Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2)
Abstract
1. Introduction
1.1. Classification of MCs
1.2. Mast Cell Receptors and Activation Mechanism
1.3. Mast Cell Mediators
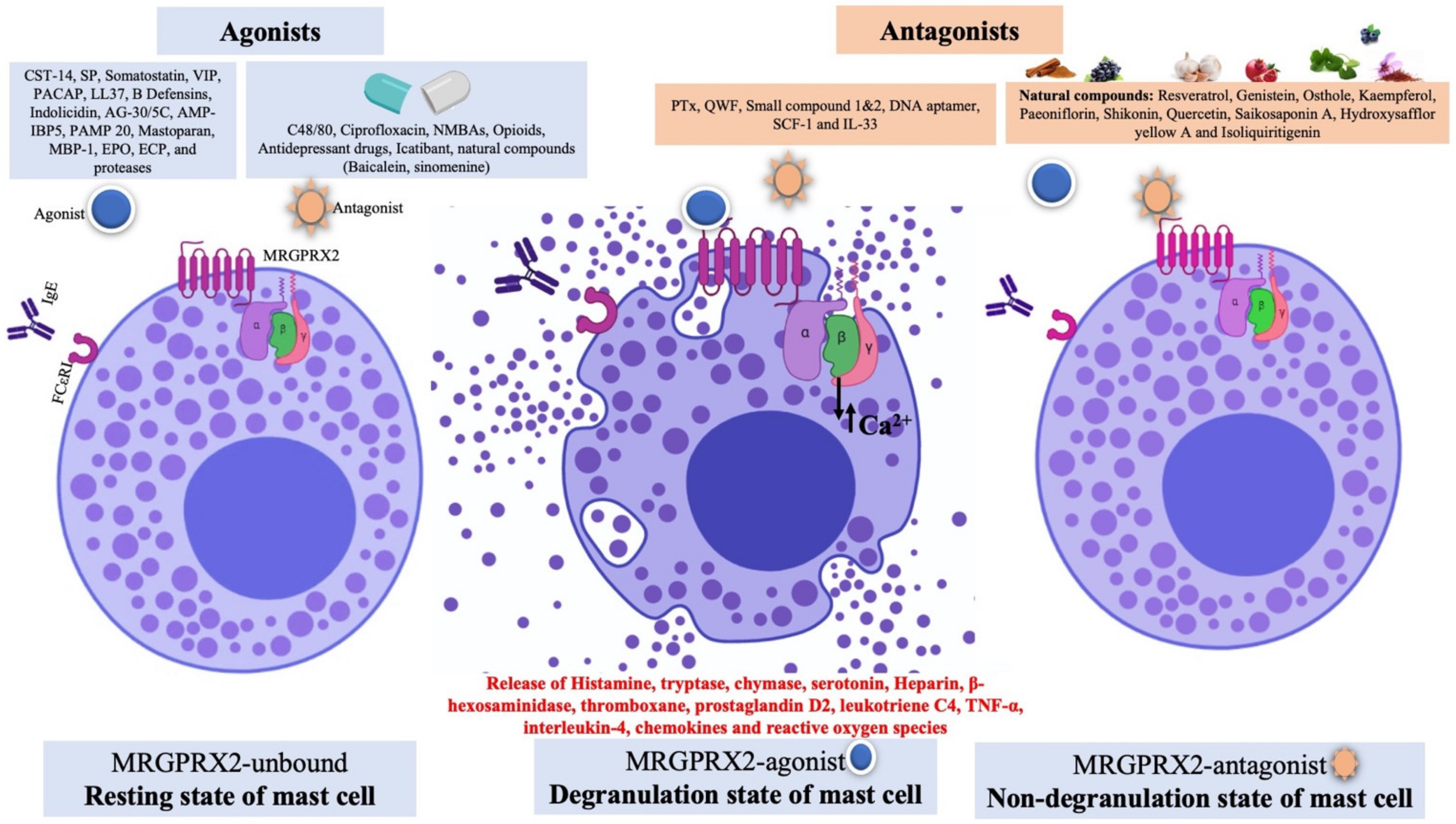
2. Pharmacology of MAS-Related G Protein-Coupled Receptors X 2 (MRGPRX2)
2.1. MRGPRX2 Ortholog in Mice and Expression Profile
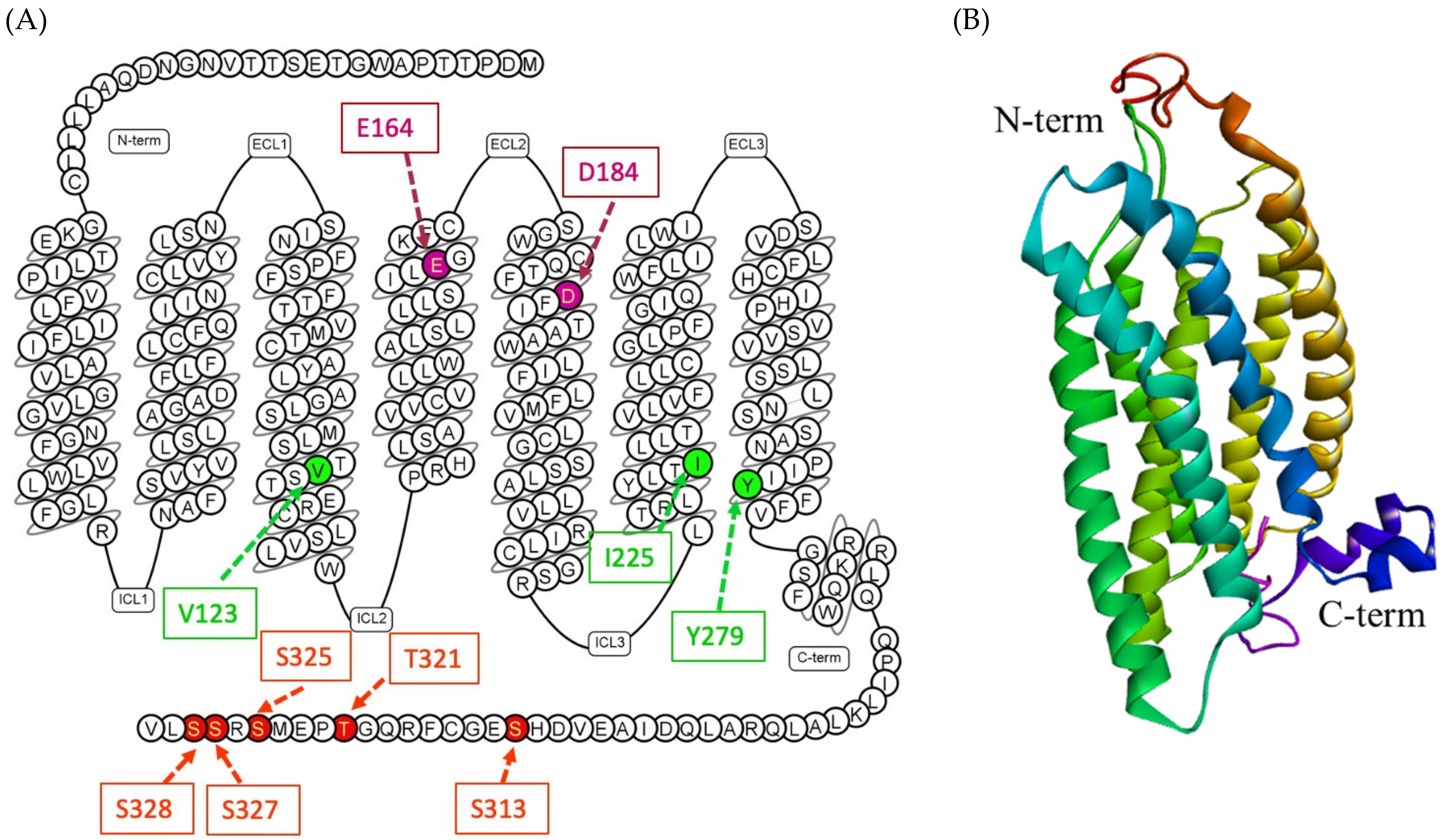
2.2. Structural Insights of MRGPRX2 and Signaling Pathway
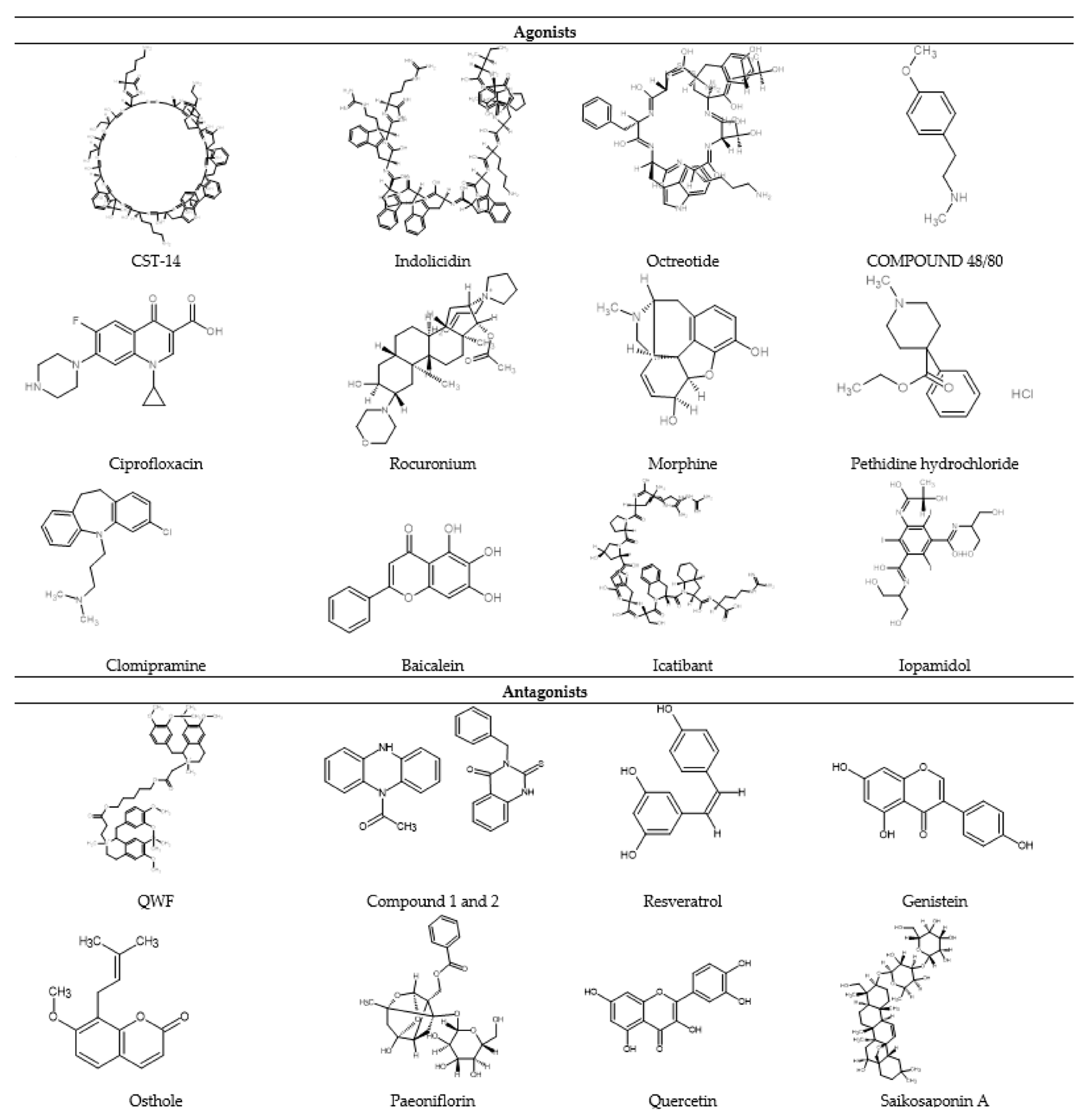
2.3. Diversity of MRGPRX2 Agonists
2.3.1. Peptide Agonists
Cortistatin-14 (CST)
Substance P (SP)
Somatostatin
Vasoactive Intestinal Polypeptide (VIP)
Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)
Human Cathelicidins
β-Defensins
Indolicidin
Angiogenic Peptide 30/5C (AG-30/5C)
AMPs Derived from Insulin-Like Growth Factor-Binding Protein 5 (AMP-IBP5)
Proadrenomedullin N-terminal peptides (PAMP 20)
Mastoparan
Eosinophil Granule Proteins
Proteases
Gonadotropin-Releasing Hormone (GnRH) Receptor Agonist and Antagonist
2.3.2. Non-Peptide Agonist
Compound 48/80 (C48/80)
Fluoroquinolone Antibiotics
Neuromuscular Blocking Agents (NMBAs)
Opioids and Their Derivatives
Antidepressant Drugs
Others
3. MRGPRX2 Unlocking the Puzzle of Non-IgE-Mediated Pseudo-Allergic Reaction
4. Development of MRGPRX2 Antagonists
4.1. Pertussis Toxin (PTx)
4.2. Tripeptide QWF (Gln-Trp-Phe)
4.3. Small Compound Antagonist
4.4. Natural Compounds
4.5. Others
5. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar]
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-related G protein-coupled receptor-X2 (MRGPRX2) in drug hypersensitivity reactions. Front. Immunol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhang, C.; Sun, M.; Wu, D.; Cheng, L.; Pan, B.; Li, T.; Che, D. MRGPRX2 mediates immediate-type pseudo-allergic reactions induced by iodine-containing iohexol. Biomed. Pharmacother. 2021, 137, 111323. [Google Scholar] [CrossRef]
- Navinés-Ferrer, A.; Serrano-Candelas, E.; Lafuente, A.; Muñoz-Cano, R.; Martín, M.; Gastaminza, G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gell, P. The classification of allergic reactions underlying disease. Clin. Asp. Immunol. 1963, 317–337. [Google Scholar]
- Rajan, T. The Gell–Coombs classification of hypersensitivity reactions: A re-interpretation. Trends Immunol. 2003, 24, 376–379. [Google Scholar] [CrossRef]
- Jurakic Tončić, R.; Marinović, B.; Lipozenčić, J. Nonallergic hypersensitivity to nonsteroidal antiinflammatory drugs, angiotensin-converting enzyme inhibitors, radiocontrast media, local anesthetics, volume substitutes and medications used in general anesthesia. Acta Dermatovenerol. Croat. 2009, 17, 54–69. [Google Scholar] [PubMed]
- He, S.-H.; Zhang, H.-Y.; Zeng, X.-N.; Chen, D.; Yang, P.-C. Mast cells and basophils are essential for allergies: Mechanisms of allergic inflammation and a proposed procedure for diagnosis. Acta Pharmacol. Sin. 2013, 34, 1270–1283. [Google Scholar] [CrossRef]
- Demoly, P.; Lebel, B.; Messaad, D.; Sahla, H.; Rongier, M.; Daures, J.; Godard, P.; Bousquet, J. Predictive capacity of histamine release for the diagnosis of drug allergy. Allergy 1999, 54, 500–506. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell proteases as protective and inflammatory mediators. In Mast Cell Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 212–234. [Google Scholar]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood J. Am. Soc. Hematol. 2014, 123, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.R.; Kawaji, H.; Rehli, M.; Baillie, J.K.; de Hoon, M.J.; Haberle, V.; Lassmann, T.; Kulakovskiy, I.V.; Lizio, M.; Itoh, M. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar] [PubMed]
- Noguchi, S.; Arakawa, T.; Fukuda, S.; Furuno, M.; Hasegawa, A.; Hori, F.; Ishikawa-Kato, S.; Kaida, K.; Kaiho, A.; Kanamori-Katayama, M.; et al. FANTOM5 CAGE profiles of human and mouse samples. Sci. Data 2017, 4, 170112. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef]
- Irani, A.; Bradford, T.R.; Kepley, C.L.; Schechter, N.M.; Schwartz, L.B. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J. Histochem. Cytochem. 1989, 37, 1509–1515. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–530. [Google Scholar] [CrossRef]
- Ruitenberg, E.; Elgersma, A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature 1976, 264, 258–260. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein–coupled receptor X2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Zhao, W.; Min, H.-K.; Xia, H.-Z.; Pozez, A.; Kiev, J.; Schwartz, L.B. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005, 115, 1162–1168. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Xia, H.-Z.; Sanchez-Muñoz, L.B.; Dellinger, A.L.; Escribano, L.; Schwartz, L.B. Generation of anaphylatoxins by human β-tryptase from C3, C4, and C5. J. Immunol. 2008, 180, 6307–6316. [Google Scholar] [CrossRef]
- Patella, V.; de Crescenzo, G.; Ciccarelli, A.; Marinò, I.; Adt, M.; Marone, G. Human heart mast cells: A definitive case of mast cell heterogeneity. Int. Arch. Allergy Immunol. 1995, 106, 386–393. [Google Scholar] [CrossRef]
- Ali, H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol. Lett. 2010, 128, 36–45. [Google Scholar] [CrossRef]
- Schäfer, B.; Piliponsky, A.M.; Oka, T.; Song, C.H.; Gerard, N.P.; Gerard, C.; Tsai, M.; Kalesnikoff, J.; Galli, S.J. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J. Allergy Clin. Immunol. 2013, 131, 541–548.e9. [Google Scholar] [CrossRef]
- Ogasawara, T.; Murakami, M.; Suzuki-Nishimura, T.; Uchida, M.K.; Kudo, I. Mouse bone marrow-derived mast cells undergo exocytosis, prostanoid generation, and cytokine expression in response to G protein-activating polybasic compounds after coculture with fibroblasts in the presence of c-kit ligand. J. Immunol. 1997, 158, 393–404. [Google Scholar]
- Erdei, A.; Andrásfalvy, M.; Péterfy, H.; Tóth, G.; Pecht, I. Regulation of mast cell activation by complement-derived peptides. Immunol. Lett. 2004, 92, 39–42. [Google Scholar] [CrossRef]
- Ali, H. Emerging roles for MAS-related G protein-coupled receptor-X2 in host defense peptide, opioid, and neuropeptide-mediated inflammatory reactions. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 136, pp. 123–162. [Google Scholar]
- Babina, M.; Guhl, S.; Artuc, M.; Trivedi, N.N.; Zuberbier, T. Phenotypic variability in human skin mast cells. Exp. Dermatol. 2016, 25, 434–439. [Google Scholar] [CrossRef]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef]
- Steinhoff, M.; Ständer, S.; Seeliger, S.; Ansel, J.C.; Schmelz, M.; Luger, T. Modern aspects of cutaneous neurogenic inflammation. Arch. Dermatol. 2003, 139, 1479–1488. [Google Scholar] [CrossRef]
- Shtessel, M.; Limjunyawong, N.; Oliver, E.T.; Chichester, K.; Gao, L.; Dong, X.; Saini, S.S. MRGPRX2 Activation Causes Increased Skin Reactivity in Chronic Spontaneous Urticaria Patients. J. Investig. Dermatol. 2020, 141, 678–681.e2. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D. MRGPRX2 and Immediate Drug Hypersensitivity: Insights from Cultured Human Mast Cells. J. Investig. Allergol. Clin. Immunol. 2020, 31. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Wershil, B.K. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am. J. Pathol. 1993, 142, 965. [Google Scholar] [PubMed]
- Tkaczyk, C.; Horejsi, V.; Iwaki, S.; Draber, P.; Samelson, L.E.; Satterthwaite, A.B.; Nahm, D.-H.; Metcalfe, D.D.; Gilfillan, A.M. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and FcϵRI aggregation. Blood 2004, 104, 207–214. [Google Scholar] [CrossRef]
- Qiao, H.; Andrade, M.V.; Lisboa, F.A.; Morgan, K.; Beaven, M.A. FcϵR1 and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood 2006, 107, 610–618. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Gilfillan, A.M. G protein-coupled receptors and the modification of FcɛRI-mediated mast cell activation. Immunol. Lett. 2007, 113, 59–69. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Kabesch, M. Current concepts of IgE regulation and impact of genetic determinants. Clin. Exp. Allergy 2012, 42, 852–871. [Google Scholar] [CrossRef]
- Babina, M. The pseudo-allergic/neurogenic route of mast cell activation via MRGPRX2: Discovery, functional programs, regulation, relevance to disease, and relation with allergic stimulation. Itch 2020, 5, e32. [Google Scholar] [CrossRef]
- Muñoz-Cano, R.; Pascal, M.; Araujo, G.; Goikoetxea, M.; Valero, A.L.; Picado, C.; Bartra, J. Mechanisms, cofactors, and augmenting factors involved in anaphylaxis. Front. Immunol. 2017, 8, 1193. [Google Scholar] [CrossRef]
- Jimenez-Rodriguez, T.W.; Garcia-Neuer, M.; Alenazy, L.A.; Castells, M. Anaphylaxis in the 21st century: Phenotypes, endotypes, and biomarkers. J. Asthma Allergy 2018, 11, 121. [Google Scholar] [CrossRef]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Mousli, M.; Bronner, C.; Bockaert, J.; Rouot, B.; Landry, Y. Interaction of substance P, compound 4880 and mastoparan with the α-subunit C-terminus of G protein. Immunol. Lett. 1990, 25, 355–357. [Google Scholar] [CrossRef]
- Mousli, M.; Bueb, J.-L.; Bronner, C.; Rouot, B.; Landry, Y. G protein activation: A receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol. Sci. 1990, 11, 358–362. [Google Scholar] [CrossRef]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, D.; Kashiwakura, J.-I.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633.e9. [Google Scholar] [CrossRef]
- Kajiwara, N.; Sasaki, T.; Bradding, P.; Cruse, G.; Sagara, H.; Ohmori, K.; Saito, H.; Ra, C.; Okayama, Y. Activation of human mast cells through the platelet-activating factor receptor. J. Allergy Clin. Immunol. 2010, 125, 1137–1145.e6. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Song, P.; Yue, H.; Sutthammikorn, N.; Umehara, Y.; Okumura, K.; Ogawa, H. Antimicrobial peptide derived from insulin-like growth factor-binding protein 5 activates mast cells via Mas-related G protein-coupled receptor X2. Allergy 2020, 75, 203–207. [Google Scholar] [CrossRef]
- Kanazawa, K.; Okumura, K.; Ogawa, H.; Niyonsaba, F. An antimicrobial peptide with angiogenic properties, AG-30/5C, activates human mast cells through the MAPK and NF-κB pathways. Immunol. Res. 2016, 64, 594–603. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Guo, Q.; Price, R.; Ali, H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 2011, 286, 44739–44749. [Google Scholar] [CrossRef]
- Geppetti, P.; Veldhuis, N.A.; Lieu, T.; Bunnett, N.W. G protein-coupled receptors: Dynamic machines for signaling pain and itch. Neuron 2015, 88, 635–649. [Google Scholar] [CrossRef]
- Kempkes, C.; Buddenkotte, J.; Cevikbas, F.; Buhl, T.; Steinhoff, M. 11 Role of PAR-2 in Neuroimmune Communication and Itch. In Itch Mechanisms and Treatment; CRC Press: Boca Raton, FL, USA, 2014; Volume 193. [Google Scholar]
- Tsujii, K.; Andoh, T.; Ui, H.; Lee, J.-B.; Kuraishi, Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J. Pharmacol. Sci. 2009, 109, 388–395. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Buyuktiryaki, B.; Gungor, H.E.; Sahiner, N.; Turasan, A.; Torun, Y.A.; Sekerel, B.E. Factors that predict disease severity in atopic dermatitis: The role of serum basal tryptase. Allergy Asthma Proc. 2018, 39, 5. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.H.; Wang, X.R.; Liu, Y.; Chen, M.; Xu, X.G.; Liao, W.Q.; Hu, J.H. Tryptase and protease-activated receptor-2 stimulate scratching behavior in a murine model of ovalbumin-induced atopic-like dermatitis. Int. Immunopharmacol. 2015, 28, 507–512. [Google Scholar] [CrossRef]
- Gaudenzio, N.; Sibilano, R.; Marichal, T.; Starkl, P.; Reber, L.L.; Cenac, N.; McNeil, B.D.; Dong, X.; Hernandez, J.D.; Sagi-Eisenberg, R.; et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Investig. 2016, 126, 3981–3998. [Google Scholar] [CrossRef]
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 2019, 50, 1163–1171.e5. [Google Scholar] [CrossRef]
- Solinski, H.J.; Gudermann, T.; Breit, A. Pharmacology and signaling of MAS-related G protein–coupled receptors. Pharmacol. Rev. 2014, 66, 570–597. [Google Scholar] [CrossRef]
- Hamamura-Yasuno, E.; Iguchi, T.; Kumagai, K.; Tsuchiya, Y.; Mori, K. Identification of the dog orthologue of human MAS-related G protein coupled receptor X2 (MRGPRX2) essential for drug-induced pseudo-allergic reactions. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Dong, X.; Han, S.-K.; Zylka, M.J.; Simon, M.I.; Anderson, D.J. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 2001, 106, 619–632. [Google Scholar] [CrossRef]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.-G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Katritch, V.; Cherezov, V.; Stevens, R.C. Structure-function of the G protein–coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531–556. [Google Scholar] [CrossRef]
- Davenport, A.P.; Alexander, S.P.; Sharman, J.L.; Pawson, A.J.; Benson, H.E.; Monaghan, A.E.; Liew, W.C.; Mpamhanga, C.P.; Bonner, T.I.; Neubig, R.R.; et al. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: Recommendations for new pairings with cognate ligands. Pharmacol. Rev. 2013, 65, 967–986. [Google Scholar] [CrossRef]
- Karhu, T.; Akiyama, K.; Vuolteenaho, O.; Bergmann, U.; Naito, T.; Tatemoto, K.; Herzig, K.-H. Mast cell degranulation via MRGPRX2 by isolated human albumin fragments. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2530–2534. [Google Scholar] [CrossRef]
- Alexander, S.P.; Battey, J.; Benson, H.E.; Benya, R.V.; Bonner, T.I.; Davenport, A.P.; Singh, K.D.; Eguchi, S.; Harmar, A.; Holliday, N.; et al. Class A Orphans (version 2020.5) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR BPS Guide Pharmacol. CITE 2020, 2020. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Z.; Surdenikova, L.; Kim, S.; Patel, K.N.; Kim, A.; Ru, F.; Guan, Y.; Weng, H.-J.; Geng, Y.; et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009, 139, 1353–1365. [Google Scholar] [CrossRef]
- Lembo, P.M.; Grazzini, E.; Groblewski, T.; O’Donnell, D.; Roy, M.-O.; Zhang, J.; Hoffert, C.; Cao, J.; Schmidt, R.; Pelletier, M.; et al. Proenkephalin A gene products activate a new family of sensory neuron–specific GPCRs. Nat. Neurosci. 2002, 5, 201–209. [Google Scholar] [CrossRef]
- Zylka, M.J.; Dong, X.; Southwell, A.L.; Anderson, D.J. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc. Natl. Acad. Sci. USA 2003, 100, 10043–10048. [Google Scholar] [CrossRef]
- Reddy, V.B.; Graham, T.A.; Azimi, E.; Lerner, E.A. A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J. Allergy Clin. Immunol. 2017, 140, 1726. [Google Scholar] [CrossRef]
- Azimi, E.; Reddy, V.B.; Pereira, P.J.S.; Talbot, S.; Woolf, C.J.; Lerner, E.A. Substance P activates Mas-related G protein–coupled receptors to induce itch. J. Allergy Clin. Immunol. 2017, 140, 447–453.e3. [Google Scholar] [CrossRef]
- Azimi, E.; Reddy, V.B.; Shade, K.-T.C.; Anthony, R.M.; Talbot, S.; Pereira, P.J.S.; Lerner, E.A. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 2016, 1, e89362. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef]
- Robas, N.; Mead, E.; Fidock, M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J. Biol. Chem. 2003, 278, 44400–44404. [Google Scholar] [CrossRef]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 2019, 101, 412–420.e3. [Google Scholar] [CrossRef] [PubMed]
- Kamohara, M.; Matsuo, A.; Takasaki, J.; Kohda, M.; Matsumoto, M.; Matsumoto, S.-I.; Soga, T.; Hiyama, H.; Kobori, M.; Katou, M. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem. Biophys. Res. Commun. 2005, 330, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Chompunud Na Ayudhya, C.; Roy, S.; Thapaliya, M.; Ali, H. Roles of a Mast Cell–Specific Receptor MRGPRX2 in Host Defense and Inflammation. J. Dent. Res. 2020, 99, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, K.; Duraisamy, K.; Allam, A.A.; Ajarem, J.; Kwok Chong Chow, B. Protective effect of genistein against compound 48/80 induced anaphylactoid shock via inhibiting MAS related G protein-coupled receptor X2 (MRGPRX2). Molecules 2020, 25, 1028. [Google Scholar] [CrossRef]
- Li, W.; Yosipovitch, G. The role of the microbiome and microbiome-derived metabolites in atopic dermatitis and non-histaminergic itch. Am. J. Clin. Dermatol. 2020, 21, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kiatsurayanon, C.; Niyonsaba, F.; Chieosilapatham, P.; Okumura, K.; Ikeda, S.; Ogawa, H. Angiogenic peptide (AG)-30/5C activates human keratinocytes to produce cytokines/chemokines and to migrate and proliferate via MrgX receptors. J. Dermatol. Sci. 2016, 83, 190–199. [Google Scholar] [CrossRef]
- Ogasawara, H.; Furuno, M.; Edamura, K.; Noguchi, M. Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J. Leukoc. Biol. 2019, 106, 1069–1077. [Google Scholar] [CrossRef]
- Bader, M.; Alenina, N.; Andrade-Navarro, M.A.; Santos, R.A. Mas and its related G protein–coupled receptors, Mrgprs. Pharmacol. Rev. 2014, 66, 1080–1105. [Google Scholar] [CrossRef]
- Choi, S.S.; Lahn, B.T. Adaptive evolution of MRG, a neuron-specific gene family implicated in nociception. Genome Res. 2003, 13, 2252–2259. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, M.; Cho, E.Y.; Jiang, W.; Sha, O. The origin, expression, function and future research focus of a G protein-coupled receptor, Mas-related Gene X2 (MrgX2). Prog. Histochem. Cytochem. 2015, 50, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.-N.; Ping, N.-N.; Cao, Y.-X. Ligands and Signaling of Mas-Related G Protein-Coupled Receptor-X2 in Mast Cell Activation. Rev. Physiol. Biochem. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Chompunud Na Ayudhya, C.; Amponnawarat, A.; Roy, S.; Oskeritzian, C.A.; Ali, H. MRGPRX2 Activation by Rocuronium: Insights from Studies with Human Skin Mast Cells and Missense Variants. Cells 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Chompunud Na Ayudhya, C.; Roy, S.; Alkanfari, I.; Ganguly, A.; Ali, H. Identification of gain and loss of function missense variants in MRGPRX2′s transmembrane and intracellular domains for mast cell activation by substance P. Int. J. Mol. Sci. 2019, 20, 5247. [Google Scholar] [CrossRef] [PubMed]
- Lansu, K.; Karpiak, J.; Liu, J.; Huang, X.-P.; McCorvy, J.D.; Kroeze, W.K.; Che, T.; Nagase, H.; Carroll, F.I.; Jin, J.; et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 2017, 13, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Alkanfari, I.; Gupta, K.; Jahan, T.; Ali, H. Naturally occurring missense MRGPRX2 variants display loss of function phenotype for mast cell degranulation in response to substance P, hemokinin-1, human β-defensin-3, and icatibant. J. Immunol. 2018, 201, 343–349. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Heydenreich, F.M.; Flock, T.; Miljus, T.; Balaji, S.; Bouvier, M.; Veprintsev, D.B.; Tate, C.G.; et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature 2016, 536, 484–487. [Google Scholar] [CrossRef]
- Isberg, V.; Mordalski, S.; Munk, C.; Rataj, K.; Harpsøe, K.; Hauser, A.S.; Vroling, B.; Bojarski, A.J.; Vriend, G.; Gloriam, D.E. GPCRdb: An information system for G protein-coupled receptors. Nucleic Acids Res. 2016, 44, D356–D364. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef]
- Liu, R.; Che, D.; Zhao, T.; Pundir, P.; Cao, J.; Lv, Y.; Wang, J.; Ma, P.; Fu, J.; Wang, N.; et al. MRGPRX2 is essential for sinomenine hydrochloride induced anaphylactoid reactions. Biochem. Pharmacol. 2017, 146, 214–223. [Google Scholar] [CrossRef]
- Mori, K.; Maru, C.; Takasuna, K.; Furuhama, K. Mechanism of histamine release induced by levofloxacin, a fluoroquinolone antibacterial agent. Eur. J. Pharmacol. 2000, 394, 51–55. [Google Scholar] [CrossRef]
- Barrocas, A.M.; Cochrane, D.E.; Carraway, R.E.; Feldberg, R.S. Neurotensin stimulation of mast cell secretion is receptor-mediated, pertussis-toxin sensitive and requires activation of phospholipase C. Immunopharmacology 1999, 41, 131–137. [Google Scholar] [CrossRef]
- Roy, S.; Ganguly, A.; Haque, M.; Ali, H. Angiogenic host defense peptide AG-30/5C and Bradykinin B2 receptor antagonist icatibant are G protein biased agonists for MRGPRX2 in mast cells. J. Immunol. 2019, 202, 1229–1238. [Google Scholar] [CrossRef]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Matsumoto, K.; Saito, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; et al. Antimicrobial peptides human β-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur. J. Immunol. 2007, 37, 434–444. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human β-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Hu, S.; Ge, S.; Jia, M.; Wang, N. Resveratrol inhibits MRGPRX2-mediated mast cell activation via Nrf2 pathway. Int. Immunopharmacol. 2021, 93, 107426. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Iwabuchi, K.; Someya, A.; Hirata, M.; Matsuda, H.; Ogawa, H.; Nagaoka, I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 2002, 106, 20–26. [Google Scholar] [CrossRef]
- Yoshioka, M.; Fukuishi, N.; Kubo, Y.; Yamanobe, H.; Ohsaki, K.; Kawasoe, Y.; Murata, M.; Ishizumi, A.; Nishii, Y.; Matsui, N.; et al. Human cathelicidin CAP18/LL-37 changes mast cell function toward innate immunity. Biol. Pharm. Bull. 2008, 31, 212–216. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; van Harten, R.M.; Veldhuizen, E.J.; Haagsman, H.P.; Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. Front. Immunol. 2020, 11, 1137. [Google Scholar] [CrossRef]
- Gupta, K.; Subramanian, H.; Ali, H. Modulation of host defense peptide-mediated human mast cell activation by LPS. Innate Immun. 2016, 22, 21–30. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Lee, D.; Bayir, A.K.; Ahn, H.; Ali, H. β-Defensins activate human mast cells via Mas-related gene X2. J. Immunol. 2013, 191, 345–352. [Google Scholar] [CrossRef]
- Occhiuto, C.J.; Kammala, A.K.; Yang, C.; Nellutla, R.; Garcia, M.; Gomez, G.; Subramanian, H. Store-operated calcium entry via STIM1 contributes to MRGPRX2 induced mast cell functions. Front. Immunol. 2020, 10, 3143. [Google Scholar] [CrossRef]
- Nothacker, H.-P.; Wang, Z.; Zeng, H.; Mahata, S.K.; O’Connor, D.T.; Civelli, O. Proadrenomedullin N-terminal peptide and cortistatin activation of MrgX2 receptor is based on a common structural motif. Eur. J. Pharmacol. 2005, 519, 191–193. [Google Scholar] [CrossRef]
- Ogasawara, H.; Furuno, M.; Edamura, K.; Noguchi, M. Peptides of major basic protein and eosinophil cationic protein activate human mast cells. Biochem. Biophys. Rep. 2020, 21, 100719. [Google Scholar] [CrossRef]
- Reddy, V.B.; Sun, S.; Azimi, E.; Elmariah, S.B.; Dong, X.; Lerner, E.A. Redefining the concept of protease-activated receptors: Cathepsin S evokes itch via activation of Mrgprs. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Chung, K.; Pitcher, T.; Grant, A.D.; Hewitt, E.; Lindstrom, E.; Malcangio, M. Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour. Neurobiol. Pain 2019, 6, 100032. [Google Scholar] [CrossRef]
- Kashem, S.W.; Subramanian, H.; Collington, S.J.; Magotti, P.; Lambris, J.D.; Ali, H. G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: Roles of Mas-related genes MrgX1 and MrgX2. Eur. J. Pharmacol. 2011, 668, 299–304. [Google Scholar] [CrossRef]
- Ajima, S.; Sano, Y.; Hashizume, H. Quinolone immediate hypersensitivity due to topical ophthalmic preparations: A case report and review of literature. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Liu, R.; Hu, S.; Zhang, Y.; Che, D.; Cao, J.; Wang, J.; Zhao, T.; Jia, Q.; Wang, N.; Zhang, T. Mast cell-mediated hypersensitivity to fluoroquinolone is MRGPRX2 dependent. Int. Immunopharmacol. 2019, 70, 417–427. [Google Scholar] [CrossRef]
- Che, D.; Wang, J.; Ding, Y.; Liu, R.; Cao, J.; Zhang, Y.; Hou, Y.; An, H.; Gao, Z.; Zhang, T. Mivacurium induce mast cell activation and pseudo-allergic reactions via MAS-related G protein coupled receptor-X2. Cell. Immunol. 2018, 332, 121–128. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Zhao, T.; Cao, J.; Che, D.; Ma, P.; Zhang, Y.; Zhang, T.; Wang, N. Relationship between MRGPRX 2 and pethidine hydrochloride-or fentanyl citrate-induced LAD 2 cell degranulation. J. Pharm. Pharmacol. 2018, 70, 1596–1605. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Eddinger, K.A.; Kokubu, S.; Wang, Z.; DiNardo, A.; Ramachandran, R.; Zhu, Y.; He, Y.; Weren, F.; Quang, D.; et al. Mast cell degranulation and fibroblast activation in the morphine-induced spinal mass: Role of mas-related G protein-coupled receptor signaling. Anesthesiology 2019, 131, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Kühn, H.; Boehm, F.; Gebhardt, L.; Glaudo, M.; Agelopoulos, K.; Ständer, S.; Ectors, P.; Zahn, D.; Riedel, Y.K.; et al. A Group of Cationic Amphiphilic Drugs Activates MRGPRX2 and Induces Scratching Behavior in Mice. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Che, D.; Zeng, Y.; Wu, Y.; Qin, Q.; Wang, N. Baicalin induces Mrgprb2-dependent pseudo-allergy in mice. Immunol. Lett. 2020, 226, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Spier, A.D.; de Lecea, L. Cortistatin: A member of the somatostatin neuropeptide family with distinct physiological functions. Brain Res. Rev. 2000, 33, 228–241. [Google Scholar] [CrossRef]
- Lu, L.; Kulka, M.; Unsworth, L.D. Peptide-mediated mast cell activation: Ligand similarities for receptor recognition and protease-induced regulation. J. Leukoc. Biol. 2017, 102, 237–251. [Google Scholar] [CrossRef]
- Vena, G.A.; Cassano, N.; di Leo, E.; Calogiuri, G.; Nettis, E. Focus on the role of substance P in chronic urticaria. Clin. Mol. Allergy 2018, 16, 1–6. [Google Scholar] [CrossRef]
- Liu, L.; Tan, Q.; Hu, B.; Wu, H.; Wang, C.; Tang, C. Somatostatin Inhibits the Production of Interferon-γ by Intestinal Epithelial Cells During Intestinal Ischemia–Reperfusion in Macaques. Dig. Dis. Sci. 2014, 59, 2423–2432. [Google Scholar] [CrossRef]
- Delgado, M.; Pozo, D.; Ganea, D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol. Rev. 2004, 56, 249–290. [Google Scholar] [CrossRef]
- Ødum, L.; Petersen, L.; Skov, P.; Ebskov, L. Pituitary adenylate cyclase activating polypeptide (PACAP) is localized in human dermal neurons and causes histamine release from skin mast cells. Inflamm. Res. 1998, 47, 488–492. [Google Scholar] [CrossRef]
- Sherwood, N.M.; Krueckl, S.L.; McRory, J.E. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr. Rev. 2000, 21, 619–670. [Google Scholar]
- Hazlett, L.; Wu, M. Defensins in innate immunity. Cell Tissue Res. 2011, 343, 175–188. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Bąbolewska, E.; Brzezińska-Błaszczyk, E. Human-derived cathelicidin LL-37 directly activates mast cells to proinflammatory mediator synthesis and migratory response. Cell. Immunol. 2015, 293, 67–73. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Someya, A.; Hirata, M.; Ogawa, H.; Nagaoka, I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur. J. Immunol. 2001, 31, 1066–1075. [Google Scholar] [CrossRef]
- Yang, D.; Chertov, O.; Bykovskaia, S.; Chen, Q.; Buffo, M.; Shogan, J.; Anderson, M.; Schröder, J.; Wang, J.; Howard, O.; et al. β-defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999, 286, 525–528. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Iwabuchi, K.; Matsuda, H.; Ogawa, H.; Nagaoka, I. Epithelial cell-derived human β-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int. Immunol. 2002, 14, 421–426. [Google Scholar] [CrossRef]
- Osaki, T.; Sasaki, K.; Minamino, N. Peptidomics-based discovery of an antimicrobial peptide derived from insulin-like growth factor-binding protein 5. J. Proteome Res. 2011, 10, 1870–1880. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshida, H.; Kitaichi, K.; Hiramatsu, K.; Kimura, T.; Ito, Y.; Kume, H.; Yamaki, K.; Suzuki, R.; Shibata, E.; et al. Adrenomedullin and proadrenomedullin N-terminal 20 peptide induce histamine release from rat peritoneal mast cell. Regul. Pept. 2001, 101, 163–168. [Google Scholar] [CrossRef]
- Mousli, M.; Bronner, C.; Bueb, J.-L.; Tschirhart, E.; Gies, J.; Landry, Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J. Pharmacol. Exp. Ther. 1989, 250, 329–335. [Google Scholar]
- Krüger, P.-G.; Mahata, S.K.; Helle, K.B. Catestatin (CgA344–364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regul. Pept. 2003, 114, 29–35. [Google Scholar] [CrossRef]
- O’Connell, A.E.; Hess, J.A.; Santiago, G.A.; Nolan, T.J.; Lok, J.B.; Lee, J.J.; Abraham, D. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect. Immun. 2011, 79, 2770–2778. [Google Scholar] [CrossRef]
- Abu-Ghazaleh, R.I.; Gleich, G.J.; Prendergast, F.G. Interaction of eosinophil granule major basic protein with synthetic lipid bilayers: A mechanism for toxicity. J. Membr. Biol. 1992, 128, 153–164. [Google Scholar] [CrossRef]
- Patella, V.; de Crescenzo, G.; Marinò, I.; Genovese, A.; Adt, M.; Gleich, G.J.; Marone, G. Eosinophil granule proteins activate human heart mast cells. J. Immunol. 1996, 157, 1219–1225. [Google Scholar]
- Subramanian, H.; Kashem, S.W.; Collington, S.J.; Qu, H.; Lambris, J.D.; Ali, H. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol. Pharmacol. 2011, 79, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.J.; Bond, J.S. Proteolytic Enzymes: A Practical Approach; Oxford University Press Oxford: Oxford, UK, 2001; Volume 2. [Google Scholar]
- Reddy, V.B.; Iuga, A.O.; Shimada, S.G.; LaMotte, R.H.; Lerner, E.A. Cowhage-evoked itch is mediated by a novel cysteine protease: A ligand of protease-activated receptors. J. Neurosci. 2008, 28, 4331–4335. [Google Scholar] [CrossRef]
- Kim, N.; Bae, K.B.; Kim, M.O.; Yu, D.H.; Kim, H.J.; Yuh, H.S.; Ji, Y.R.; Park, S.J.; Kim, S.; Son, K.-H.; et al. Overexpression of cathepsin S induces chronic atopic dermatitis in mice. J. Investig. Dermatol. 2012, 132, 1169–1176. [Google Scholar] [CrossRef]
- Schönefuß, A.; Wendt, W.; Schattling, B.; Schulten, R.; Hoffmann, K.; Stuecker, M.; Tigges, C.; Lübbert, H.; Stichel, C. Upregulation of cathepsin S in psoriatic keratinocytes. Exp. Dermatol. 2010, 19, e80–e88. [Google Scholar] [CrossRef]
- McGee, E.U.; Samuel, E.; Boronea, B.; Dillard, N.; Milby, M.N.; Lewis, S.J. Quinolone allergy. Pharmacy 2019, 7, 97. [Google Scholar] [CrossRef]
- Rothschild, A. Mechanisms of histamine release by compound 48/80. Br. J. Pharmacol. 1970, 38, 253. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.; Gonçalves, D.G.; Zanandréa, A.; de Castro, R.B.; Garro, L.S.; Kalil, J.; Castells, M. Anaphylaxis to quinolones in mastocytosis: Hypothesis on the mechanism. J. Allergy Clin. Immunol. Pract. 2019, 7, 2089–2090. [Google Scholar] [CrossRef] [PubMed]
- Ciobotaru, O.R.; Stoleriu, G.; Ciobotaru, O.C.; Grigorovici, A.; Voinescu, D.C.; Matei, M.N.; Cobzaru, R.G.; Manolache, N.; Lupu, M.N. Postanesthetic skin erythema due to succinylcholine versus atracurium. Exp. Ther. Med. 2020, 20, 2368–2372. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, D.; Roux-Lombard, P.; Harr, T.; Czarnetzki, C. Non-IgE-dependent hypersensitivity to rocuronium reversed by sugammadex: Report of three cases and hypothesis on the underlying mechanism. Int. Arch. Allergy Immunol. 2016, 169, 256–262. [Google Scholar] [CrossRef]
- Suzuki, Y.; Liu, S.; Kadoya, F.; Takasaki, Y.; Yorozuya, T.; Mogi, M. Association between mutated Mas-related G protein-coupled receptor-X2 and rocuronium-induced intraoperative anaphylaxis. Br. J. Anaesth. 2020, 125, e446–e448. [Google Scholar] [CrossRef]
- Casale, T.B.; Bowman, S.; Kaliner, M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: Evidence for opiate and nonopiate receptor participation. J. Allergy Clin. Immunol. 1984, 73, 775–781. [Google Scholar] [CrossRef]
- Sheen, C.; Schleimer, R.; Kulka, M. Codeine induces human mast cell chemokine and cytokine production: Involvement of G-protein activation. Allergy 2007, 62, 532–538. [Google Scholar] [CrossRef]
- Nasser, S.; Ewan, P. Opiate-sensitivity: Clinical characteristics and the role of skin prick testing. Clin. Exp. Allergy 2001, 31, 1014–1020. [Google Scholar] [CrossRef]
- Li, P.H.; Ue, K.L.; Wagner, A.; Rutkowski, R.; Rutkowski, K. Opioid hypersensitivity: Predictors of allergy and role of drug provocation testing. J. Allergy Clin. Immunol. Pract. 2017, 5, 1601–1606. [Google Scholar] [CrossRef]
- Varricchi, G.; Pecoraro, A.; Loffredo, S.; Poto, R.; Rivellese, F.; Genovese, A.; Marone, G.; Spadaro, G. Heterogeneity of human mast cells with respect to MRGPRX2 receptor expression and function. Front. Cell. Neurosci. 2019, 13, 299. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, S.; Che, D.; An, H.; Liu, R. A mast-cell-specific receptor mediates Iopamidol induced immediate IgE-independent anaphylactoid reactions. Int. Immunopharmacol. 2019, 75, 105800. [Google Scholar] [CrossRef]
- Azimi, E.; Reddy, V.B.; Lerner, E.A. MRGPRX2, atopic dermatitis, and red man syndrome. Itch 2017, 2, e5. [Google Scholar] [CrossRef]
- Huang, L.; Dong, Y.; Wu, J.; Wang, P.; Zhou, H.; Li, T.; Liu, L. Sinomenine-induced histamine release-like anaphylactoid reactions are blocked by tranilast via inhibiting NF-κB signaling. Pharmacol. Res. 2017, 125, 150–160. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, R.; Han, Y.; Fei, Q.; Cai, R.; Qi, Y. Shuang-Huang-Lian injection induces an immediate hypersensitivity reaction via C5a but not IgE. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Han, S.; Zhang, T.; Huang, J.; Cui, R.; He, L. New method of screening allergenic components from Shuanghuanglian injection: With RBL-2H3/CMC model online HPLC/MS system. J. Pharm. Biomed. Anal. 2014, 88, 602–608. [Google Scholar] [CrossRef]
- National Center for Health Statistics. FastStats—Allergies and Hay Fever; CDC: Atlanta, GA, USA, 2019.
- American College of Allergy, Asthma, and Immunology. Allergy Facts|ACAAI Public Website. 2015. Available online: https://acaai.org/ (accessed on 10 May 2020).
- Chan, A.W.; Chan, J.K.; Tam, A.Y.; Leung, T.; Lee, T. Guidelines for allergy prevention in Hong Kong. Hong Kong Med. J. 2016, 22, 279–285. [Google Scholar] [CrossRef]
- Lee, S.L.; Wong, W.; Lau, Y.L. Increasing prevalence of allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood). Pediatric Allergy Immunol. 2004, 15, 72–78. [Google Scholar] [CrossRef]
- Wang, F.; Yang, T.-B.; Kim, B.S. The return of the mast cell: New roles in neuroimmune itch biology. J. Investig. Dermatol. 2020, 140, 945–951. [Google Scholar] [CrossRef]
- Dothel, G.; Barbaro, M.R.; Boudin, H.; Vasina, V.; Cremon, C.; Gargano, L.; Bellacosa, L.; de Giorgio, R.; le Berre-Scoul, C.; Aubert, P.; et al. Nerve fiber outgrowth is increased in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2015, 148, 1002–1011.e4. [Google Scholar] [CrossRef]
- Borici-Mazi, R.; Kouridakis, S.; Kontou-Fili, K. Cutaneous responses to substance P and calcitonin gene-related peptide in chronic urticaria: The effect of cetirizine and dimethindene. Allergy 1999, 54, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Atkinson, B.; Lee, T.; Morris, R.; Hayes, N.; Foreman, J. Cutaneous responses to vasoactive intestinal polypeptide in chronic idiopathic urticaria. Lancet 1992, 339, 91–93. [Google Scholar] [CrossRef]
- Douglas, S.D.; Leeman, S.E. Neurokinin-1 receptor: Functional significance in the immune system in reference to selected infections and inflammation. Ann. N. Y. Acad. Sci. 2011, 1217, 83. [Google Scholar] [CrossRef] [PubMed]
- Manorak, W.; Idahosa, C.; Gupta, K.; Roy, S.; Panettieri, R.; Ali, H. Upregulation of Mas-related G Protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respir. Res. 2018, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Keynan, Y. Vancomycin revisited–60 years later. Front. Public Health 2014, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.N.; Kammala, A.K.; Syed, M.; Yang, C.; Occhiuto, C.J.; Nellutla, R.; Chumanevich, A.P.; Oskeritzian, C.A.; Das, R.; Subramanian, H. Osthole, a Natural Plant Derivative Inhibits MRGPRX2 Induced Mast Cell Responses. Front. Immunol. 2020, 11, 703. [Google Scholar] [CrossRef]
- Dondalska, A.; Rönnberg, E.; Ma, H.; Pålsson, S.A.; Magnusdottir, E.; Gao, T.; Adam, L.; Lerner, E.A.; Nilsson, G.; Lagerström, M.; et al. Amelioration of compound 48/80-mediated itch and LL-37-induced inflammation by a single-stranded oligonucleotide. Front. Immunol. 2020, 11, 559589. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.-J.; Hao, D.; Wen, X.; Du, D.; He, G.; Jiang, X. The Theranostics Role of Mast Cells in the Pathophysiology of Rosacea. Front. Med. 2020, 6, 324. [Google Scholar] [CrossRef]
- Mencarelli, A.; Gunawan, M.; Yong, K.S.M.; Bist, P.; Tan, W.W.S.; Tan, S.Y.; Liu, M.; Huang, E.K.; Fan, Y.; Chan, J.K.Y.; et al. A humanized mouse model to study mast cells mediated cutaneous adverse drug reactions. J. Leukoc. Biol. 2020, 107, 797–807. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Li, C.; Ding, Y.; Hu, S.; An, H. Inhibitory function of Shikonin on MRGPRX2-mediated pseudo-allergic reactions induced by the secretagogue. Phytomedicine 2020, 68, 153149. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Hu, S.; Ding, Y.; Jia, Q.; Zhu, J.; An, H. Kaempferol ameliorates secretagogue-induced pseudo-allergic reactions via inhibiting intracellular calcium fluctuation. J. Pharm. Pharmacol. 2020, 72, 1221–1231. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, J.; Liu, R.; Zhang, G.; Dong, K.; Zhang, T. Paeoniflorin inhibits MRGPRX2-mediated pseudo-allergic reaction via calcium signaling pathway. Phytother. Res. 2020, 34, 401–408. [Google Scholar] [CrossRef]
- Qiao, C.; Hu, S.; Che, D.; Wang, J.; Gao, J.; Ma, R.; Jiang, W.; Zhang, T.; Liu, R. The anti-anaphylactoid effects of Piperine through regulating MAS-related G protein-coupled receptor X2 activation. Phytother. Res. 2020, 34, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Che, D.; Li, C.; Cao, J.; Wang, J.; Ma, P.; Zhao, T.; An, H.; Zhang, T. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int. Immunopharmacol. 2019, 66, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Che, D.; Zhang, T.; Liu, R.; Cao, J.; Wang, J.; Zhao, T.; Ma, P.; Dong, X.; He, L. Saikosaponin A inhibits compound 48/80-induced pseudo-allergy via the Mrgprx2 pathway in vitro and in vivo. Biochem. Pharmacol. 2018, 148, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Lv, Y.; Ma, P.; Ding, Y.; Wang, N.; Wang, X.; et al. The anti-anaphylactoid effects of hydroxysafflor yellow A on the suppression of mast cell Ca2+ influx and degranulation. Phytomedicine 2018, 48, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Che, D.; Ma, P.; Zhao, T.; Zeng, Y.; Wang, N. Anti-pseudo-allergy effect of isoliquiritigenin is MRGPRX2-dependent. Immunol. Lett. 2018, 198, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Wang, Z.; Artuc, M.; Guhl, S.; Zuberbier, T. MRGPRX 2 is negatively targeted by SCF and IL-4 to diminish pseudo-allergic stimulation of skin mast cells in culture. Exp. Dermatol. 2018, 27, 1298–1303. [Google Scholar] [CrossRef]
- Suzuki, Y.; Liu, S.; Ogasawara, T.; Sawasaki, T.; Takasaki, Y.; Yorozuya, T.; Mogi, M. A novel MRGPRX2-targeting antagonistic DNA aptamer inhibits histamine release and prevents mast cell-mediated anaphylaxis. Eur. J. Pharmacol. 2020, 878, 173104. [Google Scholar] [CrossRef]
- Wang, Z.; Guhl, S.; Franke, K.; Artuc, M.; Zuberbier, T.; Babina, M. IL-33 and MRGPRX2-triggered activation of human skin mast cells—elimination of receptor expression on chronic exposure, but reinforced degranulation on acute priming. Cells 2019, 8, 341. [Google Scholar] [CrossRef]
- Mangmool, S.; Kurose, H. Gi/o protein-dependent and-independent actions of pertussis toxin (PTX). Toxins 2011, 3, 884–899. [Google Scholar] [CrossRef]
- Ferry, X.E.V.; Daeffler, L.; Landry, Y. Activation of betagamma subunits of G(i2) and G(i3) proteins by basic secretagogues induces exocytosis through phospholipase Cβ and arachidonate release through phospholipase Cγ in mast cells. J. Immunol. 2001, 167, 4805–4813. [Google Scholar] [CrossRef]
- Hagiwara, D.; Miyake, H.; Morimoto, H.; Murai, M.; Fujii, T.; Matsuo, M. Studies on neurokinin antagonists. 2. Design and structure-activity relationships of novel tripeptide substance P antagonists, N. alpha.-[N. alpha.-(N. alpha.-acetyl-L-threonyl)-N1-formyl-D-tryptophyl]-N-methyl-N-(phenylmethyl)-L-phenylalaninamide and its related compounds. J. Med. Chem. 1992, 35, 3184–3191. [Google Scholar]
- Cao, J.; Li, C.; Ma, P.; Ding, Y.; Gao, J.; Jia, Q.; Zhu, J.; Zhang, T. Effect of kaempferol on IgE-mediated anaphylaxis in C57BL/6 mice and LAD2 cells. Phytomedicine 2020, 79, 153346. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Lennartsson, J.; Rönnstrand, L. Stem cell factor receptor/c-Kit: From basic science to clinical implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef]
- Babina, M.; Guhl, S.; Artuc, M.; Zuberbier, T. Allergic Fcε RI-and pseudo-allergic MRGPRX 2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 2018, 73, 256–260. [Google Scholar] [CrossRef]
- Thapaliya, M.; Ayudhya, C.C.N.; Amponnawarat, A.; Roy, S.; Ali, H. Mast Cell-Specific MRGPRX2: A Key Modulator of Neuro-Immune Interaction in Allergic Diseases. Curr. Allergy Asthma Rep. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Bernstein, I.L.; Li, J.T.; Bernstein, D.I.; Hamilton, R.; Spector, S.L.; Tan, R.; Sicherer, S.; Golden, D.B.; Khan, D.A.; Nicklas, R.A.; et al. Allergy diagnostic testing: An updated practice parameter. Ann. Allergy Asthma Immunol. 2008, 100, S1–S148. [Google Scholar] [CrossRef]
- Oppenheimer, J.; Durham, S.; Nelson, H.; Wolthers, O.D. Allergy Diagnostic Testing; World Allergy Organization: Milwaukee, WI, USA, 2014. [Google Scholar]
- Mayorga, C.; Ebo, D.G.; Lang, D.M.; Pichler, W.J.; Sabato, V.; Park, M.A.; Makowska, J.; Atanaskovic-Markovic, M.; Bonadonna, P.; Jares, E. Controversies in drug allergy: In vitro testing. J. Allergy Clin. Immunol. 2019, 143, 56–65. [Google Scholar] [CrossRef]
- Díaz, I.D. Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Quinolones. J. Investig. Allergol. Clin. Immunol. 2021, 31. [Google Scholar] [CrossRef]
- Decuyper, I.; Mangodt, E.; van Gasse, A.; Claesen, K.; Uyttebroek, A.; Faber, M.; Sabato, V.; Bridts, C.; Mertens, C.; Hagendorens, M.; et al. In vitro diagnosis of immediate drug hypersensitivity anno 2017: Potentials and limitations. Drugs R D 2017, 17, 265–278. [Google Scholar] [CrossRef]
- Spoerl, D.; Nigolian, H.; Czarnetzki, C.; Harr, T. Reclassifying anaphylaxis to neuromuscular blocking agents based on the presumed patho-mechanism: IgE-mediated, pharmacological adverse reaction or “innate hypersensitivity”? Int. J. Mol. Sci. 2017, 18, 1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Che, D.; Liu, R.; Han, S.; Wang, N.; Zhan, Y.; Pundir, P.; Cao, J.; Lv, Y.; Yang, L.; et al. Typical antimicrobials induce mast cell degranulation and anaphylactoid reactions via MRGPRX2 and its murine homologue MRGPRB2. Eur. J. Immunol. 2017, 47, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.A.; Shultz, L.D.; Luban, J.; Greiner, D.L. Overcoming current limitations in humanized mouse research. J. Infect. Dis. 2013, 208, S125–S130. [Google Scholar] [CrossRef] [PubMed]
| SN | Agonist | Major Findings | References |
|---|---|---|---|
| Peptide Agonist | |||
| 1 | CST-14 |
| [72,75,104] |
| 2 | Substance P |
| [46,67,70] |
| 3 | Somatostatin |
| [45,73] |
| 4 | PACAP |
| [45] |
| 5 | VIP |
| [45] |
| 6 | LL-37 |
| [50,103] |
| 7 | β-defensins |
| [103] |
| 8 | Indolicidin |
| [45] |
| 9 | PAMP 20 |
| [75,105] |
| 10 | Mastoparan |
| [72,91] |
| 11 | Human eosinophil granules |
| [45,46,106] |
| 12 | Proteases |
| [107,108] |
| 13 | AG-30/5C |
| [95] |
| 14 | AMP-IBP5 |
| [48] |
| 15 | GnRHR agonist and antagonist |
| [72] |
| Non-peptide agonist | |||
| 1 | Compound 48/80 |
| [45,72,109] |
| 2 | Fluoroquinolone antibiotics (ciprofloxacin) |
| [72,110,111] |
| 3 | NMBAs (rocuronium, mivacurium, and atracurium) |
| [72,85,112] |
| 4 | Opioids (morphine, pethidine chloride, dextrorphan, levorphanol) |
| [87,113,114] |
| 5 | Antidepressant drugs (clomipramine paroxetine and desipramine |
| [115] |
| 6 | Others (Icatibant, iopamidol, vancomycin, baicalein, and sinomemnin) |
| [72,92,95,116] |
| S.N. | Antagonist | Major findings | References |
|---|---|---|---|
| 1 | Pertussis Toxin (PTx) |
| [50,97] |
| 2 | Peptide QWF |
| [70] |
| 3 | Small compounds (compound 1 and 2) |
| [80] |
| Natural compounds | |||
| 4 | Resveratrol |
| [98] |
| 5 | Genistein |
| [77] |
| 6 | Shikonin |
| [174] |
| 7 | Osthole |
| [170] |
| 8 | Kaempferol |
| [175] |
| 9 | Paeoniflorin |
| [176] |
| 10 | Piperine |
| [177] |
| 12 | Quercetin |
| [178] |
| 12 | Saikosaponin A |
| [179] |
| 13 | Hydroxysafflor yellow A |
| [180] |
| 14 | Isoliquiritigenin |
| [181] |
| 15 | Others (DNA aptamer, cytokines, and stem cell factor) |
| [182,183,184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Duraisamy, K.; Chow, B.-K.-C. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. https://doi.org/10.3390/cells10051033
Kumar M, Duraisamy K, Chow B-K-C. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells. 2021; 10(5):1033. https://doi.org/10.3390/cells10051033
Chicago/Turabian StyleKumar, Mukesh, Karthi Duraisamy, and Billy-Kwok-Chong Chow. 2021. "Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2)" Cells 10, no. 5: 1033. https://doi.org/10.3390/cells10051033
APA StyleKumar, M., Duraisamy, K., & Chow, B.-K.-C. (2021). Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells, 10(5), 1033. https://doi.org/10.3390/cells10051033






