, 41, 88–95. [Google Scholar] [CrossRef]
- Drenckhan, A.; Freytag, M.; Supuran, C.T.; Sauter, G.; Izbicki, J.R.; Gros, S.J. CAIX furthers tumour progression in the hypoxic tumour microenvironment of esophageal carcinoma and is a possible therapeutic target. J. Enzym. Inhib. Med. Chem. 2018, 33, 1024–1033. [Google Scholar] [CrossRef]
- Alibert, C.; Goud, B.; Manneville, J.B. Are cancer cells really softer than normal cells? Biol. Cell 2017, 109, 167–189. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Tse, H.T.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.; Clark, A.T.; Di Carlo, D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef] [PubMed]
- Beri, P.; Matte, B.F.; Fattet, L.; Kim, D.; Yang, J.; Engler, A.J. Biomaterials to model and measure epithelial cancers. Nat. Rev. Mater. 2018, 3, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflug. Arch. 2008, 456, 693–700. [Google Scholar] [CrossRef]
- Reticker-Flynn, N.E.; Malta, D.F.; Winslow, M.M.; Lamar, J.M.; Xu, M.J.; Underhill, G.H.; Hynes, R.O.; Jacks, T.E.; Bhatia, S.N. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat. Commun. 2012, 3, 1122. [Google Scholar] [CrossRef]
- Yates, C.M.; McGettrick, H.M.; Nash, G.B.; Rainger, G.E. Adhesion of tumor cells to matrices and endothelium. Methods Mol. Biol. 2014, 1070, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Beri, P.; Popravko, A.; Yeoman, B.; Kumar, A.; Chen, K.; Hodzic, E.; Chiang, A.; Banisadr, A.; Placone, J.K.; Carter, H.; et al. Cell Adhesiveness Serves as a Biophysical Marker for Metastatic Potential. Cancer Res. 2020, 80, 901–911. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Banisadr, A.; Beri, P.; Tlsty, T.D.; Engler, A.J. Metastatic State of Cancer Cells May Be Indicated by Adhesion Strength. Biophys. J. 2017, 112, 736–745. [Google Scholar] [CrossRef]
- Wadso, I. Isothermal microcalorimetry in applied biology. Thermochim. Acta 2002, 394, 305–311. [Google Scholar] [CrossRef]
- Lemos, D.; Oliveira, T.; Martins, L.; de Azevedo, V.R.; Rodrigues, M.F.; Ketzer, L.A.; Rumjanek, F.D. Isothermal Microcalorimetry of Tumor Cells: Enhanced Thermogenesis by Metastatic Cells. Front. Oncol. 2019, 9, 1430. [Google Scholar] [CrossRef]
- Scheunemann, P.; Izbicki, J.R.; Pantel, K. Tumorigenic potential of apparently tumor-free lymph nodes. N. Engl. J. Med. 1999, 340, 1687. [Google Scholar] [CrossRef] [PubMed]
- Gros, S.J.; Dohrmann, T.; Peldschus, K.; Schurr, P.G.; Kaifi, J.T.; Kalinina, T.; Reichelt, U.; Mann, O.; Strate, T.G.; Adam, G.; et al. Complementary use of fluorescence and magnetic resonance imaging of metastatic esophageal cancer in a novel orthotopic mouse model. Int. J. Cancer. 2010, 126, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Drenckhan, A.; Grob, T.; Dupree, A.; Dohrmann, T.; Mann, O.; Izbicki, J.R.; Gros, S.J. Esophageal carcinoma cell line with high EGFR polysomy is responsive to gefitinib. Langenbeck’s Arch. Surg. 2014, 399, 879–888. [Google Scholar] [CrossRef]
- Braissant, O.; Keiser, J.; Meister, I.; Bachmann, A.; Wirz, D.; Gopfert, B.; Bonkat, G.; Wadso, I. Isothermal microcalorimetry accurately detects bacteria, tumorous microtissues, and parasitic worms in a label-free well-plate assay. Biotechnol. J. 2015, 10, 460–468. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Engler, A.J. Acute shear stress direction dictates adherent cell remodeling and verifies shear profile of spinning disk assays. Phys. Biol. 2015, 12, 016011. [Google Scholar] [CrossRef]
- Gallant, N.D.; Garcia, A.J. Quantitative analyses of cell adhesion strength. Methods Mol. Biol. 2007, 370, 83–96. [Google Scholar] [CrossRef]
- Boettiger, D. Quantitative measurements of integrin-mediated adhesion to extracellular matrix. Methods Enzym. 2007, 426, 1–25. [Google Scholar] [CrossRef]
- Hosch, S.; Kraus, J.; Scheunemann, P.; Izbicki, J.R.; Schneider, C.; Schumacher, U.; Witter, K.; Speicher, M.R.; Pantel, K. Malignant potential and cytogenetic characteristics of occult disseminated tumor cells in esophageal cancer. Cancer Res. 2000, 60, 6836–6840. [Google Scholar]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef]
- Drenckhan, A.; Kurschat, N.; Dohrmann, T.; Raabe, N.; Koenig, A.M.; Reichelt, U.; Kaifi, J.T.; Izbicki, J.R.; Gros, S.J. Effective inhibition of metastases and primary tumor growth with CTCE-9908 in esophageal cancer. J. Surg. Res. 2013, 182, 250–256. [Google Scholar] [CrossRef]
- Gros, S.J.; Graeff, H.; Drenckhan, A.; Kurschat, N.; Blessmann, M.; Rawnaq, T.; Izbicki, J.R. CXCR4/SDF-1alpha-mediated chemotaxis in an in vivo model of metastatic esophageal carcinoma. In Vivo 2012, 26, 711–718. [Google Scholar] [PubMed]
- Gros, S.J.; Kurschat, N.; Dohrmann, T.; Drenckhan, A.; Reichelt, U.; Schultze, A.; Effenberger-Harms, K.; Rawnaq, T.; Peldschus, K.; Eicke-Kohlmorgen, U.; et al. Significant reduction of primary growth and metastases through combined CXCR4 and HER-2 inhibition in an esophageal carcinoma orthotopic model. Cancer Res. 2011, 71, 1674. [Google Scholar]
- Gros, S.J.; Kurschat, N.; Dohrmann, T.; Reichelt, U.; Dancau, A.M.; Peldschus, K.; Adam, G.; Hoffman, R.M.; Izbicki, J.R.; Kaifi, J.T. Effective therapeutic targeting of the overexpressed HER-2 receptor in a highly metastatic orthotopic model of esophageal carcinoma. Mol. Cancer Ther. 2010, 9, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Axelson, H. The Notch signaling cascade in neuroblastoma: Role of the basic helix-loop-helix proteins HASH-1 and HES-1. Cancer Lett. 2004, 204, 171–178. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 71–103. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- DiMilla, P.A.; Stone, J.A.; Quinn, J.A.; Albelda, S.M.; Lauffenburger, D.A. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 1993, 122, 729–737. [Google Scholar] [CrossRef]
- Bijian, K.; Lougheed, C.; Su, J.; Xu, B.; Yu, H.; Wu, J.H.; Riccio, K.; Alaoui-Jamali, M.A. Targeting focal adhesion turnover in invasive breast cancer cells by the purine derivative reversine. Br. J. Cancer 2013, 109, 2810–2818. [Google Scholar] [CrossRef]
- Indra, I.; Undyala, V.; Kandow, C.; Thirumurthi, U.; Dembo, M.; Beningo, K.A. An in vitro correlation of mechanical forces and metastatic capacity. Phys. Biol. 2011, 8, 015015. [Google Scholar] [CrossRef]
- Braissant, O.; Muller, G.; Egli, A.; Widmer, A.; Frei, R.; Halla, A.; Wirz, D.; Gasser, T.C.; Bachmann, A.; Wagenlehner, F.; et al. Seven hours to adequate antimicrobial therapy in urosepsis using isothermal microcalorimetry. J. Clin. Microbiol. 2014, 52, 624–626. [Google Scholar] [CrossRef]
- Galindo, F.G.; Rocculi, P.; Wadso, L.; Sjohlm, I. The potential of isothermal calorimetry in monitoring and predicting quality changes during processing and storage of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2005, 16, 325–331. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Fathi, M.H.; Karbasi, S.; Braissant, O.; Daniels, A.U. Direct cytotoxicity evaluation of 63S bioactive glass and bone-derived hydroxyapatite particles using yeast model and human chondrocyte cells by microcalorimetry. J. Mater. Sci. 2011, 22, 2293–2300. [Google Scholar] [CrossRef]
- Gros, S.J.; Holland-Cunz, S.G.; Supuran, C.T.; Braissant, O. Personalized Treatment Response Assessment for Rare Childhood Tumors Using Microcalorimetry-Exemplified by Use of Carbonic Anhydrase IX and Aquaporin 1 Inhibitors. Int. J. Mol. Sci. 2019, 20, 4984. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Diez, G.; Koch, T.M.; Marg, S.; Ziegler, W.H.; Goldmann, W.H.; Fabry, B. Vinculin facilitates cell invasion into three-dimensional collagen matrices. J. Biol. Chem. 2010, 285, 13121–13130. [Google Scholar] [CrossRef]
- Kraning-Rush, C.M.; Califano, J.P.; Reinhart-King, C.A. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE 2012, 7, e32572. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.; Lomora, M.; Kym, U.; Palivan, C.; Holland-Cunz, S.G.; Gros, S.J. AQP1 Is Up-Regulated by Hypoxia and Leads to Increased Cell Water Permeability, Motility, and Migration in Neuroblastoma. Front. Cell Dev. Biol. 2021, 9, 605272. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. The morphogenesis of evolutionary developmental biology. Int. J. Dev. Biol. 2003, 47, 467–477. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Z.; Sá Santos, M.; Drenckhan, A.; Holland-Cunz, S.; Izbicki, J.R.; Nash, M.A.; Gros, S.J. Metastatic Esophageal Carcinoma Cells Exhibit Reduced Adhesion Strength and Enhanced Thermogenesis. Cells 2021, 10, 1213. https://doi.org/10.3390/cells10051213
Huo Z, Sá Santos M, Drenckhan A, Holland-Cunz S, Izbicki JR, Nash MA, Gros SJ. Metastatic Esophageal Carcinoma Cells Exhibit Reduced Adhesion Strength and Enhanced Thermogenesis. Cells. 2021; 10(5):1213. https://doi.org/10.3390/cells10051213
Chicago/Turabian StyleHuo, Zihe, Mariana Sá Santos, Astrid Drenckhan, Stefan Holland-Cunz, Jakob R. Izbicki, Michael A. Nash, and Stephanie J. Gros. 2021. "Metastatic Esophageal Carcinoma Cells Exhibit Reduced Adhesion Strength and Enhanced Thermogenesis" Cells 10, no. 5: 1213. https://doi.org/10.3390/cells10051213
APA StyleHuo, Z., Sá Santos, M., Drenckhan, A., Holland-Cunz, S., Izbicki, J. R., Nash, M. A., & Gros, S. J. (2021). Metastatic Esophageal Carcinoma Cells Exhibit Reduced Adhesion Strength and Enhanced Thermogenesis. Cells, 10(5), 1213. https://doi.org/10.3390/cells10051213






