General Patterns and Species-Specific Differences in the Organization of the Tubulin Cytoskeleton in Indeterminate Nodules of Three Legumes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Bacterial Strains
2.2. Microscopy
2.2.1. Scanning Electron Microscopy
2.2.2. Transmission Electron Microscopy
2.2.3. Immunolocalization and Laser Scanning Confocal Microscopy
2.3. Quantitative Analysis
2.4. Bacteroid Isolation
2.5. Measurement of the Intensity of Antibody-Labeled Endoplasmic Microtubules
3. Results
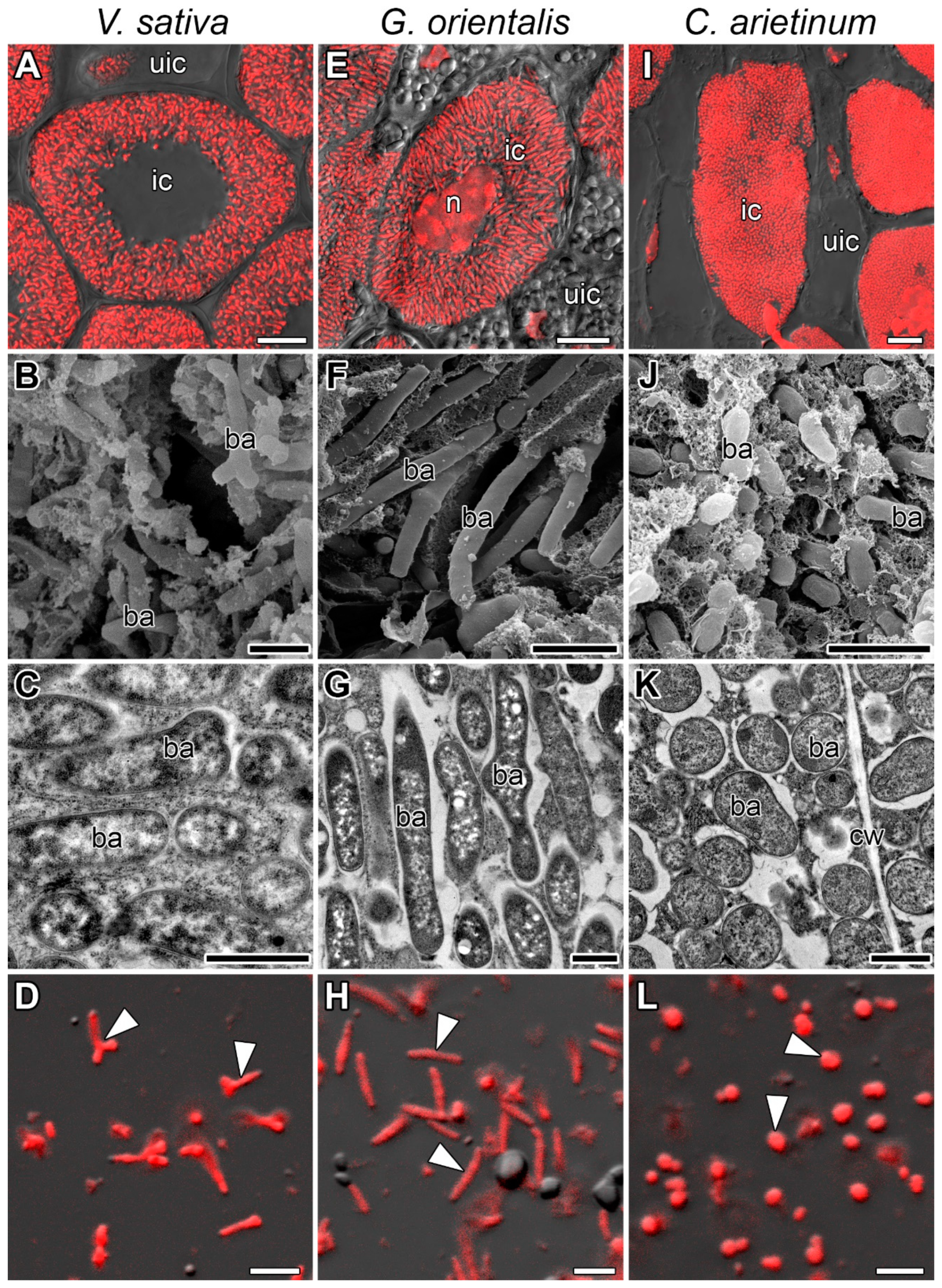
3.1. Symbiosome Distribution in Infected Cells and Bacteroid Morphology
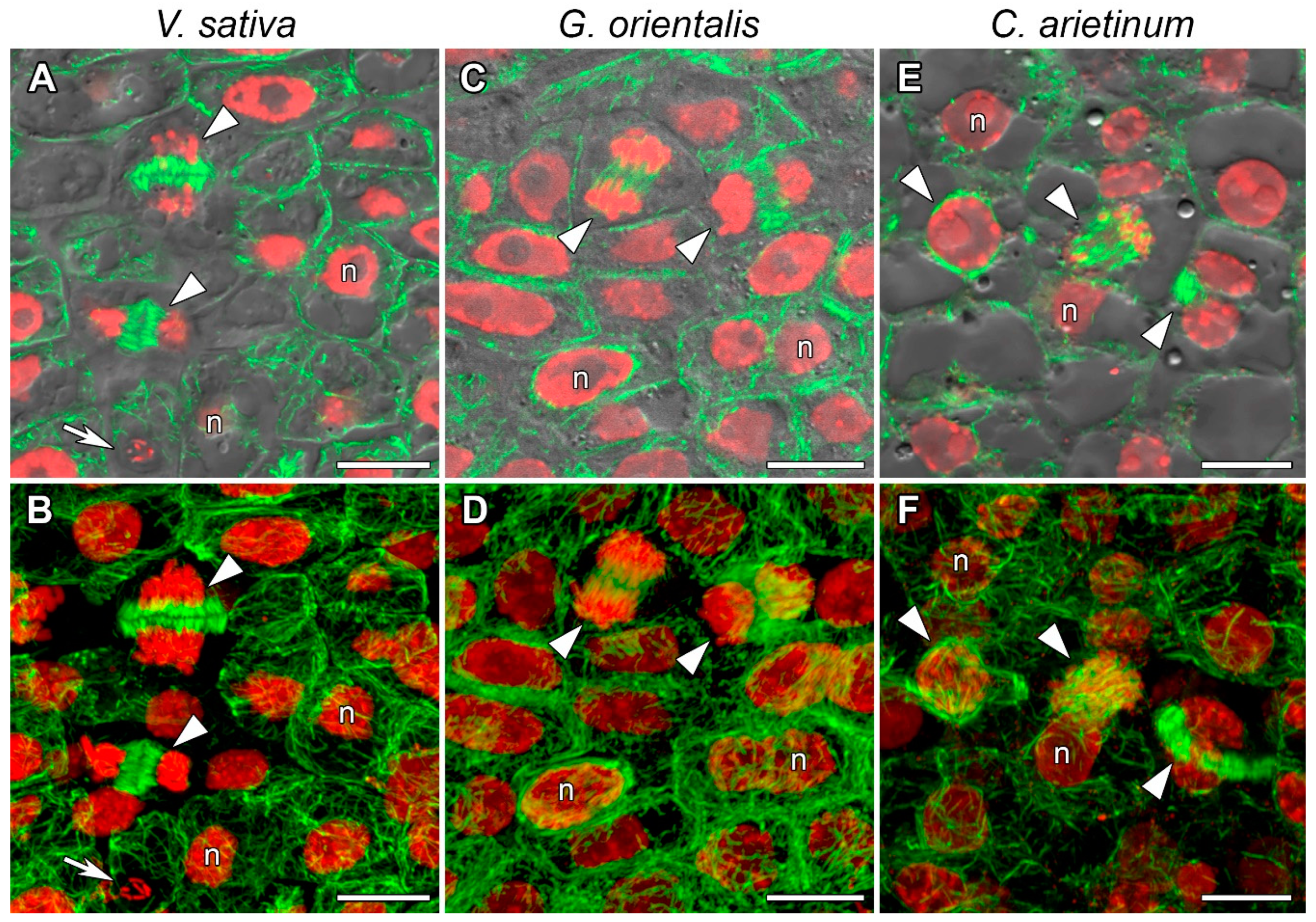
3.2. Microtubule Organization in Meristematic Cells
3.3. Microtubule Organization in Infected Cells in the Infection Zone
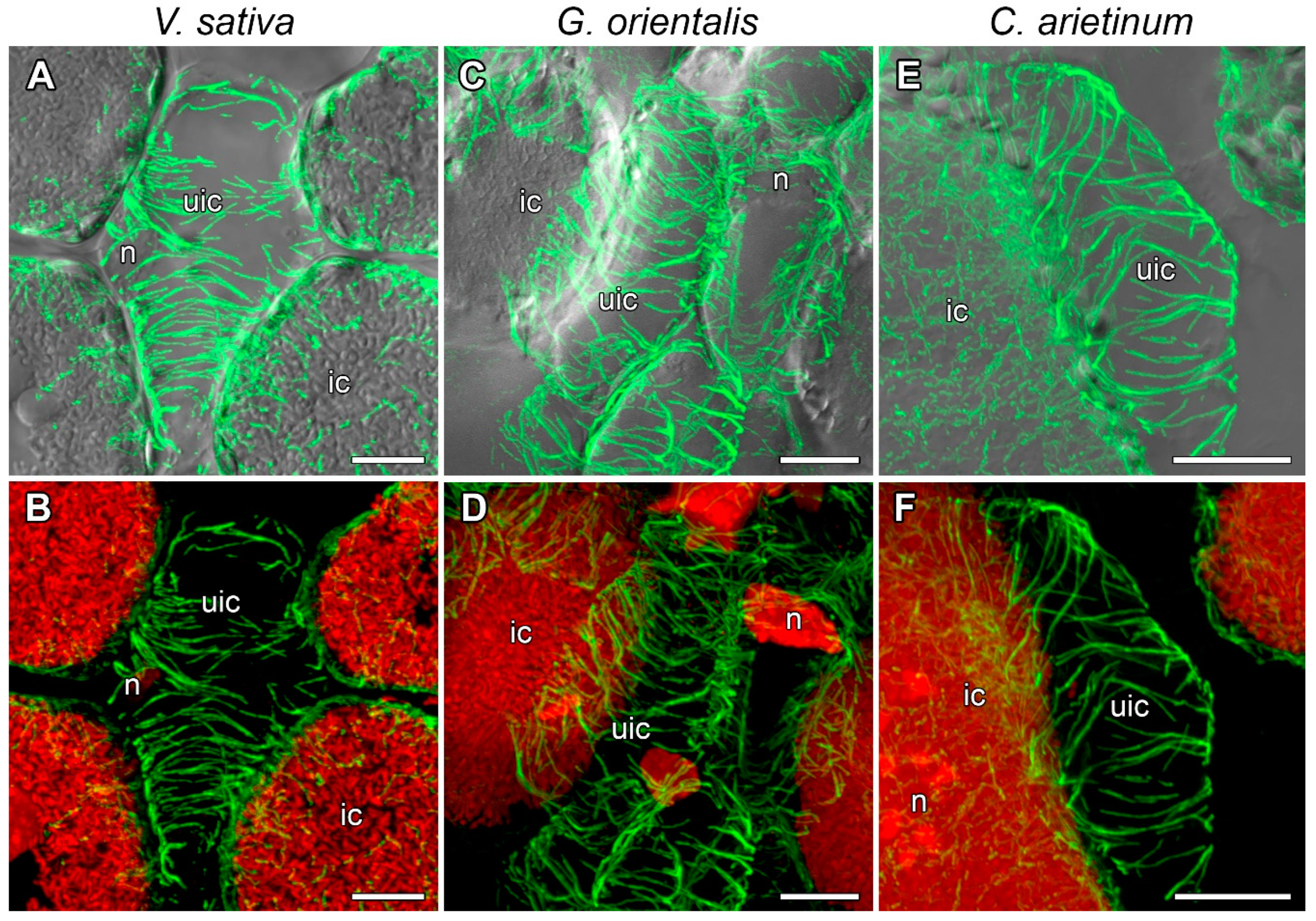
3.4. Microtubule Organization in Uninfected and Colonized Cells
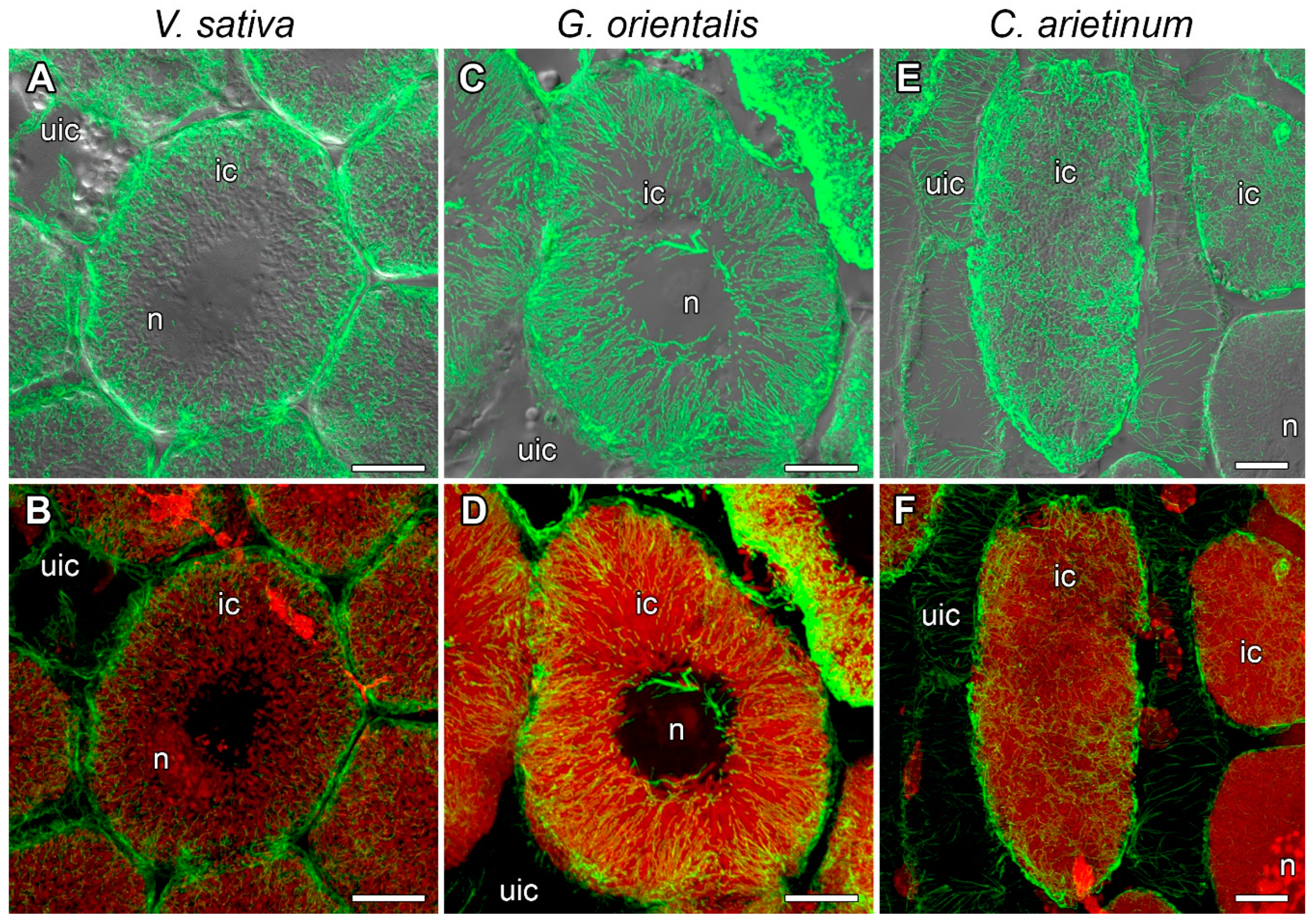
3.5. Microtubule Organization in Infected Cells in the Nitrogen Fixation Zone
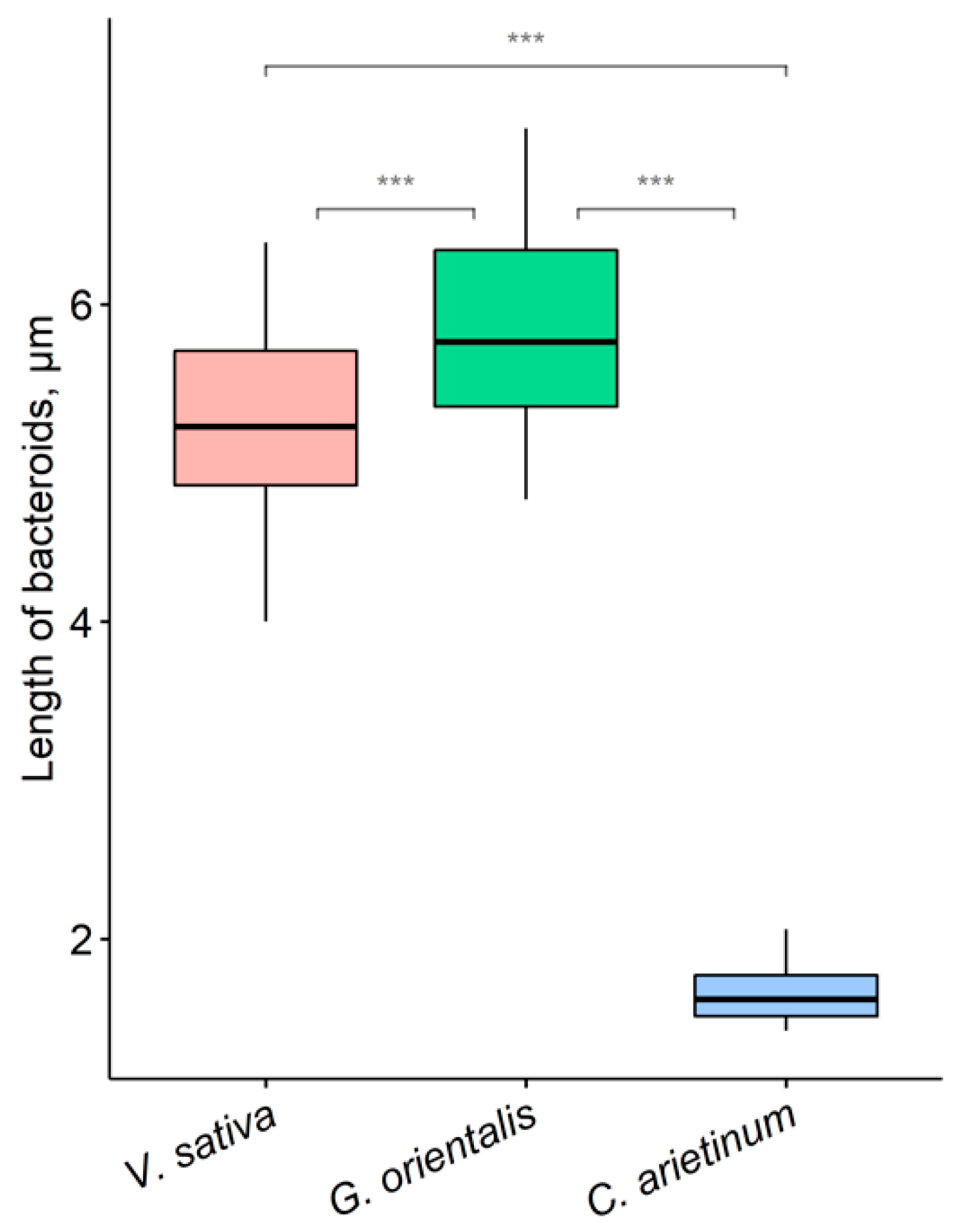
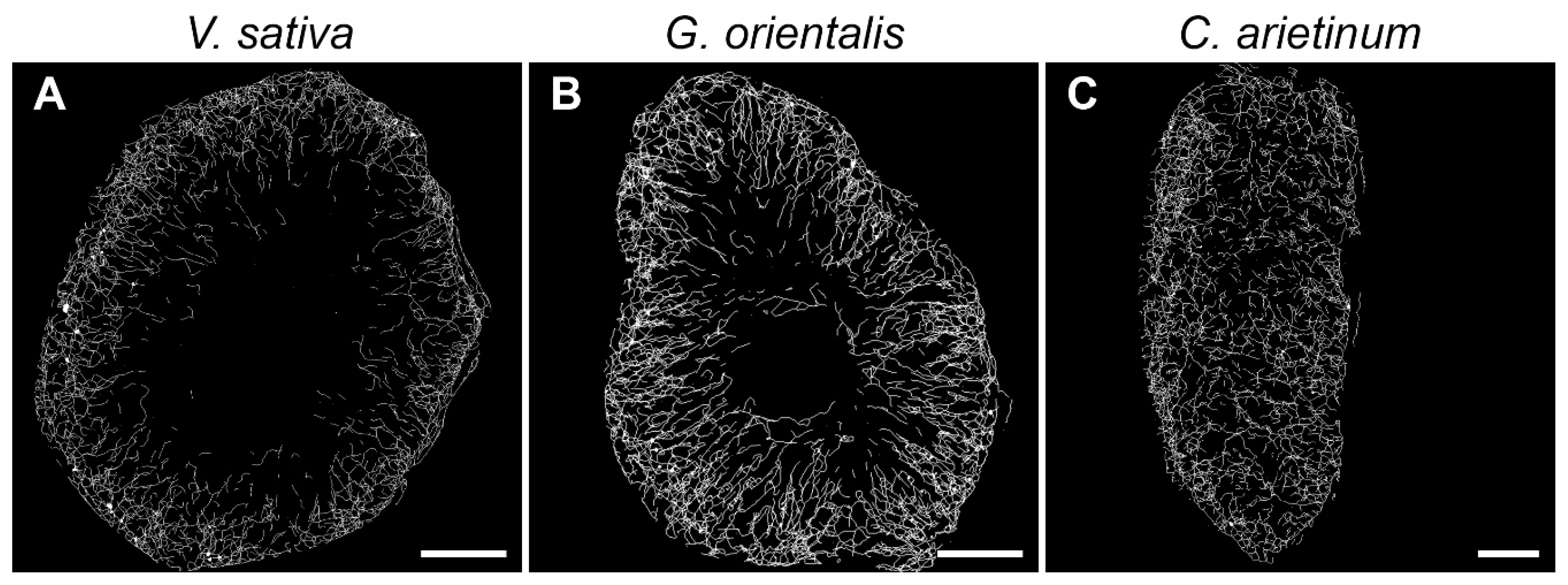
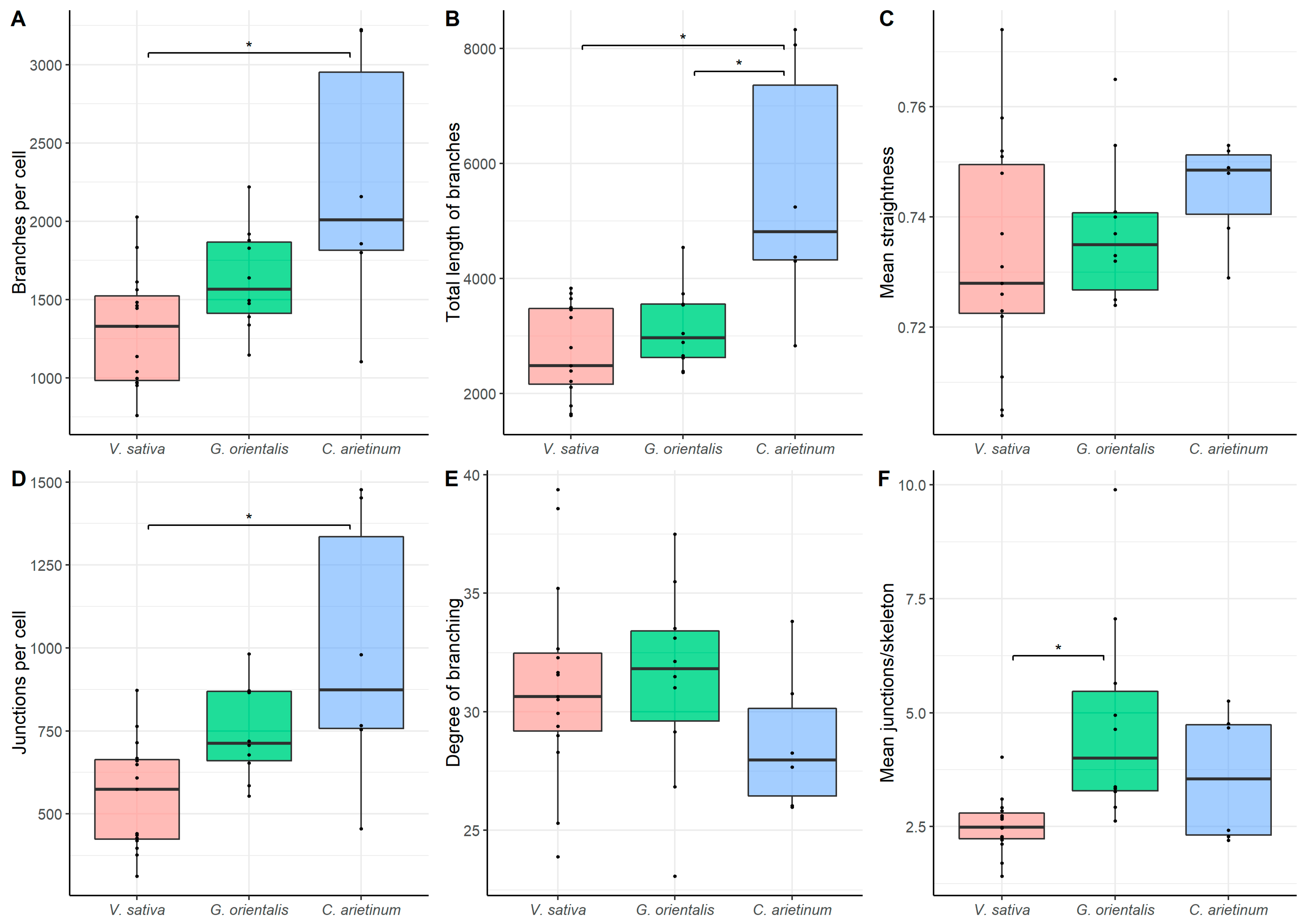
3.6. Quantitative Analysis
3.7. Mean Intensity of Antibody-Labeled Endoplasmic Microtubules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewin, N.J. Development of the legume root nodule. Annu. Rev. Cell Biol. 1991, 7, 191–226. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Tsyganova, A.V.; Tsyganov, V.E. Plant genetic control over infection thread development during legume-Rhizobium symbiosis. In Symbiosis; Rigobelo, E.C., Ed.; IntechOpen: London, UK, 2018; pp. 23–52. [Google Scholar] [CrossRef]
- Timmers, A.C.; Auriac, M.C.; Truchet, G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 1999, 126, 3617–3628. [Google Scholar]
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate map of Medicago truncatula root nodules. Development 2014, 141, 3517–3528. [Google Scholar] [CrossRef]
- Brewin, N.J. Plant cell wall remodelling in the Rhizobium–legume symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Kitaeva, A.B.; Tsyganov, V.E. Cell differentiation in nitrogen-fixing nodules hosting symbiosomes. Funct. Plant Biol. 2018, 45, 47–57. [Google Scholar] [CrossRef]
- Kondorosi, E.; Kondorosi, A. Endoreduplication and activation of the anaphase-promoting complex during symbiotic cell development. FEBS Lett. 2004, 567, 152–157. [Google Scholar] [CrossRef]
- Maróti, G.; Kondorosi, É. Nitrogen-fixing Rhizobium-legume symbiosis: Are polyploidy and host peptide-governed symbiont differentiation general principles of endosymbiosis? Front. Microbiol. 2014, 5, 326. [Google Scholar] [CrossRef]
- Alunni, B.; Gourion, B. Terminal bacteroid differentiation in the legume–rhizobium symbiosis: Nodule-specific cysteine-rich peptides and beyond. New Phytol. 2016, 211, 411–417. [Google Scholar] [CrossRef]
- Timmers, A.C.J. The role of the plant cytoskeleton in the interaction between legumes and rhizobia. J. Microsc. 2008, 231, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, V.E.; Kitaeva, A.B.; Demchenko, K.N. Comparative analysis of tubulin cytoskeleton rearrangements in nodules of Medicago truncatula and Pisum sativum. In The Model Legume Medicago truncatula; De Bruijn, F.J., Ed.; JohnWiley & Sons Inc.: Hoboken, NJ, USA, 2019; pp. 543–547. [Google Scholar] [CrossRef]
- Timmers, A.C.; Vallotton, P.; Heym, C.; Menzel, D. Microtubule dynamics in root hairs of Medicago truncatula. Eur. J. Cell Biol. 2007, 86, 69–83. [Google Scholar] [CrossRef]
- Sieberer, B.J.; Timmers, A.C.; Emons, A.M.C. Nod factors alter the microtubule cytoskeleton in Medicago truncatula root hairs to allow root hair reorientation. Mol. Plant-Microbe Interact. 2005, 18, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, V.N.; Kouchi, H.; Ridge, R.W. Microtubule dynamics in living root hairs: Transient slowing by lipochitin oligosaccharide nodulation signals. Plant Cell 2005, 17, 1777–1787. [Google Scholar] [CrossRef][Green Version]
- Su, C.; Klein, M.-L.; Hernández-Reyes, C.; Batzenschlager, M.; Ditengou, F.A.; Lace, B.; Keller, J.; Delaux, P.-M.; Ott, T. The Medicago truncatula DREPP protein triggers microtubule fragmentation in membrane nanodomains during symbiotic infections. Plant Cell 2020, 32, 1689–1702. [Google Scholar] [CrossRef]
- Davidson, A.L.; Newcomb, W. Organization of microtubules in developing pea root nodule cells. Can. J. Bot. 2001, 79, 777–786. [Google Scholar] [CrossRef]
- Fedorova, E.E.; de Felipe, M.R.; Pueyo, J.J.; Lucas, M.M. Conformation of cytoskeletal elements during the division of infected Lupinus albus L. nodule cells. J. Exp. Bot. 2007, 58, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, L.F.; Day, D.A.; Hardham, A.R. Cytoskeletal arrays in the cells of soybean root nodules: The role of actin microfilaments in the organisation of symbiosomes. Protoplasma 1998, 203, 194–205. [Google Scholar] [CrossRef]
- Timmers, A.C.; Auriac, M.C.; de Billy, F.; Truchet, G. Nod factor internalization and microtubular cytoskeleton changes occur concomitantly during nodule differentiation in alfalfa. Development 1998, 125, 339–349. [Google Scholar] [PubMed]
- Kitaeva, A.B.; Tsyganov, V.E. Influence of mutation in the gene Sym26 of the garden pea (Pisum sativum L.) on the organization of tubulin cytoskeleton in nodules. Agric. Biol. 2019, 54, 1014–1023. [Google Scholar] [CrossRef]
- Kitaeva, A.B.; Demchenko, K.N.; Tikhonovich, I.A.; Timmers, A.C.J.; Tsyganov, V.E. Comparative analysis of the tubulin cytoskeleton organization in nodules of Medicago truncatula and Pisum sativum: Bacterial release and bacteroid positioning correlate with characteristic microtubule rearrangements. New Phytol. 2016, 210, 168–183. [Google Scholar] [CrossRef]
- Bonaldi, K.; Gargani, D.; Prin, Y.; Fardoux, J.; Gully, D.; Nouwen, N.; Goormachtig, S.; Giraud, E. Nodulation of Aeschynomene afraspera and A. indica by photosynthetic Bradyrhizobium sp. strain ORS285: The Nod-dependent versus the Nod-independent symbiotic interaction. Mol. Plant-Microbe Interact. 2011, 24, 1359–1371. [Google Scholar] [CrossRef]
- Vasse, J.; de Billy, F.; Camut, S.; Truchet, G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 1990, 172, 4295–4306. [Google Scholar] [CrossRef]
- Sen, D.; Weaver, R.W. A comparison of nitrogen-fixing ability of peanut, cowpea and siratro plants nodulated by different strains of Rhizobium. Plant Soil 1981, 60, 317–319. [Google Scholar] [CrossRef]
- Oono, R.; Denison, R.F. Comparing symbiotic efficiency between swollen versus nonswollen rhizobial bacteroids. Plant Physiol. 2010, 154, 1541–1548. [Google Scholar] [CrossRef]
- Lamouche, F.; Gully, D.; Chaumeret, A.; Nouwen, N.; Verly, C.; Pierre, O.; Sciallano, C.; Fardoux, J.; Jeudy, C.; Szücs, A.; et al. Transcriptomic dissection of Bradyrhizobium sp. strain ORS285 in symbiosis with Aeschynomene spp. inducing different bacteroid morphotypes with contrasted symbiotic efficiency. Environ. Microbiol. 2019, 21, 3244–3258. [Google Scholar] [CrossRef]
- Bergersen, F.J. The cytology of bacteroids from root nodules of subterranean clover (Trifolium subterraneum L.). Microbiology 1955, 13, 411–419. [Google Scholar] [CrossRef]
- Montiel, J.; Szűcs, A.; Boboescu, I.Z.; Gherman, V.D.; Kondorosi, É.; Kereszt, A. Terminal bacteroid differentiation is associated with variable morphological changes in legume species belonging to the inverted repeat-lacking clade. Mol. Plant-Microbe Interact. 2016, 29, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Montiel, J.; Downie, J.A.; Farkas, A.; Bihari, P.; Herczeg, R.; Bálint, B.; Mergaert, P.; Kereszt, A.; Kondorosi, É. Morphotype of bacteroids in different legumes correlates with the number and type of symbiotic NCR peptides. Proc. Natl. Acad. Sci. USA 2017, 114, 5041–5046. [Google Scholar] [CrossRef] [PubMed]
- Fåhraeus, G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tsyganov, V.E. Early nodule senescence is activated in symbiotic mutants of pea (Pisum sativum L.) forming ineffective nodules blocked at different nodule developmental stages. Protoplasma 2018, 255, 1443–1459. [Google Scholar] [CrossRef]
- Kitaeva, A.B.; Kusakin, P.G.; Demchenko, K.N.; Tsyganov, V.E. Key methodological features of tubulin cytoskeleton studies in nodules of legume plants. Agric. Biol. 2018, 53, 634–644. [Google Scholar] [CrossRef]
- VandenBosch, K.A.; Bradley, D.J.; Knox, J.P.; Perotto, S.; Butcher, G.W.; Brewin, N.J. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 1989, 8, 335–341. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Fernández-González, R.; Muñoz-Barrutia, A.; Ortiz-De-Solorzano, C. 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc. Res. Tech. 2010, 73, 1019–1029. [Google Scholar] [CrossRef]
- Wojciechowski, M.F.; Lavin, M.; Sanderson, M.J. A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. Am. J. Bot. 2004, 91, 1846–1862. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Copeland, L. Ultrastructure of chickpea nodules. Protoplasma 1994, 182, 32–38. [Google Scholar] [CrossRef]
- Baluška, F.; Parker, J.S.; Barlow, P.W. Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.). J. Cell Sci. 1992, 103, 191–200. [Google Scholar]
- Adamakis, I.D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten affects the cortical microtubules of Pisum sativum root cells: Experiments on tungsten-molybdenum antagonism. Plant Biol. 2010, 12, 114–124. [Google Scholar] [CrossRef]
- Hamada, T. Microtubule organization and microtubule-associated proteins in plant cells. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2014; Volume 312, pp. 1–52. [Google Scholar]
- Oda, Y. Cortical microtubule rearrangements and cell wall patterning. Front. Plant Sci. 2015, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.; Shaw, S.L. Update: Plant cortical microtubule arrays. Plant Physiol. 2018, 176, 94–105. [Google Scholar] [CrossRef]
- Bichet, A.; Desnos, T.; Turner, S.; Grandjean, O.; Höfte, H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 2001, 25, 137–148. [Google Scholar] [CrossRef]
- Chen, X.; Grandont, L.; Li, H.; Hauschild, R.; Paque, S.; Abuzeineh, A.; Rakusová, H.; Benkova, E.; Perrot-Rechenmann, C.; Friml, J. Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 2014, 516, 90–93. [Google Scholar] [CrossRef]
- True, J.H.; Shaw, S.L. Exogenous auxin induces transverse microtubule arrays through TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX receptors. Plant Physiol. 2020, 182, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.C.; Abuzeineh, A.; Kopf, A.; Juanes-Garcia, A.; Ötvös, K.; Petrášek, J.; Sixt, M.; Benková, E. Phytohormone cytokinin guides microtubule dynamics during cell progression from proliferative to differentiated stage. EMBO J. 2020, 39, e104238. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, T.; Yano, K.; Ito, M.; Umehara, Y.; Suganuma, N.; Kawaguchi, M. Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 2012, 139, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Dolgikh, E.A.; Kusakin, P.G.; Kitaeva, A.B.; Tsyganova, A.V.; Kirienko, A.N.; Leppyanen, I.V.; Dolgikh, A.V.; Ilina, E.L.; Demchenko, K.N.; Tikhonovich, I.A.; et al. Mutational analysis indicates that abnormalities in rhizobial infection and subsequent plant cell and bacteroid differentiation in pea (Pisum sativum) nodules coincide with abnormal cytokinin responses and localization. Ann. Bot. 2020, 125, 905–923. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Morzhina, E.V.; Stefanov, S.Y.; Borisov, A.Y.; Lebsky, V.K.; Tikhonovich, I.A. The pea (Pisum sativum L.) genes sym33 and sym40 control infection thread formation and root nodule function. Mol. Gen. Genet. 1998, 259, 491–503. [Google Scholar] [CrossRef]
- Gavrin, A.; Jansen, V.; Ivanov, S.; Bisseling, T.; Fedorova, E. ARP2/3-mediated actin nucleation associated with symbiosome membrane is essential for the development of symbiosomes in infected cells of Medicago truncatula root nodules. Mol. Plant-Microbe Interact. 2015, 28, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, L.; Wang, Q.; Zhang, C.; Yu, Y.; Tian, J.; Kong, Z. The host actin cytoskeleton channels rhizobia release and facilitates symbiosome accommodation during nodulation in Medicago truncatula. New Phytol. 2019, 221, 1049–1059. [Google Scholar] [CrossRef]

| Vicia sativa | Galega orientalis | Cicer arietinum |
|---|---|---|
| 133.67 ± 9.697 b | 247.31 ± 24.262 a | 130.9 ± 24.015 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaeva, A.B.; Gorshkov, A.P.; Kirichek, E.A.; Kusakin, P.G.; Tsyganova, A.V.; Tsyganov, V.E. General Patterns and Species-Specific Differences in the Organization of the Tubulin Cytoskeleton in Indeterminate Nodules of Three Legumes. Cells 2021, 10, 1012. https://doi.org/10.3390/cells10051012
Kitaeva AB, Gorshkov AP, Kirichek EA, Kusakin PG, Tsyganova AV, Tsyganov VE. General Patterns and Species-Specific Differences in the Organization of the Tubulin Cytoskeleton in Indeterminate Nodules of Three Legumes. Cells. 2021; 10(5):1012. https://doi.org/10.3390/cells10051012
Chicago/Turabian StyleKitaeva, Anna B., Artemii P. Gorshkov, Evgenii A. Kirichek, Pyotr G. Kusakin, Anna V. Tsyganova, and Viktor E. Tsyganov. 2021. "General Patterns and Species-Specific Differences in the Organization of the Tubulin Cytoskeleton in Indeterminate Nodules of Three Legumes" Cells 10, no. 5: 1012. https://doi.org/10.3390/cells10051012
APA StyleKitaeva, A. B., Gorshkov, A. P., Kirichek, E. A., Kusakin, P. G., Tsyganova, A. V., & Tsyganov, V. E. (2021). General Patterns and Species-Specific Differences in the Organization of the Tubulin Cytoskeleton in Indeterminate Nodules of Three Legumes. Cells, 10(5), 1012. https://doi.org/10.3390/cells10051012








